1. Acute promyelocytic leukemia: an emblem of the success of precision medicine
Acute promyelocytic leukemia (APL) accounts for up to 10% of all childhood acute myeloid leukemia cases with a median age at presentation of 9–12 years and a higher incidence in Italy, China, and Latin America countries [Citation1]. While no differences have been found for gender distribution, some reports identified obesity as a possible risk factor because of polyunsaturated fatty acid metabolism disturbances, in particular in cases harboring FLT3 mutations (occurring in up to 40% of APL patients) [Citation2]. The hallmark of the disease is the typical chromosomal translocation t(15;17)(q24.1;q21.1) detected at diagnosis in 95% of cases, which leads to the oncogenic fusion gene PML-RARA responsible for the differentiation block at the promyelocytic stage and for the characteristic clinical picture [Citation3,Citation4]. Indeed, APL blasts express procoagulant factors and fibrinolytic proteins, which along with secretion of inflammatory cytokines, generate a perturbation of the thrombo-hemostatic balance resulting in a scenario resembling disseminated intravascular coagulation (DIC), a life-threatening condition still responsible for the majority of early death (ED) during induction [Citation5]. This severe complication generally occurs within 30 days from diagnosis and is registered at a various frequency (3–10% in clinical trials vs. up to 30% in real-life scenarios) [Citation6]. In particular, ED is predominantly deriving from hemorrhagic events, with the brain as the main bleeding site and characterized by majority of fatal cases (up to 70%). Specific pediatric series have reported major bleeding in up to 16% of cases with ED rate of 10% [Citation7–9]. Taking into account the modern therapeutic success of APL, DIC at presentation and ED still represent a hurdle to face during induction and, for this reason, many studies have tried to identify early predictors of such complications with various results. Among others, higher white blood cells (WBC), increased lactate dehydrogenase (LDH) levels and lower fibrinogen have been the ones consistently found as predictors of fatal outcomes, underscoring that the profound coagulation disturbance and its consequences are directly related to the circulating disease burden [Citation6,Citation10].
Despite the impressive results obtained with the all-trans retinoic acid (ATRA) plus anthracycline-based chemotherapy combination with long-term cure rates approaching 80%, myelosuppression during therapy along with long-term cardiac toxicities and therapy-related myeloid neoplasms still constituted impending issues, especially in the pediatric setting. Regardless of the efforts in lowering such complications by sparing anthracycline cumulative dose (from 650 mg/m2 in the AIDA-0493 trial to 355 mg/m2 in standard risk (SR)-APL in the last ICC-APL-01 trial) while maintaining an appropriate anti-leukemic efficacy, the option of an exclusively chemotherapy-free regimen has been eagerly pursued by the entire hematology community [Citation11]. Indeed, APL blasts have been found exquisitely sensitive to ATRA and arsenic trioxide (ATO) combination, now representing the modern standard of care for SR-APL (i.e. WBC <10x109/L at diagnosis) in adults [Citation12]. This completely chemotherapy-free regimen has led to response rates exceeding 90%, demonstrating also a long-term anti-leukemic efficacy as shown by the updated results of the seminal APL 0406 trial [Citation13]. While ATRA/ATO regimen has been set as a gold standard for adult SR-APL treatment almost a decade ago, pediatric patients have been sporadically treated as such.
2. ATRA/ATO mechanism of function and drug-associated clinical situations
In physiologic conditions, the nuclear retinoic acid (RA) receptor RARA generates heterodimers with the retinoid X receptor (RXR), which regulate gene expression (and thereby cellular differentiation) via binding to specific RA-responsive elements in a retinoid-dependent fashion [Citation14]. In absence of ligands, RARA-RXR heterodimers recruit co-repressors ultimately leading to suppression of transcription of myeloid differentiation genes [Citation15]. Specifically, in the presence of the oncogenic fusion gene PML-RARA, the heterodimers are not responsive to physiological levels of retinoids with resultant block of differentiation at the promyelocyte stage [Citation16]. In this scenario, ATRA is able to overcome this resistance and degrade the aberrant PML-RARA fusion transcript via the ubiquitin-proteasome pathway, ultimately restoring the myeloid differentiation program (). However, the block of differentiation is only one of the pathophysiologic consequences of PML-RARA, as underlined by the fact that ATRA is not curative when used as a single agent. Indeed, in vitro studies have also shown that in APL, PML is unable to exert its tumor suppressor functions via p53 interaction, generating a disorganization of macromolecular structures called nuclear bodies (PML-NBs) and producing nuclear microspeckles whose identification is used as a diagnostic hallmark of APL [Citation17]. In this context, ATO is able to bind to PML-RARA oxidizing 2 cysteine residues in the B2 domain of PML, generating disulfide bonds and leading to Small Ubiquitin-like MOdifier-(SUMO)ylation of lysine 160 and reorganization of PML-NBs with restoration of apoptosis [Citation18]. Moreover, the interaction of ATO with PML induces its oligomerization and interaction with the SUMO–conjugating enzyme UBC9 promoting the polyubiquitination and degradation of PML-RARA [Citation17].
