ABSTRACT
Introduction
Neuropathic pain (NeP) is a chronic and refractory condition in many patients, and its treatment is a challenge for physicians. A new voltage-gated Ca2+ channel α2δ ligand, mirogabalin, has a high specific binding affinity for the α2δ subunit, with a slower dissociation rate for α2δ-1 than α2δ-2 compared to that of pregabalin. Mirogabalin was shown to be effective in NeP animal models, with a margin of safety between central nervous system side effects and the analgesic effect of the dose. It exerted a favorable analgesic effect, was well tolerated in patients with peripheral NeP (P-NeP), and was first approved in Japan in 2019 and subsequently in Korea and Taiwan in 2020.
Areas covered
The purpose of this article is to review the pharmacological characteristics, pharmacokinetics, and efficacy and safety of mirogabalin for NeP based on the results of non-clinical and clinical studies.
Expert opinion
Although there are several first-line therapies for NeP, insufficient efficacy and adverse drug reactions of NeP drugs often cause patient dissatisfaction. Mirogabalin was effective and well tolerated with a step-wise dose increase in clinical studies on P-NeP patients. Thus, mirogabalin is expected to be a useful treatment option for patients with P-NeP.
1. Introduction
Neuropathic pain (NeP) is usually a chronic and refractory condition that does not resolve even if the underlying disorder is cured [Citation1]. NeP can be induced by a variety of diseases, such as herpes zoster (HZ), diabetes mellitus (DM), spinal disorders, cancer, interventions, and trauma [Citation2–4], and is subclassified into peripheral NeP (P-NeP) and central NeP according to the region of nerve injury causing the pain [Citation2]. The reported number of patients with DM in 2019 was 463 million (aged 20 to 79 years, approximately 9.3% of the world population) [Citation5], and as approximately 20–30% of diabetic patients reported experiencing diabetic peripheral neuropathic pain (DPNP) [Citation6–10], the estimated number of DPNP patients in 2017 was 93–139 million. In Japan, approximately 10 million patients were strongly suspected of having diabetes [Citation11], and as approximately 9–22% of the diabetic patients in Japan have DPNP [Citation12], the estimated number of DPNP patients in 2017 was 0.9–2.2 million. The incidence rate of HZ was estimated as 3.4 cases/1000 persons/year in Europe [Citation13] and 3.15 cases/1000 persons/year in the USA [Citation14], and approximately 1.7 million and 1 million new patients, respectively, can be expected each year. As 5.4% to 17.6% of HZ patients reported experiencing post-herpetic neuralgia (PHN) [Citation15], the estimated number of new PHN patients each year in Europe and the USA was approximately 0.1 to 0.3 million and 0.06 to 0.18 million, respectively. In Japan, the annual number of patients with HZ is estimated to be approximately 0.6 million [Citation16]. As 10–25% of the HZ patients in Japan reported experiencing PHN [Citation17], the estimated numbers of PHN patients were 0.06–0.15 million. NeP not only causes severe pain but also markedly reduces the quality of life (QOL) of patients; therefore, the economic loss due to the consequent decrease in the labor force has become a major social problem [Citation18,Citation19].
Currently, Ca2+ channel α2δ ligands, tricyclic antidepressants (TCAs), and serotonin norepinephrine reuptake inhibitors (SNRIs) are used as first-line drugs for NeP [Citation1,Citation20,Citation21]. In a large-scale prescription database study of oral analgesic drugs in Japan in 2017, pregabalin and duloxetine were among the top 10 drugs prescribed for many patients with chronic pain [Citation22]. However, insufficient pain relief and adverse drug reactions (ADRs) are some of the problems associated with NeP drugs [Citation23–25]. In a cross-sectional study of satisfaction with oral analgesic drugs in Japanese patients with chronic pain in 2019, only 26.1% of NeP patients were satisfied with NeP drugs [Citation26]. Therefore, new drugs with more favorable analgesic efficacy and fewer ADRs are required as alternative therapeutic options for patients with NeP.
Mirogabalin besylate (herein referred to as mirogabalin) is a novel voltage-gated Ca2+ channel α2δ ligand developed by Daiichi Sankyo Co., Ltd. (Tokyo, Japan). It was approved for the indication of P-NeP in Japan in January 2019 [Citation27–29] and subsequently for P-NeP in Korea [Citation29] and for PHN and DPNP in Taiwan [Citation30] in 2020. Based on the results of phase 1 [Citation29,Citation31] and phase 2 studies [Citation32,Citation33], pivotal phase 3 studies were conducted and clarified that mirogabalin had a favorable analgesic effect and was well tolerated in Asian patients with PHN and DPNP [Citation34,Citation35]. We prepared the review article of mirogabalin mainly using the results of in vitro/in vivo studies and pivotal phase 3 studies conducted in Asian countries including Japan.
The purpose of this review article is to provide an overview of the pharmacological characteristics, pharmacokinetics, efficacy, and safety of mirogabalin.
2. Overview of the market
Current systemic treatment options for NeP include Ca2+ channel α2δ ligands, TCAs, SNRIs, tramadol, and strong opioids [Citation23,Citation36]. For many patients, the efficacy of these treatment options is limited and can be associated with a range of adverse effects [Citation37]. Although some potential pharmacological candidates, including cannabis-deprived compounds and transient receptor potential vanilloid 1 antagonists, have proved effective for the treatment of NeP in phase 3 clinical studies, further evaluations are needed [Citation38]. The guidelines for the pharmacologic management of NeP published by the Japan Society of Pain Clinicians recommended pregabalin, gabapentin (this drug is not approved for NeP in Japan, but NeP patients could claim insurance reimbursement for gabapentin), duloxetine, amitriptyline, nortriptyline, and imipramine as drugs for NeP in 2016 [Citation1]. Mirogabalin was added as a drug that can be used for P-NeP in 2019 [Citation39]. Mirogabalin was launched in Japan, with the reported sales to be approximately 8 billion Japanese yen between April, 2020 and March, 2021, whereas in Taiwan and Korea, mirogabalin was approved in 2020 but they are still preparing for its launch in their markets.
