1. Introduction
Nearly 30 years have passed since the first selective 5-HT1 receptor agonist sumatriptan became a choice of treatment for migraineurs. Recently, a new class of antimigraine drugs has joined antimigraine armamentarium, targeting the calcitonin gene-related peptide (CGRP) receptor; however, 5-HT1 receptor agonists remain a mainstream of migraine therapy and antimigraine drug research. This editorial discusses 5-HT1 receptor agonists approved for clinical use, the pathogenesis of migraine, and the tryptophan (TRP)-kynurenine (KYN) pathway that imposes a significant impact on serotonin metabolism and thus migraine, in search of a novel target for the treatment of migraine. Migraine is a prevalent and debilitating neurological disorder that causes throbbing headache and pulsatile sensation, often accompanied by nausea, vomiting, numbness, tingling, and severe sensitivity to light and sound. The global prevalence of migraine is 14.7%; it is three times more common in women than men; and women are less responsive to treatment. A migraine attack may last 72 h. Migraine is attributed to complex multifactorial conditions with underlying heterogeneity, which arise to considerable variations of inter-patient and longitudinal intra-patient responses to treatments. The first-line treatment for mild to moderate migraine is acetaminophen and nonsteroidal anti-inflammatory drugs, while the first-line treatment for mild to severe migraine is triptans, a group of the indole ring-containing tryptamine-based compounds which act as agonists for serotonin (5-hydroxytryptamine) 5-HT1B and 5-HT1D receptors. Sumatriptan is the first antimigraine drug that belongs to this family and is available in tablet, nasal spray, and subcutaneous injection. Previously, the action mechanism of the triptans was considered to be attributed to their vasoconstrictive properties in the central nervous system. However, a selective 5-HT1F receptor agonist lasmiditan lacks vasoconstrictive properties. Lasmiditan was approved for clinical use in 2019 by the US Food and Drug Administration and is a choice of treatment for migraineurs refractory to the triptans or having cardiovascular diseases, but the mechanism of its antimigraine action remains unclear [Citation1]. Recently a new class of drugs joined the repertoire of antimigraine treatment. Rimegepant and ubrogepant are CGRP receptor antagonists, while humanized CGRP monoclonal antibodies include erenumab, fremanezumab, and galcanezumab. Triptans and lasmiditan are used for the treatment of acute attack; the gepants both for an acute attack and prevention; and CGRP receptor antibodies for prevention. They are indicated for the prevention of episodic or chronic migraine in adults [Citation2]. Nevertheless, 5-HT1 receptor agonists have been a mainstream for antimigraine drug research, but little is known about the exact mechanism of action of antimigraine 5-HT1 receptor agonists.
2. Antimigraine drugs targeting 5-hydroxytryptamine 1 receptor
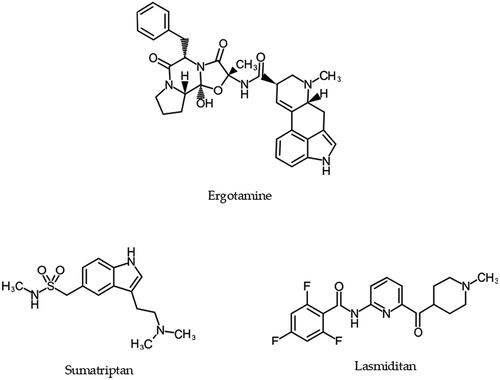
2.1. Ergotamine
The ergot alkaloid ergotamine is an antimigraine drug isolated from the ergot fungus in 1918, which targets 5-HT1/2-, adrenaline-, and dopamine receptors (). Dihydroergotamine relieves migraine headache through the activation of 5-HT1D receptors of intracranial blood vessels, which leads to vasoconstriction, and increase of 5-HT1D receptor expression of the trigeminal sensory nerve endings, which leads to the inhibition of the release of inflammatory neuropeptides, such as CGRP ( and ). Furthermore, ergotamine targets 5-HT1B receptor, 5-HT2A receptor, and adrenergic receptors alpha (α) 1A/B/D subtypes, prolonging vasoconstriction through the inhibition of norepinephrine uptake and stimulation of α -adrenergic receptors. Generally, ergotamine is not considered as the first-line treatment for migraine [Citation3].