Figure 1. Mechanism of action of ATRA/ATO combination.
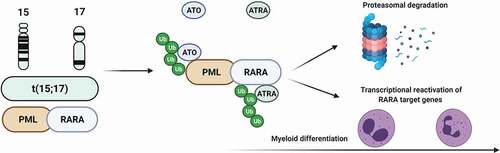
However, it is noteworthy to mention that, despite sharing similar clinical features, only the classical PML-RARA positive APL shows this exquisite sensitivity to ATRA/ATO combination, while other RARA fusion variants (particularly ZTBTB16-RARA and STAT5B-RARA) display a much more limited sensitivity [Citation19,Citation20].
The above mentioned molecular mechanism of action of ATRA/ATO underlies the typical clinical occurrence of leukocytosis during induction, an epiphenomenon deriving from the myeloid differentiation generated by the treatment. This peculiar aspect is usually managed with hydroxyurea or low chemotherapy doses and is directly correlated to the occurrence of a ‘differentiation syndrome’ (DS), a complication caused by the endothelial damage provoked by differentiating promyelocytes and registered in up to 20% of pediatric patients treated as such [Citation1]. DS constitutes a life-threatening condition and one of the major contributor of ED in induction together with the aforementioned thrombo-hemorrhagic diathesis of APL. The diagnosis of DS is based on Montesinos criteria, which include fever, dyspnea with pulmonary infiltrates, weight gain, renal failure, hypotension, and pleuro-pericardial effusions [Citation21]. The prompt recognition of DS, typically manifesting at a median of 12 days from treatment start, is essential for the establishment of the appropriate treatment, which consists of dexamethasone and diuretics as well as ATRA/ATO withdrawal [Citation22]. Administration of steroids from the first day of induction is now a standard prophylactic measure to prevent DS.
A peculiarity of children treated with ATRA/ATO combination and the reason for a specific pediatric dose of ATRA (25 instead of 45 mg/m2) is the increased risk of pseudotumor cerebri (PTC) [Citation1]. This condition is caused by the alteration of cerebrospinal fluid (CSF) recirculation due to ATRA neurotoxicity and is registered in up to 15% of children undergoing ATRA therapy [Citation23]. The clinical picture includes headache with vision disturbances which may culminate in permanent blindness if left untreated or not promptly recognized [Citation24]. However, the ‘Dandy criteria’ used for the diagnosis of PTC include, among others, an elevated lumbar puncture opening pressure, a maneuver difficult to perform in children with APL because of the typical coagulation disturbances and thrombocytopenia. Therefore, new criteria based on clinico-radiological (i.e. MRI) evaluation have been proposed for the noninvasive diagnosis of ‘probable’ PTC, allowing ATRA discontinuation (sufficient in up to 30% of cases) and establishment of specific supportive measures or further specific treatment (e.g. acetazolamide, topiramate) [Citation1,Citation25].