3. Introduction to the compound
Mirogabalin has a high binding affinity for the voltage-gated Ca2+ channel α2δ-1 subunit [Citation40]; moreover, it exerted a favorable analgesic effect and was well tolerated in Asian patients with PHN or DPNP in phase 3 studies [Citation34,Citation35]. This drug was first approved for the treatment of P-NeP in Japan. Currently, an Asian phase 3 study (the AMELA study, clinical trial registration number: NCT03901352) is underway to evaluate the efficacy and safety of mirogabalin for Asian patients with post-spinal cord injury (SCI) NeP [Citation41]. The details of the pharmacological characteristics, pharmacokinetics, clinical efficacy and safety, and approval and marketing status of mirogabalin are introduced in the following sections.
4. Chemistry
The chemical name of mirogabalin besylate is [(1R,5S,6S) -6-(aminomethyl)-3-ethylbicyclo [3.2.0] hept-3-en-6-yl] acetic acid monobenzenesulfonate (Drug summary box). Its molecular formula is C12H19NO2∙C6H6O3S and molecular weight is 367.46. Mirogabalin is a white to pale yellowish powder and its water solubility is 42 mg/mL at 20°C; its dissociation constants (pKa1 and pKa2) are 4.1 and 11.0, respectively [Citation29].
5. Pharmacodynamics
5.1. In vitro studies
5.1.1. Binding-dissociation profile of mirogabalin to human α2δ subunits
The binding-dissociation profiles of 3H-mirogabalin and 3H-pregabalin for the human α2δ-1 and α2δ-2 subunits were evaluated using cell membrane fractions prepared from 293A cells expressing human α2δ-1 or α2δ-2 [Citation40]. The binding-dissociation kinetic parameters of mirogabalin and pregabalin for the α2δ-1 and α2δ-2 subunits are shown in . The dissociation constants (Kd) of mirogabalin for the α2δ-1 and α2δ-2 subunits were 13.5 nmol/L and 22.7 nmol/L, respectively, while those of pregabalin were 62.5 nmol/L and 125.0 nmol/L, respectively. These results suggested that the binding affinities of mirogabalin to the α2δ-1 and α2δ-2 subunits were higher than those of pregabalin. Furthermore, no clear subtype selectivity of the two drugs for α2δ-1 and α2δ-2 was observed. The dissociation half-life (t1/2) of mirogabalin from the α2δ-1 and α2δ-2 subunits was 11.1 h and 2.4 h, respectively, while that of pregabalin from both subunits was 1.4 h (, ). The dissociation t1/2 for a target protein is considered an important factor for determining the duration of pharmacological effects and target selectivity in vivo [Citation42].
Figure 1. Dissociation curves of mirogabalin and pregabalin for the human α2δ-1 and human α2δ-2 subunits
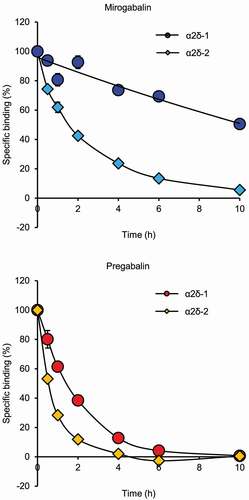
Table 1. Binding-dissociation kinetic parameters of 3H-mirogabalin and 3H-pregabalin for the α2δ subunits
5.1.2. Inhibitory effect of mirogabalin on N-type Ca2+ channel current in rat dorsal root ganglion (DRG) neurons
Cultured DRG neurons isolated from rats were treated with mirogabalin and pregabalin, and then N-type Ca2+ channel current was measured using a whole-cell patch-clamp technique [Citation43]. Mirogabalin (50 μmol/L) and pregabalin (200 μmol/L) reduced N-type Ca2+ channel current in rat DRG neurons. Thus, mirogabalin could exert its analgesic effect through the same mechanism of action – by reducing excessive excitatory neurotransmitter release via Ca2+ influx inhibition – as pregabalin and gabapentin [Citation40,Citation44,Citation45].
5.2. In vivo studies
Mirogabalin dose levels are expressed as those of the free form unless specified otherwise.
5.2.1. Analgesic effect of mirogabalin in partial sciatic nerve ligation (PSL) rats
PSL rats were prepared by tight ligation of 1/3 to 1/2 of the sciatic nerve in the upper thigh using surgical threads [Citation46]. Mirogabalin (1, 3 and 10 mg/kg) or pregabalin (3, 10 and 30 mg/kg) was orally administered to PSL rats, and paw withdrawal threshold to mechanical stimulation using von Frey test was measured [Citation40]. The paw withdrawal thresholds for mirogabalin at 3 mg/kg and 10 mg/kg peaked at 4 h and remained effective for 6–8 h, while that for pregabalin at 30 mg/kg peaked at 4 h but decreased to the baseline level (control rat level) within 6 h. Furthermore, the area under the paw withdrawal threshold curve up to 8 h for both mirogabalin (3 mg/kg and 10 mg/kg) and pregabalin (10 mg/kg and 30 mg/kg) increased in a dose-dependent manner. These results showed that mirogabalin exerted a more sustained analgesic effect than pregabalin in PSL rats.
5.2.2. Analgesic effect of mirogabalin in streptozotocin (STZ)-induced diabetic rats
Mirogabalin (2.5, 5 and 10 mg/kg, twice daily [BID]) or pregabalin (10, 20 and 40 mg/kg, BID) was orally administered for 5 days in diabetic rats at 18 weeks after a 60 mg/kg STZ injection [Citation40]. The paw withdrawal threshold for mirogabalin and pregabalin peaked at 4 h on Days 1, 3, and 5, with no apparent difference in the maximum effects between the two drugs. The paw withdrawal threshold for mirogabalin at 10 mg/kg in diabetic rats was comparable to that in normal rats for 2–12 h, while that of pregabalin at 40 mg/kg was increased up to 2–8 h but decreased to baseline level (control level) within 12 h (). Moreover, for 10 mg/kg mirogabalin, the pain thresholds before administration on Day 3 and Day 5 were at the same level as those of normal controls but were higher than those on Day 1, regardless of almost undetectable plasma drug concentrations. The above phenomena and differences from pregabalin might be potentially explained by the sustained binding affinity of mirogabalin for the α2δ-1 subunit, rather than its pharmacokinetic parameters. The 50% effective dose (ED50) values for the analgesic effects of mirogabalin and pregabalin on Day 1 were 4.4 mg/kg and 26.8 mg/kg, respectively; thus, mirogabalin showed a 6.09-fold greater potency than pregabalin in the diabetic rats (). These results suggested that mirogabalin exerted a more potent and sustained analgesic effect than pregabalin in STZ-induced diabetic rats.