Table 1. Clinically approved antimigraine drugs and their targets. 5-HT1A-D: 5-hydroxytryptamine receptors subtypes, A-D: adrenergic receptors subtypes, D1-2: dopamine receptor subtypes [Citation3–5]
Table 2. 5-HT1 receptor subtypes, location of expression pertaining to migraine, functions and ligands clinically approved for migraine [Citation3–5]
2.2. Triptans
Sumatriptan is the first clinically available drug introduced in 1991 among triptans (). Sumatriptan is a 5-HT1B/D agonist, but its efficacy is considered based on its action on the 5-HT1D receptors, which leads to vasoconstriction of the basilar artery and the blood vessels of the dura mater, increasing blood flow velocity in the middle cerebral artery and the internal carotid artery, and reducing carotid arterial flow. Sumatriptan blocks the plasma leakage caused by vasoactive compounds including CGRP, substance P, and neurokinin A from the trigeminovascular neurons. However, the affinity to 5-HT1B precipitates vasoconstriction of the cerebral artery, which may limit the efficacy as an antimigraine agent and is responsible for uncomfortable side effects including a mild allergic reaction, such as flush ( and ). Due to its peripheral vasoconstrictive action, furthermore, sumatriptan is contraindicated to patients who suffered from cardiovascular diseases, cerebrovascular diseases, and uncontrollable hypertension. Other triptans approved for clinical use are naratriptan, zolmitriptan, rizatriptan, almotriptan, frovatriptan, and eletriptan, which are indicated according to the age of migraineurs, the duration of migraine, or menstruation-induced migraine. The triptans are considered to act as antimigraine drugs by stimulating the presynaptic 5-HT1D receptor to inhibit the dural vasodilation and neurogenic inflammation, inhibiting the trigeminal nuclei cell excitability through 5-HT1B/D activation in the brainstem, and stimulating the vascular 5-HT1B receptors triggering meningeal, dural cerebral, and pial vasoconstriction. They are indicated for the acute phase of migraine attack and almotriptan is the only oral triptan approved for the treatment of migraine between 12 and 17 years old in the United States of America [Citation4]. Main side effects of triptans are chest pressure or heaviness, flushing, weakness, drowsiness, dizziness, malaise, a feeling of warmth, and paresthesia.
2.3. Ditans
Another target for the treatment of migraine is the 5-HT1F receptor expressed centrally in the cortex, the hypothalamus, the trigeminal ganglia, the locus coeruleus, the middle cerebral artery, and the upper cervical cord. The 5-HT1F receptor is also expressed peripherally, but the expression is low in the coronary artery and absent in the heart. Lasmiditan is the first clinically approved drug of the pyridine/piperidine-containing ditans which have a high affinity for 5-HT1F receptors (). Lasmiditan does not cause vasoconstriction. The precise mechanism of action is unknown, but the high selectivity for 5-HT1F receptors successfully terminates migraine by inhibiting the release of CGRP and probably substance P from the peripheral trigeminal endings in the dura and centrally acting in the trigeminal nucleus caudalis or the thalamus ( and ) [Citation5]. Drowsiness, dizziness, tiredness, and numbness or tingling of the skin or mouth are main side effects of Lasmiditan.
3. Searching for a cause of migraine to explore therapeutic options
The exact cause of migraine is unknown. A pain attack was linked to vasodilation and thus, the vascular theory of Wolff proposed that migraine headache was initiated by vasospasm causing focal cerebral ischemia leading to transient neurologic symptoms and followed by compensatory vasodilation. The brain acidosis and the stimulation of the pain nerve endings cause a headache [Citation6]. However, the vascular events are considered to be a part of mechanism in a migraine headache. The neurovascular theory of De Vries holds that a series of neural and vascular incidents cause migraine in which a neurogenic initiator leads to the alteration of the cerebral vasculature. The vascular pulsation activates stretch receptors, leading to the release of neuropeptides including CGRP from the perivascular nerves [Citation7].
The neurogenic theory of Moskowitz assumes that pain is caused by an inflammatory reaction with neuropeptides CGRP and substance P release from the trigeminal axons of the dura and pia mater [Citation8]. The neurological theory states that migraine arises as a result of abnormal neural functions including neuronal firings and neurotransmitter release. A state of neuronal hyperexcitability in the cerebral cortex, especially in the occipital cortex was observed in migraineurs who are not in migraine attack. The theory explicates migraine with aura in which depolarization of cortical neurons from the occipital lobe to the frontal lobe, causes sensory or motor dysfunction leading to sensory symptoms, loss of vision, and speech symptoms. This cortical spreading depression (CSD) is associated with a period of hyperemia followed by oligemia and the release of the vasodilator nitric oxide [Citation9].