3. The modern therapeutic scenario of childhood APL: toward a chemotherapy free-approach
After a Chinese experience of 43 newly diagnosed children with APL treated with ATRA/ATO in induction, a first series of 11 pediatric cases by the AML Berlin-Frankfurt-Münster (BFM) group demonstrated the safety and feasibility of ATRA/ATO in both induction and consolidation phases () [Citation26,Citation27]. Although three patients received additional doses of chemotherapy to control leukocytosis, this was the first experience reporting an APL0406-like protocol to treat pediatric patients with APL. Major differences included lower doses of ATRA (25 instead of 45 mg/m2 daily in two divided doses for the aforementioned risk of PTC in children, established as the standard of care for pediatric patients), a delayed start of ATO (given from day 10 to avoid leukocytosis), and one-week break from ATRA after the first 14 days of treatment (intermittent schedule). Following these encouraging results, our group reported the first entirely chemotherapy-free approach in 18 children with newly diagnosed APL using the same APL0406 schedule, with the only difference of a pediatric ATRA dose [Citation28]. In this retrospective case series, all patients achieved molecular complete remission (CR) and were alive at a median follow-up of 24 months. Of note, two patients were classified as high-risk (HR, WBC >10×109/L), but none received any anti-neoplastic agent other than hydroxyurea for the control of leukocytosis. Moreover, the side effects were manageable and included hematological (grade 3–4 neutropenia in 67% and grade 3–4 thrombocytopenia in 56% of patients), hepatic (elevation of serum transaminases in 50% of cases), and cardiac toxicities (17% experienced a prolongation of QTc interval). Comparable results were described by a French report on 21 children with the only differences of the addition of two doses of idarubicin (12 mg/m2 at day 1 and 3 of induction) for the three HR patients included in the study, and the absence of any registered hepatic toxicity [Citation29]. A recent Chinese study reported on 17 patients aged <14 years treated with a similar approach without the addition of any chemotherapy other than hydroxyurea. However, in this study, one patient died during induction because of intracranial hemorrhage (ICH – for an observed ED rate of 6%), while, similarly to our experience, hepatic toxicity was reported in 64% of cases [Citation30].
Table 1. Studies reporting ATRA/ATO combinations for induction and consolidation in children with newly diagnosed APL
The recently published multicenter prospective trial CCLG-APL2016, enrolling 193 patients from 38 Chinese hospitals, is the first to prove the safety and effectiveness of ATRA plus arsenic in the pediatric population [Citation31]. Different from prior experiences, the protocol defined as HR not only patients with WBC >10x109/L at diagnosis but also those harboring FLT3-ITD mutations, despite previously published evidences pointing toward the abrogation of the negative impact of this mutation in the context of arsenic use [Citation32]. Moreover, while SR patients were treated with an entirely chemo-free approach, HR cases still received additional anthracyclines (idarubicin 10 mg/m2 or daunorubicin 40 mg/m2 on alternate days for a total of 2–3 doses). Translating the optimal results obtained in adults, the CCLG-APL2016 trial was designed to include the option of oral arsenic (Realgar/Indigo naturalis Formula, RIF at a dose of 60 mg/kg daily in two or three divided doses), depending on local drug availability, but a direct comparison of the effectiveness of the two (intravenous vs. oral) options was not foreseen. Besides induction and consolidation, the protocol included also a maintenance phase with RIF (60 mg/kg daily in two divided doses)/ATO(0.15 mg/kg daily) in a 2 weeks on/off schedule plus ATRA in a 1 week on/off regimen, for a total of 8 (SR-APL) or 10 (HR-APL) months. The ED rate was 3% with ICH as the leading cause of mortality. At a median follow-up of 29 months, the reported 2-year overall survival (OS) and event-free survival (EFS) were 99% and 97% in the SR and 95% and 90% in the HR group, respectively (p = 0.088 and 0.252). All side effects were transient and manageable, with around 10% of cases registering hepatic toxicity while no patient had abnormal QTc elongation. An important consideration is that the arsenic levels measured in plasma, urine, hair and nail rapidly decreased after 6 months from the cessation of treatment, and no patient developed signs or symptoms of chronic arseniasis.
4. Expert opinion
In the last two decades, APL became the most curable subtype of acute leukemia thanks to the success of the ATRA/ATO combination. Childhood APL has been traditionally treated using adults’ protocols, and because of the rarity of the condition, dedicated pediatric trials have been designed only in the last two decades. However, the aforementioned impressive results obtained using ATRA/ATO combinations along with those of the recent CCLG-APL2016 study may soon expedite a very welcome increase of the use of this chemo-free option also in the pediatric setting () [Citation31]. To that end, the North-American NCT01409161 (for children aged 10 years and older) and in particular the International ICC-APL-02 (Eudract no. 2017–002383-40) trials are now recruiting newly diagnosed children with APL. Based on a completely ATRA/ATO chemo-free regimen for both SR and HR groups, similar to the ongoing APOLLO trial for adults (EudraCT 2015–001151-68), these protocols are designed to give HR patients additional doses of CHT (gemtuzumab-ozogamicin GO-3 mg/m2 in the ICC-APL-02 study) for the control of leukocytosis, with slightly different schedules (on days 2 and 4 of induction in the ICC-APL-02, and once weekly for the first 4 weeks in the North American trial). Furthermore, all these studies only include induction and consolidation phases (i.e. without maintenance) because of the established long-lasting anti-leukemic efficacy of the ATRA/ATO combination [Citation13,Citation33]. This, along with the evidence that relapses are typically registered in the first 24 months of follow-up, questions the necessity of a more prolonged molecular disease monitoring in patients treated as such, with obvious consequences when translating these recommendations to the youngsters, potentially less burdened by invasive procedures [Citation32].