Figure 2. Analgesic effect of mirogabalin in streptozotocin-induced diabetic rats
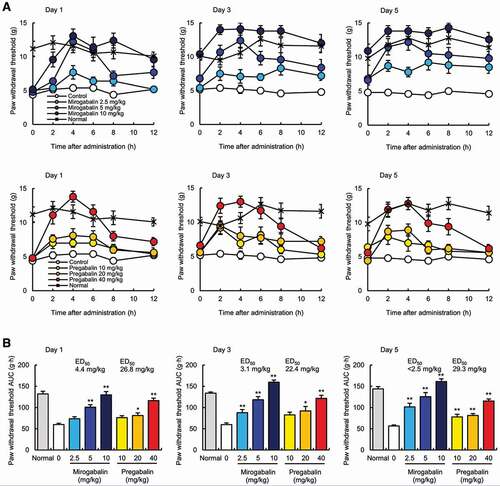
5.3. Mechanism of analgesic action of mirogabalin
5.3.1. Role of the α2δ subunit in the analgesic effect of mirogabalin
The α2δ-1 subunit protein and mRNA were found to be upregulated in the spinal dorsal horn or DRG of sciatic nerve ligation rats and SCI rats [Citation47–49]. Whether mirogabalin exerts its analgesic effect via the α2δ-1 or α2δ-2 subunit was investigated using PSL mutant mice lacking ligand binding affinity to α2δ-1 (by substituting arginine at position 217 with alanine [R217A] on the α2δ-1 subunit) or to α2δ-2 (by substituting arginine at position 282 with alanine [R282A] on the α2δ-2 subunit) [Citation50]. The paw withdrawal threshold to mechanical stimulation induced using von Frey filament in mutant mice was measured to evaluate the analgesic effect of mirogabalin. The analgesic effect of mirogabalin (10 mg/kg, p.o.) was not observed in α2δ-1 (R217A) mutant mice but was observed in α2δ-2 (R282A) mutant mice as well as wild-type mice, indicating that mirogabalin exerts its analgesic effect via binding to the α2δ-1 subunit, not the α2δ-2 subunit (). Similarly, the analgesic effects of pregabalin and gabapentin were not observed in α2δ-1 mutant mice, indicating that pregabalin and gabapentin are considered to exert their analgesic effects via binding to the α2δ-1 subunit [Citation51]. However, it has not been reported that the analgesic effects of pregabalin and gabapentin were not affected in α2δ-2 mutant mice.
Figure 3. Analgesic effect of mirogabalin in a PSL model of α2δ subunit mutant mice
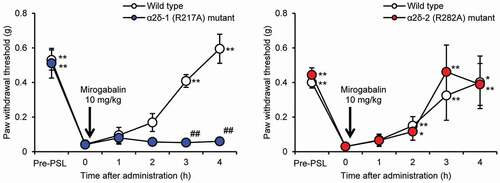
5.3.2. Mirogabalin-induced activation of the descending noradrenergic pain inhibitory system involving its binding to the α2δ-1 subunit to produce an analgesic effect
Mirogabalin dose levels are expressed as the salt form. Mirogabalin had analgesic effects on mechanical (von Frey test) and thermal (plantar test) hypersensitivities in PSL mice when administered both systemically (10 and 30 mg/kg, intraperitoneally [i.p.]) or locally (10 and 30 μg, intracerebroventricularly [i.c.v.] or intrathecally [i.t.]). The analgesic effects of mirogabalin (30 mg/kg, i.p. and 30 μg, i.c.v.) were largely reduced in mice pretreated with the α2-adrenoceptor antagonist yohimbine HCl (3 μg, i.t.). Moreover, in α2δ-1 (R217A) mutant mice, the analgesic effects of mirogabalin (30 μg, i.c.v.) were almost completely suppressed. These results suggest that mirogabalin activates the descending noradrenergic pain inhibitory system supraspinally, and that the binding of mirogabalin to the α2δ-1 subunit may be necessary for pain relief [Citation52].
5.4. Safety pharmacology
5.4.1. Effect on motor coordination and spontaneous locomotor activities and safety margins
To evaluate the central nervous system (CNS)-related effects of mirogabalin and pregabalin, motor coordination and spontaneous locomotor activities were measured using the rota-rod test and Supermex system, respectively. Motor coordination was significantly reduced by mirogabalin (10, 30 mg/kg and 100 mg/kg, respectively) and pregabalin (30, 100 mg/kg and 300 mg/kg, respectively) administration. Similarly, spontaneous locomotor activities were significantly reduced by mirogabalin (30 mg/kg and 100 mg/kg, respectively) and pregabalin (100 mg/kg and 300 mg/kg, respectively) administration. ED50 values are shown in . The safety margins of mirogabalin and pregabalin were calculated by dividing the ED50 values for the CNS side-effects by those for their analgesic effects in STZ-induced diabetic rats. The safety margins of mirogabalin were approximately 2.4–5.3 fold higher than those of pregabalin.
Table 2. Safety margins calculated from analgesic effects and CNS side-effect of mirogabalin and pregabalin
Several reports have shown that the α2δ-1 subunit is associated with induction and maintenance of NeP, while the α2δ-2 subunit is related to ataxia, dyskinesia, and absence seizures, respectively [Citation40,Citation53–56]. The higher safety margin of mirogabalin could be related to the slower dissociation rate of mirogabalin for the α2δ-1 subunit than the α2δ-2 subunit compared with those of pregabalin, as described in sub-section 5.1.1. ‘Binding-dissociation profile of mirogabalin to human α2δ subunits.’ In the non-clinical study, the safety margin was higher in mirogabalin than in pregabalin. However, as no head-to-head comparison was performed between mirogabalin and pregabalin in the clinical study, it is unclear whether mirogabalin has an improved safety margin over pregabalin in clinical practice.
6. Pharmacokinetics and metabolism
6.1. Absorption
In a single-dose phase 1 study conducted in the USA, mirogabalin at 3, 5, 10, and 30, 50, and 75 mg, was orally administered to healthy adult volunteers (6 participants/group). Mirogabalin was rapidly absorbed and reached maximum plasma concentration (Cmax) at 1 h after treatment [Citation57]. Both Cmax and area under the plasma concentration-time curve up to infinity (AUCinf) of mirogabalin increased in a dose-dependent manner (Cmax: 49, 78, 205, 433, 671, and 1060 ng/mL, respectively; AUCinf: 184, 276, 614, 1682, 3231, and 4896 ng×h/mL, respectively). The t1/2 was 2.96–3.37 h, and mirogabalin up to 30 mg in a single dose study was well tolerated [Citation57]. Similarly, a phase 1 study was conducted on healthy adult Japanese volunteers receiving mirogabalin at 10 mg (6 participants) or 20 mg (5 participants) [Citation29]. The Cmax, AUCinf, and t1/2 of mirogabalin at 10 mg were consistent with those in the phase 1 study described above.