The comorbidity of migraine may be an essential clue to the pathogenesis of migraine. Migraine occurs at a significantly higher rate with other illness such as cardiovascular diseases, respiratory diseases, psychiatric diseases, and restless legs syndrome. The damage of vascular endothelium, inflammation, and/or disturbance of serotonin, sex hormone, and/or stress hormone homeostasis links migraine to the comorbid diseases. Downregulation of the serotonergic nervous system, upregulation of the sympathetic nervous system, and/or activation of the hypothalamic-pituitary-adrenal axis links migraine to mood disorders and obesity [Citation10].
Consensus has been gained on that a migraine attack is caused by the activation of the trigeminovascular system (TVS), which is the final common pathway. The TVS consists of the nociceptive neurons innervating the dural blood vessels from the trigeminal ganglions and upper cervical dorsal nerve roots, releasing neuropeptides, such as CGRP, neurokinin A, substance P, and pituitary adenylate cyclase-activating peptide. Stimulation of 5-HT1B-, 5-HT1D, and 5-HT1F receptors disrupts signaling between the central and peripheral trigeminovascular neurons, thereby aborting migraine [Citation7].
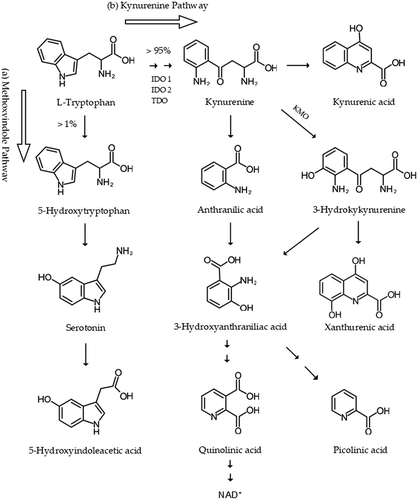
Serotonin is produced from TRP through the methoxyindole pathway that utilizes only about 1% of L-TRP available in the body. More than 95% of L-TRP is catabolized through the KYN pathway to biosynthesize various bioactive metabolites, such as oxidants, antioxidants, neurotoxins, neuroprotectants, and immunomodulators (). The KYN pathway was observed to influence serotonin metabolism [Citation11]. Activation of the KYN pathway and resultant alteration of KYN metabolites were reported in neurodegenerative and neuropsychiatric diseases including migraine [Citation12]. The altered levels of KYN metabolites of migraineurs includes KYN, kynurenic acid (KYNA), 3-hydroxykinurenine, 3-hydroxyanthuranilic acid, 5-hydroxyindoleacetic acid, and quinolinic acid. The excitotoxic KYN metabolites activated the N-methyl-D-aspartic acid or N-methyl-D-aspartate receptor of the glutamatergic nervous system. The levels of xanthurenic acid (XA) and anthranilic acid (ANA) were observed increased in migraineurs. The increased levels of XA were considered to be endogenous analgesic mechanisms through metabotropic glutamate receptor 2 activation, but the significance of elevated ANA levels remains unknown. The levels of glutamate were increased in blood of migraineurs both during attacks and between episodes. The levels of glutamate were also increased in patients with migraine with aura and thus, elevated glutamatergic neuron activity was associated with CSD. The peripheral KYNs were proposed as possible biomarkers for neurodegenerative diseases and psychiatric disorders [Citation13]. An animal model of orofacial pain showed elevated levels of glutamate, KYN, and KYNA in the caudal trigeminal nucleus, following a complete Freund’s adjuvant whisker pad injection. The levels of 5-HT and KYNA were significantly increased in the somatosensory cortex, which occurred in narrow time period between 24 and 48 hours, suggesting that a compensatory mechanism is in action to reduce glutamate sensitization and the TVS activation. Thus, the glutamatergic excitotoxicity plays an important role in the pathogenesis of migraine and excitotoxic KYN metabolites certainly contribute to the process [Citation14]. Thus, activation of KYN pathways and their bioactive metabolites play a significant role in the pathogenesis of migraine.
Figure 2. L-tryptophan metabolism: the methoxyindole and kynurenine pathways. A majority of tryptophan (TRP) is metabolized through the TRP-kynurenine (KYN) pathway, catalyzed by several key enzymes including indoleamine 2,3-dioxygenase (IDO) 1, IDO 2, tryptophan-2,3-dioxygenase (TDO), and kynurenine 3-monooxygenase (KMO) into several bioactive metabolites including kynurenine (KYN), kynurenic acid (KYNA), 3-hydroxykinurenine, anthranilic acid (ANA), xanthurenic acid (XA), 3-hydroxyanthuranilic acid, 5-hydroxyindoleacetic acid, xanthurenic acid (XA), and quinolinic acid. IDO 1, IDO 2, and KMO enzymes and KYN metabolites were shown to be involved in the pathogenesis of migraine
4. Expert opinion
The mechanism of 5-HT1B/D receptor agonist triptans for migraine was once considered to be their vasoconstrictive properties, whereas the mechanism of 5-HT1F receptor agonist lasmiditan is currently considered to occur through inhibition of the trigeminal nerves by hyperpolarization. Inhibition of 5-HT1B and 5-HT7 receptors is also under investigation for possible targets in migraine treatment [Citation15]. However, little is known about the pathophysiology of 5-HT1 receptors in migraine and mechanisms of antimigraine action through 5-HT1 receptors in migraine treatment. An uncharted area is the roles of the TRP-KYN metabolic pathway in migraine, which certainly has impacts on serotonin metabolism. Upon activation of the KYN pathway consequence for the methoxyindole pathway in migraine is expected to be investigated in search of possible interventional targets.