To this end, a special remark deserves the case of FLT3-ITD mutation, whose prognostic role in the pre-ATO era has been controversial and possibly linked with a negative impact on disease characteristics at presentation, being associated with increased WBC, poor survival and risk of relapse [Citation34]. Nevertheless, the long-term results of the seminal APL0406 study confirmed that ATRA–ATO combination is effective in SR-APL regardless of the presence of FLT3–ITD mutation, thereby abrogating the poor outcome associated with this mutation in the ATRA-chemotherapy setting [Citation32].
Another milestone achieved after the introduction of ATO-containing regimens is represented by the improved relapse rate (4% vs 17–27% in the pre-ATO era) [Citation35]. For this reason, and taken into consideration the rarity of the disorder, future prospective trials focusing on relapsing/refractory patients are difficult to envision. As a result, the current management of such cases is based on specific recommendations from International Expert Panels and evidence derived from retrospective case series [Citation35,Citation36]. According to these proposed criteria, children with relapsed APL are stratified in standard-risk (>18 months, late; >36 months, very late relapse; ATO naïve <18 months; extramedullary relapse promptly responding to salvage treatment), and high-risk (<18 months, early relapse; refractory to first-line or salvage treatment) [Citation36]. ATO-containing regimens have been used for the re-induction of relapsed cases along with gemtuzumab-ozogamicin (GO), an anti-CD33 antibody-drug conjugate linked to calicheamicin, in particular in cases with prior ATO exposure. Post-remission strategies include both autologous and allogeneic hematopoietic stem cell transplantation with the preferential use of the latter in cases without clearance of the PML-RARA transcript (MRD positive), although no significant differences in terms of EFS and OS have been observed between the two options [Citation35,Citation36]. These results have been recently confirmed by a report on an Italian retrospective case series of 51 pediatric patients with relapsed APL, which also showed a better OS in cases classified as high-risk (see above) receiving ATO regimens as opposed to those treated before ATO introduction (94.4% vs. 72.1% at 10-year, p = 0.08), despite not reaching a statistical significance [Citation35].
An important issue of childhood APL in the post-ATO era is the role of central nervous system (CNS) prophylaxis. Although little is known as to the CNS involvement at diagnosis because of the bleeding complications associated with early lumbar punctures, CNS is one of the main site of extramedullary disease at relapse, along with skin and testes. In particular, HR-APL as well as those experiencing intracranial hemorrhages during induction are more prone to develop this complication, with a 5-year cumulative incidence of CNS relapse as high as 18.7% for the latter group, as shown by an analysis of 739 adults patients treated with ATRA and chemotherapy [Citation37]. However, given that ATO overcomes the blood–brain barrier, the role of Triple Intrathecal Therapy (ITT) with methotrexate, hydrocortisone and cytarabine (if excluding the actual treatment of CNS relapses) remains unclear, and ITTs are recommended for CNS prophylaxis mainly in HR-APL and those patients experiencing ICH during induction [Citation4,Citation36].
Besides the possibility of an entirely chemo-free approach overcoming the long-term sequelae associated with the use of antineoplastic agents, the availability of oral formulations is also of paramount importance for the pediatric population. To that end, data deriving from the Chinese experience provide encouraging results as to the safety profile of RIF, which has been proven to have a better pharmacokinetic profile than ATO, resulting in lower cardiac and hepatic toxicities, and opening a new horizon for a more ‘home-based’ treatment, at least for the post-induction phase [Citation31]. Moreover, the data on arsenic elimination kinetics further emphasize the safety profile of this agent, and reassure as to the virtually absent likelihood of long-term toxicities, especially when envisioning the prolonged life expectancy in children. However, the follow-up time of studies on arsenic accumulation in pediatric patients is currently very short, and more mature data are needed to establish the safety of this agent as to long-term sequelae possibly arising from its use during childhood. In particular, if the newer protocols have reduced the risks of prolonged arsenic exposure and its complications (typically involving the skin, the urinary tract as well as both neurologic and endocrine systems) by diminishing the duration of post-remission arsenic administration, concerns have been raised as to the possible interference with growth and development. Finally, the use of oral arsenic formulations has also socio-economic consequences, reducing both hospital stays and impacts on health systems, and repercussions on the psychological burden of young patients facing a diagnosis of malignancy.