In a repeated dose phase 1 study on healthy adult Japanese volunteers (6 participants/group), mirogabalin (10 mg BID and 15 mg BID) was administered for 7 days. Mirogabalin reached the steady state by Day 3, and tmax of mirogabalin (10 mg BID and 15 mg BID) on Day 7 was 1.5 h and 0.5 h, respectively; t1/2 was 2.4 h and 2.8 h, respectively [Citation31]. Cmax and area under the plasma concentration-time curve during dosing interval (AUCtau) on Day 7 increased in a dose-dependent manner (mirogabalin 10 mg BID and 15 mg BID, Cmax: 210 ng/mL and 381 ng/mL, AUC0−12 h: 601 ng×h/mL and 1057 ng×h/mL, respectively). No accumulation of mirogabalin was observed.
6.2. Effect of food intake
In a cross-over phase 1 study on 30 healthy adult volunteers, mirogabalin at 15 mg was orally administered in the fasting and after-meal states. Cmax (SD) was 230 ± 53.1 ng/mL and 188 ± 40.1 ng/mL in the fasting and after meal states, respectively. Area under the plasma concentration-time curve up to the last quantifiable time (AUClast [SD]) was 884 ± 157 ng×h/mL and 833 ± 155 ng×h/mL, while tmax was 1 h and 1.5 h, respectively [Citation29]. No serious adverse events (AEs) or AEs leading to treatment discontinuation were reported. Thus, no clear effect of food intake was observed on the pharmacokinetic parameters and safety of mirogabalin.
6.3. Drug interactions
In a cross-over phase 1 study on 30 healthy adult volunteers, Cmax and AUClast of mirogabalin increased by 29% and 76%, respectively, when mirogabalin (15 mg) was administered with probenecid (500 mg), and by 17% and 44%, respectively, with co-administration of cimetidine (400 mg) [Citation58]. No apparent effect on the pharmacokinetic parameters of mirogabalin was observed with the co-administration of ethanol or lorazepam in healthy adult volunteers (16 and 20 participants, respectively), but reductions in attentiveness and balance functions were observed in the combination groups compared with the mirogabalin alone group [Citation29]. Concomitant tramadol administration had little effect on the pharmacokinetics of mirogabalin in healthy adult volunteers [Citation59].
At present, precautions for co-administration with mirogabalin are required for alcohol consumption and the drugs probenecid, cimetidine, and lorazepam.
6.4. Metabolism and excretion
As mirogabalin is not a substrate of cytochrome P450 in humans, in in vitro studies, it is unlikely to cause drug interactions via cytochrome P450 [Citation29,Citation60]. In healthy adult volunteers, approximately 13–21% of orally administered mirogabalin was metabolized by UDP-glucuronosyltransferase, and then approximately 76% of mirogabalin was excreted unchanged in urine [Citation60]. In addition to glomerular filtration, tubular secretion, based on the renal clearance value (13.3 L/h), was likely associated with the excretion of mirogabalin [Citation60].
7. Clinical efficacy
Mirogabalin dose levels are expressed as those of the free form.
7.1. Phase 3 studies
7.1.1. Efficacy of mirogabalin in patients with PHN
a) Placebo-controlled study (the NEUCOURSE study)
In a randomized, double-blind, placebo-controlled phase 3 study (NCT02318719) on 763 Asian patients with PHN in Japan, Korea, Taiwan, Malaysia, Thailand, and Singapore, mirogabalin was orally administered as follows: 15 mg/day (5 mg once daily [QD] for 1 week, followed by 10 mg QD for another 1 week for the dose-titration period, and 15 mg QD for 12 weeks of the fixed-dose period), 20 mg/day (5 mg BID for only 1 week for the dose-titration period, and 10 mg BID for 13 weeks of the fixed-dose period), and 30 mg/day (5 mg BID for 1 week, followed by 10 mg BID for another 1 week for the dose-titration period, and 15 mg BID for 12 weeks of the fixed-dose period). This step-wise dose-titration regimen with low initial doses could reduce the risk of incidence of ADRs.
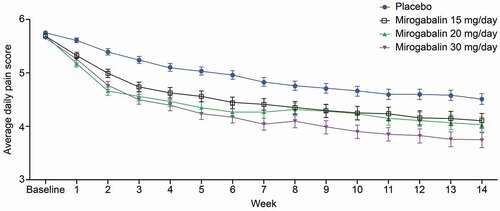
The changes in average daily pain score (ADPS) from baseline at Week 14, defined as a primary endpoint, were −1.20 (placebo), −1.61 (15 mg QD), −1.68 (10 mg BID), and −1.97 (15 mg BID); moreover, significant differences for all mirogabalin doses were observed (p = 0.017 to < 0.0001) compared with placebo. All mirogabalin groups showed a greater and more rapid decrease in ADPS compared with that in the placebo group starting at Week 1. As the daily dose of mirogabalin increased, there was a greater decrease in ADPS compared with that in the placebo group (). The proportion of patients with ≥ 30% reduction from baseline in ADPS in all mirogabalin groups was significantly higher than that in the placebo group (35.0%, 45.4%, 45.1%, and 49.7%, respectively; p = 0.0405 to 0.0035). The proportion of patients with ≥ 50% reduction from baseline in ADPS was significantly higher in the mirogabalin 15 mg BID group (29.0%) than in the placebo group (19.8%) (p < 0.05). The visual analog scale (VAS) scores in the mirogabalin groups were significantly improved from baseline (−13.6, −18.7, −19.3, and −21.4 mm, respectively; p = 0.0076 to < 0.0001). The average daily sleep interference score (ADSIS) from baseline in the mirogabalin groups was significantly improved compared with that in the placebo group (−0.95, −1.45, −1.38, and −1.69, respectively, p = 0.0014 to < 0.0001). The 36-item Short Form Health Survey (SF-36) is a widely used tool to measure health-related QOL, and SF-36 subscale scores for bodily pain, role-physical, physical functioning, vitality, social functioning, general perception of health, role-emotional, and mental health in all mirogabalin groups were improved compared with those in the placebo group [Citation34]. These results suggested that mirogabalin exerted a favorable analgesic effect and improved SF-36 scores and ADSIS in patients with PHN. As SF-36 and ADSIS are closely related to QOL, mirogabalin may help improve the QOL of NeP patients.