Vascular-, neurovascular-, neurogenic-, and neurological theories as well as the migraine comorbidity studies highlighted vascular endothelium damage, inflammation, serotonin, sex hormone, and stress hormone as participants in the pathogenesis of migraine. However, migraine mechanisms leading to the common pathway of TVS activation are not fully understood. Furthermore, the polygenic and multifactorial etiology of migraine makes it even more complex to untangle the threads of pathogenesis in migraine. Recently the TRP-KYN pathway is attracting growing attention in search of pathogenesis and interventional targets in a wide range of diseases including cancer, metabolic diseases, immune diseases, neurologic diseases, and psychiatric disorders. 5-HT1 receptor is not a single target for the treatment of migraine. Beyond the serotonergic nervous system, roles of the TRY-KYN pathway and KYN metabolites in migraine attack and under migraine treatment, and the influence of KYN metabolites on the expression of 5-HT1 receptor subtypes are to be elucidated. New findings may lead to identification of new migraine biomarkers which can be employed for the prevention, diagnosis, and intervention of migraine attacks. Furthermore, understanding of the roles of KYN metabolites may usher to their interaction with the tetrahydrobiopterin pathway and the cannabinoid system, opening an exploratory path into an unchartered territory to ensure personalized treatment plans for migraine.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors are grateful to Dr. Fanni Tóth for critical reading and English language editing.
Additional information
Funding
References
- Clemow DB, Johnson KW, Hochstetler HM, et al. Lasmiditan mechanism of action – review of a selective 5-HT1F agonist. J Headache Pain. 2020;21(1):71.
- Spindler BL, Ryan M. Medications approved for preventing migraine headaches. Am J Med. 2020;133(6):664–667.
- DRUGBANK: Ergotamine; [updated 2020 Nov 23; cited 2020 Nov 24] Available from: https://go.drugbank.com/drugs/DB00696
- DRUGBANK: triptan; [cited 2020 Nov 24] Available from: https://go.drugbank.com/unearth/q?utf8=%E2%9C%93&searcher=drugs&query=triptans
- DRUGBANK: lasmiditan; [updated 2020 Nov 2; cited 2020 Nov 24]. Available from: https://go.drugbank.com/drugs/DB11732
- Maso BN, Russo AF. Vascular contributions to migraine: time to revisit? Front Cell Neurosci. 2018;3(12):233.
- De Vrie T, Villalón CM, MaassenVanDenBrink A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther. 2020;211:107528.
- Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol. 2019;15(8):483–490.
- Borgdorff P. Arguments against the role of cortical spreading depression in migraine. Neurol Res. 2018;40(3):173–181.
- Wang SJ, Chen PK, Fuh JL. Comorbidities of migraine. Front Neurol. 2010;1:16.
- Knapp L, Szita B, Kocsis K, et al. Nitroglycerin enhances the propagation of cortical spreading depression: comparative studies with sumatriptan and novel kynurenic acid analogues. Drug Des Devel Ther. 2016;11:27–34.
- Tanaka M, Toldi J, Vécsei L. Exploring the etiological links behind neurodegenerative diseases: inflammatory cytokines and bioactive kynurenines. Int J Mol Sci. 2020;21:2431.
- Török N, Tanaka M, Vécsei L. Searching for peripheral biomarkers in neurodegenerative diseases: the tryptophan-kynurenine metabolic pathway. Int J Mol Sci. 2020;21:9338.
- Jovanovic F, Candido KD, Knezevic NN. The Role of the kynurenine signaling pathway in different chronic pain conditions and potential use of therapeutic agents. Int J Mol Sci. 2020;21:6045.
- Cortes-Altamirano JL, Olmos-Hernandez A, Jaime HB, et al. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors and their role in the modulation of pain response in the central nervous system. Curr Neuropharmacol. 2018;16(2):210–221.