In the future, children with APL will hopefully benefit from an entirely chemo-free and oral, mostly home-based, regimen for the cure of a disorder previously known as the most fatal among all types of acute leukemia.
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
One reviewer declares that their institution holds a US patent for the use of oral arsenic trioxide in the treatment of leukemia. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Gurnari C, Voso MT, Girardi K, et al. Acute promyelocytic leukemia in children: a model of precision medicine and chemotherapy-free therapy. Int J Mol Sci. 2021 Jan 11;22(2):2.
- Mazzarella L, Botteri E, Matthews A, et al. Obesity is a risk factor for acute promyelocytic leukemia: evidence from population and cross-sectional studies and correlation with FLT3 mutations and polyunsaturated fatty acid metabolism. Haematologica. 2020 Jun;105(6):1559–1566.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017 Jan 26;129(4):424–447.
- Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. 2019 Apr 11;133(15):1630–1643.
- Falanga A, Russo L, Tartari CJ. Pathogenesis and treatment of thrombohemorrhagic diathesis in acute promyelocytic leukemia. Mediterr J Hematol Infect Dis. 2011;3(1):e2011068.
- Gurnari C, Breccia M, Di Giuliano F, et al. Early intracranial haemorrhages in acute promyelocytic leukaemia: analysis of neuroradiological and clinico-biological parameters. Br J Haematol. 2020 Aug 10;193(1):129–132.
- Rajpurkar M, Alonzo TA, Wang Y-C, et al. Risk markers for significant bleeding and thrombosis in pediatric acute promyelocytic leukemia; report from the Children’s Oncology Group Study AAML0631. J Pediatr Hematol Oncol. 2019;41(1):1.
- Zhang Y, Wang L, Zhang R, et al. Long-term follow-up of children with acute promyelocytic leukemia treated with Beijing Children’s Hospital APL 2005 protocol (BCH-APL 2005). Pediatr Hematol Oncol. 2019 Oct 03;36(7):399–409.
- de Azevedo AC, Matsuda E, Cervellini JY, et al. Early mortality in children and adolescents with acute promyelocytic leukemia: experience of the Boldrini Children’s Center. J Pediatr Hematol Oncol. 2020;42(7):7.
- Naymagon L, Moshier E, Tremblay D, et al. Predictors of early hemorrhage in acute promyelocytic leukemia. Leuk Lymphoma. 2019 Oct;60(10):2394–2403.
- Testi AM, Pession A, Diverio D, et al. Risk-adapted treatment of acute promyelocytic leukemia: results from the International Consortium for Childhood APL. Blood. 2018 Jul 26;132(4):405–412.
- Lo-Coco F, Avvisati G, Vignetti M, et al., Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 369(2): 111–121. 2013.
- Cicconi L, Platzbecker U, Avvisati G, et al. Long-term results of all-trans retinoic acid and arsenic trioxide in non-high-risk acute promyelocytic leukemia: update of the APL0406 Italian-German randomized trial. Leukemia. 2020 Mar;34(3):914–918.
- Gurnari C, De Bellis E, Divona M, et al. When poisons cure: the case of arsenic in acute promyelocytic leukemia. Chemotherapy. 2019;64(5–6):238–247.
- Zeidan AM, Gore SD. New strategies in acute promyelocytic leukemia: moving to an entirely oral, chemotherapy-free upfront management approach. Clin Cancer Res. 2014;20(19):4985–4993.
- Lo-Coco F, Hasan SK. Understanding the molecular pathogenesis of acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2014 Mar 01;27(1):3–9.
- Zhang X-W, Yan X-J, Zhou Z-R, et al. Arsenic trioxide controls the fate of the PML-RARα oncoprotein by directly binding PML. Science (New York, N.Y.). 2010;328(5975):240–243.