Figure 4. Change in ADPS from baseline to Week 14 after mirogabalin treatment in patients with PHN (placebo-controlled study)
b) Open-label long-term study
In a long-term study on patients with PHN for 52 weeks after the completion of the placebo-controlled study, all patients started with a dose of mirogabalin 5 mg BID, which was then followed by a flexible dose of 10 or 15 mg BID. Mirogabalin gradually reduced the VAS score from baseline (before the initiation of the long-term study) to Week 8 in all patients and maintained the reduction until Week 52. The change in VAS score from baseline at Week 52 was −12.4 mm [Citation61].
7.1.2. Efficacy of mirogabalin in patients with DPNP
a) Placebo-controlled study (the REDUCER study)
In a randomized, double-blind, placebo-controlled phase 3 study (NCT02318706) on 824 Asian patients with DPNP in Japan, Korea, Taiwan and Malaysia, mirogabalin was orally administered as follows: 15 mg/day (5 mg QD for 1 week, followed by 10 mg QD for another 1 week for the dose-titration period, and 15 mg QD for 12 weeks of the fixed-dose period), 20 mg/day (5 mg BID for only 1 week for the dose-titration period, and 10 mg BID for 13 weeks of the fixed-dose period), and 30 mg/day (5 mg BID for 1 week, followed by 10 mg BID for another 1 week for the dose-titration period, and 15 mg BID for 12 weeks of the fixed-dose period).
The changes in ADPS from baseline at Week 14, defined as the primary endpoint, were −1.31 (placebo), −1.34 (15 mg QD), −1.47 (10 mg BID), and −1.81 (15 mg BID, p = 0.0027 versus placebo) (). Mirogabalin at 15 mg BID caused a greater and more rapid decrease in ADPS compared with the placebo starting at Week 1. The proportion of patients with ≥ 50% reduction in ADPS was higher in the 15 mg BID group (30.9%) than in the placebo group (19.4%) (p = 0.0048). The VAS score significantly decreased in the 15 mg BID group (−22.5 mm, difference vs placebo [95% CI]: −5.9 [−9.5, −2.2], p = 0.0018) compared with that in the placebo group (−16.6 mm). The ADSIS was significantly lower in the 15 mg BID group (−1.47, difference vs placebo [95% CI]: −0.60 [−9.0, −0.30], p = 0.0001) than in the placebo group (−0.91) [Citation35]. These results suggest that mirogabalin exerted a favorable analgesic effect and improved ADSIS, which is closely related to QOL, in patients with DPNP.
Table 3. Change in ADPS from baseline to Week 14 after mirogabalin treatment in patients with DPNP (placebo-controlled study)
b) Open-label long-term study
In a long-term study on patients with DPNP for 52 weeks after the completion of the placebo-controlled studies, all patients started with a dose of 5 mg mirogabalin BID, which was followed by a flexible dose of 10 or 15 mg BID. The VAS scores gradually decreased from baseline (before the initiation of the long-term study) to Week 8 after the mirogabalin treatment, and the reduction was maintained until Week 52. The change in VAS score from baseline of mirogabalin at Week 52 in all patients was −9.8 mm [Citation63].
7.1.3. Effects of mirogabalin on tingling or pins & needles sensations in patients with DPNP in a phase 3 study
A post-hoc subgroup analysis of a phase 3 study (the placebo-controlled REDUCER study [Citation35]) to evaluate the effect of mirogabalin on tingling or pins & needles sensations was conducted for 168 DPNP patients with only these symptoms. Mirogabalin (15 mg QD, 10 mg BID, or 15 mg BID) and placebo were orally administered for 14 weeks. The changes in ADPS for tingling or pins & needles sensations from baseline at Week 14 were −1.18 (placebo), −1.18 (15 mg QD), −1.26 (10 mg BID), and −1.72 (15 mg BID, difference vs placebo [95% CI]: −0.54 [−1.29, 0.21], p = 0.1553). The proportion of patients with ≥ 50% reduction in ADPS, ADSIS, patient global impression of change, and tingling or pins & needles sensations showed significant improvement in the 15 mg BID group (odd ratio vs placebo [95% CI]: 2.77 [1.06, 7.25], p = 0.0384, difference vs placebo [95% CI]: −0.73[−1.34, −0.12], p = 0.0188, odd ratio vs placebo [95% CI]: 2.62 [1.03, 6.62], p = 0.0424, and odd ratio vs placebo [95% CI]: 2.84 [1.10, 7.33], p = 0.0304, respectively). These results were similar to those of the REDUCER study [Citation35]. Thus, mirogabalin can be effective against tingling or pins & needles sensations in DPNP [Citation64].
8. Safety and tolerability
In the 14-week, placebo-controlled phase 3 studies on patients with PHN and DPNP (the NEUCOURSE study [Citation34] and the REDUCER study [Citation35], respectively), the incidence rates of ADRs were 12.9% and 10.3% (placebo), 28.3% and 23.2% (15 mg QD), 35.3% and 18.8% (10 mg BID), and 44.5% and 36.4% (15 mg BID), respectively. Major ADRs in the 15 mg BID group were somnolence (22.6% and 14.5%), dizziness (14.2% and 9.1%), peripheral edema (2.6% and 5.5%), and weight increased (4.5% and 5.5%), respectively, and most were mild or moderate () [Citation60]. In a long-term study on patients with PHN or DPNP for 52 weeks after the completion of the placebo-controlled studies, ADRs were observed in 39.7% and 27.6% of all patients, respectively. Somnolence (32/237 patients, 13.5%), dizziness (24/237, 10.1%), and weight increased (17/237, 7.2%) were the major ADRs observed in patients with PHN. Somnolence (17/214, 7.9%) and dizziness (13/214, 6.1%) were observed in patients with DPNP. Most of these ADRs were mild [Citation60]. The proportion of patients who received 10 and 15 mg BID as the most frequent dose during the treatment period was 77.0% and 80.4% of enrolled patients of each study, respectively [Citation61,Citation63].