- Lallemand-Breitenbach V, Jeanne M, Benhenda S, et al. Arsenic degrades PML or PML–RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008 May 01;10(5):547–555.
- Cicconi L, Testi AM, Montesinos P, et al. Characteristics and outcome of acute myeloid leukemia with uncommon retinoic acid receptor-alpha (RARA) fusion variants. Blood Cancer J. 2021 Oct 16;11(10):167.
- Ciangola G, Gurnari C, Paterno G, et al. STAT5b-RARa-positive acute myeloid leukemia: diagnostic and therapeutic challenges of a rare AML subtype. Leuk Res. 2019 Mar;78:21–23.
- Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009 Jan 22;113(4):775–783.
- Stahl M, Tallman MS. Differentiation syndrome in acute promyelocytic leukaemia. Br J Haematol. 2019 Oct;187(2):157–162.
- Anoop TM, Jain N, Nair SG, et al. All-trans-retinoic acid-induced pseudotumor cerebri in acute promyelocytic leukemia [Article]. J Neurosci Rural Pract. 2014;5(3):273–275.
- Montesinos P, Vellenga E, Holowiecka A, et al. Incidence, outcome and risk factors of pseudotumor cerebri after all- trans retinoic acid and anthracycline-based chemotherapy in patients with acute promyelocytic leukemia. Blood. 2008;112(11):2992.
- Coombs CC, DeAngelis LM, Feusner JH, et al. Pseudotumor cerebri in acute promyelocytic leukemia patients on intergroup protocol 0129: clinical description and recommendations for new diagnostic criteria. Clin Lymphoma Myeloma Leuk. 2016 Mar;16(3):146–151.
- Cheng Y, Zhang L, Wu J, et al. Long-term prognosis of childhood acute promyelocytic leukaemia with arsenic trioxide administration in induction and consolidation chemotherapy phases: a single-centre experience. Eur J Haematol. 2013;91(6):483–489.
- Creutzig U, Dworzak MN, Bochennek K, et al. First experience of the AML-Berlin-Frankfurt-Münster group in pediatric patients with standard-risk acute promyelocytic leukemia treated with arsenic trioxide and all-trans retinoid acid. Pediatr Blood Cancer. 2017 Aug;64(8):e26461.
- Strocchio L, Gurnari C, Santoro N, et al. Arsenic trioxide and all-trans retinoic acid treatment for childhood acute promyelocytic leukaemia. Br J Haematol. 2019 Apr;185(2):360–363.
- Garcia Spezza E, Brethon B, Petit A, et al. Tolerance to arsenic trioxide combined with all-trans-retinoic acid in children with acute promyelocytic leukaemia in France. Br J Haematol. 2020;188(1):170–173.
- Li SY, Lu Y, Liu HC, et al. Arsenic trioxide and all-trans retinoic acid in the treatment of children with newly diagnosed acute promyelocytic leukemia. Leuk Lymphoma. 2021 May;62(5):1267–1270.
- Zheng H, Jiang H, and Hu S, et al. Arsenic combined with all-trans retinoic acid for pediatric acute promyelocytic leukemia: report from the CCLG-APL2016 protocol study. J Clin Oncol. 2021 October 1;39(28):3161-3170. DOI: https://doi.org/10.1200/JCO.20.03096
- Cicconi L, Divona M, Ciardi C, et al. PML-RARα kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016 Oct;30(10):1987–1992.
- Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017 Feb 20;35(6):605–612.
- Breccia M, Loglisci G, Loglisci MG, et al. FLT3-ITD confers poor prognosis in patients with acute promyelocytic leukemia treated with AIDA protocols: long-term follow-up analysis. Haematologica. 2013 Dec;98(12):e161–3.
- Testi AM, Mohamed S, Diverio D, et al. Outcome of relapsed/refractory acute promyelocytic leukaemia in children, adolescents and young adult patients - a 25-year Italian experience. Br J Haematol. 2021 Jun;192(1):17.
- Abla O, Kutny MA, Testi AM, et al., Management of relapsed and refractory childhood acute promyelocytic leukaemia: recommendations from an international expert panel. Br J Haematol. 175(4): 588–601. 2016.
- Pau M, Joaquín D-M, Guillermo D, et al. Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal prophylaxis. Haematologica. 2009 Sep 01;94(9):1242–1249.