Table 4. Incidence of ADRs in patients with PHN or DPNP (placebo-controlled studies)
Thus, mirogabalin was found to be well tolerated in patients with PHN or DPNP in 14-week placebo-controlled studies as well as long-term studies. However, somnolence and dizziness, as reported due to treatment with mirogabalin and pregabalin, are dangerous while driving or operating machinery and could lead to falls and fractures, especially in elderly patients [Citation28,Citation65]. Thus, patients may be treated more safely using a step-wise dose-titration regimen from low initial doses of mirogabalin to reduce the incidence of ADRs.
9. Dosage and administration: pharmacokinetics, efficacy, and safety of mirogabalin in patients with renal impairment (RI)
For adults, mirogabalin is administered at an initial oral dose of 5 mg BID; thereafter, the dose is increased by 5 mg per dose with an interval of at least 1 week, up to 15 mg BID. The dose may be increased or decreased appropriately between 10 mg and 15 mg BID based on individual patient age or symptoms. shows the dosages and administration regimens of mirogabalin for patients with mild RI, moderate RI, and severe and end-stage renal disease, determined based on the results of a phase 1 study [Citation66]. The AUClast of mirogabalin at 5 mg was measured for 30 Japanese patients with normal renal function and mild RI, moderate RI, and severe RI. The AUClast of mirogabalin with decreased creatinine clearance in patients with moderate RI and severe RI increased 1.90-fold and 3.64-fold, respectively, compared to that in patients with normal renal function. The safety and efficacy of mirogabalin for patients with moderate and severe RI were also evaluated in a phase 3 study on Japanese PHN and DPNP patients [Citation67]. Mirogabalin at 7.5 mg BID and 7.5 mg QD was administered to patients with moderate RI (30 patients) and severe RI (5 patients), respectively. The major ADRs were somnolence and dizziness; most events were mild or moderate. Mirogabalin 7.5 mg QD and 7.5 mg BID reduced the change in ADPS from baseline at week 14. These results suggested that there were no clear differences in the efficacy and safety of mirogabalin between patients with impaired renal function and the patients in phase 3 studies (the NEUCOURSE study [Citation34] and REDUCER study [Citation35]).
Table 5. Recommended doses of mirogabalin for P-NeP patients with RI
10. Regulatory affairs
Mirogabalin was first approved for the treatment of P-NeP in Japan in 2019 [Citation29] and subsequently in Korea (for P-NeP) and Taiwan (for PHN and DPNP) in 2020 based on the successful results of two pivotal phase 3 studies on Asian patients with PHN or DPNP. Mirogabalin (brand name: Tarlige®) was the second Ca2+ channel α2δ ligand, after pregabalin (Lyrica®), which is approved for the treatment of P-NeP in Japan, as gabapentin (Gabapen®) was approved in 2006 as an antiepileptic drug [Citation68] but not for NeP. A phase 3 clinical study (the AMELA study) is currently underway to evaluate the efficacy and safety of mirogabalin in patients with post-SCI NeP [Citation41].
11. Conclusion
Mirogabalin is a new voltage-gated Ca2+ channel α2δ ligand with a unique binding profile for the α2δ-1 subunit, which may contribute to its target selectivity in vivo. Mirogabalin exerted a favorable analgesic effect in patients with PHN or DPNP and was well tolerated up to the maximum dose of 15 mg BID. The major ADRs were somnolence, dizziness, peripheral edema, and weight increased, but most were mild or moderate. A step-wise titration regimen from low initial doses was used to reduce the risk of ADRs in daily practice. As it has a favorable balance between efficacy and safety, mirogabalin could be more effectively used for the treatment of P-NeP.
12. Expert opinion
The first-, second-, and third-line medications recommended by the treatment guidelines for NeP do not always relieve pain and improve patient QOL. Moreover, many patients cannot continue P-NeP treatment due to the side effects of medications. Because of these reasons, it was envisaged that newer drugs would be developed, with novel modes of action and high tolerability in terms of side effects. Mirogabalin was developed to address these challenges, and results of clinical trials have shown that a step-wise increase in mirogabalin dose might be well tolerated by patients with P-NeP. Similar to the findings from longitudinal phase 3 studies of PHN and DPNP, a recent multicenter study of patients with P-NeP showed that, after switching from pregabalin to mirogabalin, most (> 70%) patients were able to safely tolerate the step-wise dose titration of the effective doses of mirogabalin (10 or 15 mg BID for patients with normal renal function; 5 or 7.5 mg BID for patients with moderately impaired renal function) [Citation69]. The early analgesic effect of mirogabalin would make it easier for patients to continue using it; thus, patients may have a higher chance of benefiting from an increase in the initial dose of mirogabalin to an effective one.
Mirogabalin showed efficacy against tingling or pins & needles sensations in the post-hoc subgroup analysis of the phase 3 study on DPNP. The tingling or pins & needles sensations are common in many P-NeP conditions, including orthopedic pain. In patients with chemotherapy-induced peripheral neuropathy (CIPN), tingling commonly exists without shooting or burning pain; it decreases the quality of life of cancer patients and halt chemotherapy. We envisage mirogabalin to relieve such a severe sensory neuropathy symptom in CIPN patients.
As P-NeP patients include many elderly patients who are indicated to receive multiple drugs for many diseases, we ought to be cautious of possible drug-drug interactions. Mirogabalin does not inhibit or activate the major CYP enzymes; it is a renal-extraction drug with limited effect on renal drug transporters. It may be relatively easy to use mirogabalin in elderly patients using multiple drugs. Moreover, to improve efficacy, a viable treatment option could be add-on or combination therapy of analgesics with mirogabalin.
There are some types of pain such as nociceptive pain, neuropathic pain, and nociplastic pain. Clinical studies require rigorous diagnosis of the disease and condition, which cannot always be conducted under actual clinical practice. The correct diagnosis of P-NeP is essential for treating P-NeP patients because inadequate diagnosis results in undertreatment or improper use of drugs. Herein, we use mirogabalin after establishing the correct diagnosis of P-NeP to ensure its maximal benefit to the patients. Finally, there are currently no results verifying the superiority of mirogabalin to pregabalin in clinical practice, and we consider that differences and similarities between pregabalin and mirogabalin will be elucidated in future clinical studies and clinical practice.
In conclusion, mirogabalin, with a benign safety profile and after increment to the effective doses, is expected to have a good analgesic effect in patients with P-NeP. Although further evidence is needed for the use of mirogabalin in clinical settings, such as the efficacy and safety in P-NeP patients, target patients, and suitable patient condition, it is expected to be a useful treatment option for P-NeP patients.
Drug summary box
Declaration of interest
T Inoue, M Yokoyama, and M Kuroha are all employees of Daiichi Sankyo Co., Ltd. J Kato reports having received personal fees from Daiichi Sankyo Co., Ltd., Pfizer Japan Inc., Eisai Co., Ltd., and Mochida Pharmaceutical Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One reviewer declares a financial relationship with Daiichi Sankyo Co., Ltd., Japan. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Japan Society of Pain Clinicians, eds. Guidelines for the pharmacologic management of neuropathic pain. 2nd. Tokyo, Japan: Shinko Trading Co., Ltd.; 2016. [cited 2020 Jul 22]. Japanese/English. Available from. http://minds4.jcqhc.or.jp/minds/Pharmacologic-management-of-neuropathic-pain/Pharmacologic-management-of-neuropathic-pain.pdf
- Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002.
- Freynhagen R, Bennett MI. Diagnosis and management of neuropathic pain. BMJ. 2009;339:b3002.
- Baron R. Neuropathic pain: a clinical perspective. Handb Exp Pharmacol. 2009;194:3–30.
- International Diabetes Federation, eds. IDF diabetes atlas. 9th. [cited 2020 Nov 27]. Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/159-idf-diabetes-atlas-ninth-edition-2019.html
- Abbott CA, Malik RA, Van Ross ER, et al. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–2224.
- Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS One. 2013;8:e74195.
- Alleman CJ, Westerhout KY, Hensen M, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diabetes Res Clin Pract. 2015;109:215–225.
- Sloan G, Shillo P, Selvarajah D, et al. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract. 2018;144:177–191.
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354.
- Ministry of Health, Labour and Welfare. Summary results of the national health and nutrition survey in Japan, 2017. [cited 2021 Jun 22]. Japanese. Available from: https://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/kekkagaiyou_7.pdf
- Hanaoka K, Ogawa S, Hotta N, et al. Current status and future prospects for neuropathic pain treatment in Japan: a proposal from the expert consensus conference. Japanese. Pain Clinic. 2009;30:1395–1408.
- Pinchinat S, Cebrián-Cuenca AM, Bricout H, et al. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis. 2013;13:170.
- Kawai K, Yawn BP, Wollan P, et al. Increasing Incidence of herpes zoster over a 60-year period from a population-based study. Clin Infect Dis. 2016;63:221–226.
- Klompas M, Kulldorff M, Vilk Y, et al. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86:1146–1153.
- Toyama N, Shiraki K. Epidemiology of herpes zoster in Miyazaki prefecture (Miyazaki study). Japanese. IASR. 2013;34:298–300.
- Inada E, eds. Herpes zoster up-to-date - from herpes zoster to PHN. Japanese. Tokyo, Japan: SHINDAN TO CHIRYO SHA, Inc.; 2012. p. 64–67.
- Inoue S, Kobayashi F, Nishihara M, et al. Chronic pain in the Japanese community–prevalence, characteristics and impact on quality of life. PLoS One. 2015;10:e0129262.
- Schaefer C, Sadosky A, Mann R, et al. Pain severity and the economic burden of neuropathic pain in the United States: BEAT neuropathic pain observational study. Clinicoecon Outcomes Res. 2014;6:483–496.
- Sommer C, Cruccu G. Topical treatment of peripheral neuropathic pain: applying the evidence. J Pain Symptom Manage. 2017;53:614–629.
- de Leon-Casasola O. New developments in the treatment algorithm for peripheral neuropathic pain. Pain Med. 2011;12(Suppl 3):S100–108.
- Ushida T, Matsui D, Inoue T, et al. Recent prescription status of oral analgesics in Japan in real-world clinical settings: retrospective study using a large-scale prescription database. Expert Opin Pharmacother. 2019;20:2041–2052.
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14:162–173.
- Yang M, Qian C, Liu Y. Suboptimal treatment of diabetic peripheral neuropathic pain in the United States. Pain Med. 2015;16:2075–2083.
- Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647.
- Ushida T, Inoue T, Matsui D, et al. Cross-sectional study of patient satisfaction with oral analgesics in patients with chronic pain in Japan. Expert Opin Pharmacother. 2020;21:983–991.
- Deeks ED Mirogabalin: first global approval. Drugs. 2019;79:463–468.
- Daiichi Sankyo Co., Ltd. Tarlige® (mirogabalin) Tablets, 2.5 mg, 5mg, 10mg, 15 mg, package insert. Japanese. [ cited 2021 Jun 22]. Available from: https://www.info.pmda.go.jp/go/pdf/430574_1190026F1028_1_05
- Daiichi Sankyo Co., Ltd. Tarlige® (mirogabalin) Tablets 2.5 mg∙5 mg∙10 mg∙15 mg, Drug interview form. Japanese. [ cited 2021 Jun 22]. Available from: https://www.info.pmda.go.jp/go/interview/1/430574_1190026F1028_1_TL6_1F.pdf
- Taiwan Food and Drug Administration. Assessment Report. TARLIGE ® F.C. Tablets 15 mg. [ cited 2020 Dec 23]. Available from: https://www.fda.gov.tw/tc/includes/GetFile.ashx?id=f637368791343691781
- Jansen M, Warrington S, Dishy V, et al. A randomized, placebo-controlled, double-blind study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and repeated doses of mirogabalin in healthy Asian volunteers. Clin Pharmacol Drug Dev. 2018;7:661–669.
- Baba M, Kuroha M, Ohwada S, et al. Results of mirogabalin treatment for diabetic peripheral neuropathic pain in Asian subjects: a phase 2, double-blind, randomized, placebo-controlled, study. Pain Ther. 2020;9:261–278.
- Vinik A, Rosenstock J, Sharma U, et al. Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator–controlled, adaptive proof-of-concept phase 2 study. Diabetes Care. 2014;37:3253–3261.
- Kato J, Matsui N, Kakehi Y, et al. Mirogabalin for the management of postherpetic neuralgia: a randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain. 2019;160:1175–1185.
- Baba M, Matsui N, Kuroha M, et al. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig. 2019;10:1299–1306.
- Amorim D. Pharmacological treatment of neuropathic pain: review of oral and topical therapy recommendations. Int J Clin Neurosci Ment Health. 2015;2:4.
- Vink S, Alewood PF. Targeting voltage-gated calcium channels: developments in peptide and small-molecule inhibitors for the treatment of neuropathic pain. Br J Pharmacol. 2012;167:970–989.
- Cavalli E, Mammana S, Nicoletti F, et al. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:1–10.
- Japan Society of Pain Clinicians, eds. Guidelines for the pharmacologic management of neuropathic pain. 2nd. Supplementary edition. Tokyo, Japan: Shinko Trading Co., Ltd.; 2016. [cited 2020 July 22]. Japanese. Available from: https://www.jspc.gr.jp/Contents/public/kaiin_guideline09.html
- Domon Y, Arakawa N, Inoue T, et al. Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels. J Pharmacol Exp Ther. 2018;365:573–582.
- Daiichi Sankyo Co., Ltd. An Asian, multicenter, randomized, double-blind, placebo-controlled, 14-week study of mirogabalin in participants with central neuropathic pain followed by a 52-week, open-label extension (NCT03901352). 2019. [cited 2020 Jul 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT03901352
- Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov. 2006;5:730–739.
- Kitano Y, Wakimoto S, Tamura S, et al. Effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels, on N-type calcium channel currents of rat dorsal root ganglion culture neurons. Pharmazie.2019;74:147–149
- Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin – alpha2-delta [Cavalpha2-delta] ligands. Pain. 2009;142:13–16.
- Burgess J, Javed S, Frank B, et al. Mirogabalin besylate in the treatment of neuropathic pain. Drugs Today. 2020;56:135–149.
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218.
- Newton RA, Bingham S, Case PC, et al. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res. 2001;95:1–8.
- Boroujerdi A, Zeng J, Sharp K, et al. Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain. 2011;152:649–655.
- Li CY, Song YH, Higuera ES, et al. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499.
- Domon Y, Arakawa N, Inoue T, et al. Analgesic effect of mirogabalin via the α2δ-1 subunit of voltage-gated calcium channels – evidence from α2δ-1 (R217A) and α2δ-2 (R282A) mutant mice. Japanese. Jpn Pharmacol Ther. 2019;47:585–591.
- Field MJ, Cox PJ, Stott E, et al. Identification of the α2δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA. 2006;103:17537–17544.
- Oyama M, Watanabe S, Iwai T, et al. Mirogabalin activates the descending noradrenergic system by binding to the α2δ-1 subunit of voltage-gated Ca2+ channels to generate analgesic effects. J Pharmacol Sci. 2021;146:33–39.
- Barclay J, Balaguero N, Mione M, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104.
- Brill J, Klocke R, Paul D, et al. Entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J Biol Chem. 2004;279:7322–7330.
- Donato R, Page KM, Koch D, et al. The ducky2J mutation in Cacna2d2 results in reduced spontaneous Purkinje cell activity and altered gene expression. J Neurosci. 2006;26:12576–12586.
- Ivanov SV, Ward JM, Tessarollo L, et al. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am J Pathol. 2004;165:1007–1018.
- Brown K, Mendell J, Ohwada S, et al. Tolerability, pharmacokinetics, and pharmacodynamics of mirogabalin in healthy subjects: results from phase 1 studies. Pharmacol Res Perspect. 2018;6(5):e00418.
- Tachibana M, Yamamura N, Atiee GJ, et al. Coadministration of probenecid and cimetidine with mirogabalin in healthy subjects: a phase 1, randomized, open-label, drug–drug interaction study. Br J Clin Pharmacol. 2018;84:2317–2324.
- Jansen M, Mendell J, Currie A, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of mirogabalin when coadministered with lorazepam, zolpidem, tramadol, or ethanol: results from drug-drug interaction studies in healthy subjects. Clin Pharmacol Drug Dev. 2018;7:597–612.
- Kitano Y, Kai K, Yamamura N, et al. Pharmacological, pharmacodynamics, and clinical profile of mirogabalin besylate (Tarlige® tablets 2.5 mg∙5 mg∙10 mg∙15 mg). Japanese. Nihon Yakurigaku Zasshi. 2019;154:352–361.
- Kato J, Matsui N, Kakehi Y, et al. Long-term safety and efficacy of mirogabalin in Asian patients with postherpetic neuralgia: results from an open-label extension of a multicenter randomized, double-blind, placebo-controlled trial. Medicine (Baltimore). 2020;99:e21976.
- Pharmaceuticals and Medical Device Agency. Common technical document of mirogabalin (module 1). Japanese. [ cited 2020 Dec 23]. Available from: https://www.pmda.go.jp/drugs/2019/P20190122001/430574000_23100AMX00014_B100_1.pdf
- Baba M, Matsui N, Kuroha M, et al. Long‐term safety and efficacy of mirogabalin in Asian patients with diabetic peripheral neuropathic pain. J Diabetes Investig. 2020;11:693–698.
- Baba M, Kuroha M, Wasaki Y, et al. Effects of mirogabalin on tingling or pins & needles in a phase 3 study of diabetic peripheral neuropathy. J Japan Soc Pain Clin. 2020;27:287–295.
- Pfizer Japan Inc. Lyrica® (pregabalin) OD Tablets 25 mg, 75 mg, 150 mg, package insert. Japanese. [ cited 2020 Oct 10]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/672212_1190017F1029_2_02
- Kato M, Tajima N, Shimizu T, et al. Pharmacokinetics and safety of a single oral dose of mirogabalin in Japanese subjects with varying degrees of renal impairment. J Clin Pharmacol. 2018;58:57–63.
- Baba M, Takatsuna H, Matsui N, et al. Mirogabalin in Japanese patients with renal impairment and pain associated with diabetic peripheral neuropathy or post-herpetic neuralgia: a phase III, open-Label, 14-week study. J Pain Res. 2020;13:1811–1821.
- Fuji Pharma Co., Ltd. Gabapen® (gabapentin) Tablets 200 mg, 300 mg, 400 mg, package insert. Japanese. [ cited 2020 Aug 10]. Available from: https://www.info.pmda.go.jp/go/pdf/670109_1139007F1022_3_01
- Kimura Y, Yamaguchi S, Suzuki T, et al. Switching from pregabalin to mirogabalin in patients with peripheral neuropathic pain: a multi-center, prospective, single-arm, open-label study (MIROP Study). Pain Ther. 2021;10:711–727.
