ABSTRACT
Background
A new voltage-gated Ca2+ channel α2δ ligand, mirogabalin, was first approved for treating peripheral neuropathic pain in Japan in 2019. This is the first report on the prescription status of mirogabalin using a large-scale prescription database.
Research Design and Methods
The authors analyzed the prescription data of 12,924 patients prescribed mirogabalin between 1 June and 31 August 2020. The endpoints were the number of patients prescribed, prescription days, prescription doses, dose changes, co-prescription patterns, medication possession ratio (MPR), and treatment discontinuation rates (TDRs).
Results
Mirogabalin was newly prescribed to 7,914 patients in the 3-month study period. Most patients were prescribed mirogabalin at about 10 mg/day during the study period, and 30.9% of patients were prescribed ≥ 20 mg/day on Day 90 after the first prescription. The most frequently prescribed concomitant drug was celecoxib. The MPR (80 to 110%) was 86.2%, indicating good treatment adherence. The cumulative TDRs during ≤ 7 Days, Days 31–60, and 61–90 were 14.0%, 70.0%, and 77.9%, respectively.
Conclusions
Mirogabalin was prescribed to a considerable number of patients. These results may be useful for optimizing mirogabalin use for patients with peripheral neuropathic pain in daily clinical practice.
Clinical trial registration number
UMIN000042592
1. Introduction
Oral analgesics are among the most frequently prescribed drugs worldwide, but optimizing the drug therapy for chronic and refractory neuropathic pain (NeP) can often be a challenge for physicians. Voltage-gated Ca2+ channel α2δ ligands, serotonin-noradrenaline reuptake inhibitors, and tricyclic antidepressants are currently used as first-line drugs for the treatment of NeP [Citation1–3]. In a previous large-scale prescription database study on oral analgesics in 2017, NeP drugs such as pregabalin, duloxetine, and tramadol & acetaminophen combination were found to be frequently prescribed to Japanese patients with chronic pain [Citation4]. However, patients were not always satisfied with conventional NeP drugs due to insufficient pain relief and/or adverse drug reactions (ADRs) [Citation5–7]. In a cross-sectional study of patient satisfaction with oral analgesics in Japanese patients with NeP in 2019, approximately 40% of NeP patients were ‘unsatisfied,’ ‘unsatisfied if anything,’ or ‘neither satisfied nor unsatisfied’ with NeP drugs [Citation7]. Therefore, the development of new NeP drugs with favorable pain relief and better safety remains an unmet medical need.
Mirogabalin is a novel voltage-gated Ca2+ channel α2δ ligand developed by Daiichi Sankyo Co., Ltd. (Tokyo, Japan). It was first approved for treating peripheral neuropathic pain (PNeP) in Japan in 2019, followed by approval for PNeP in Korea [Citation8] and for post-herpetic neuralgia (PHN) and diabetic peripheral neuropathy (DPNP) in Taiwan in 2020 [Citation8,Citation9]. After gabapentin and pregabalin, it is the third Ca2+ channel α2δ ligand. In in vitro and in vivo studies, mirogabalin has a high binding affinity to the calcium channel α2δ subunit and exerts its analgesic effect via binding to the α2δ-1 subunit, but not the α2δ-2 subunit, as the analgesic effect of mirogabalin was not observed in α2δ-1 (R217A) mutant mice, but was observed in α2δ-2 (R282A) mutant mice [Citation10]. It also has good analgesic effects in NeP rat models [Citation11,Citation12], and has been shown to have a favorable pain relief and safety profile in Asian patients with DPNP or PHN in phase 3 studies [Citation13–16]. As somnolence, dizziness, peripheral edema, and weight gain are known to be the major side effects of Ca2+ channel α2δ ligands, step-wise titration regimens from low initial doses of mirogabalin are carefully used to reduce their risk [Citation8,Citation17]. However, although the efficacy and safety of mirogabalin have been evaluated in preclinical and clinical studies, the prescribing status of mirogabalin in daily clinical practice has not been reported to date.
The objective of this study was to clarify the prescription status of mirogabalin in daily clinical practice using a large-scale prescription database.
2. Patients and methods
2.1. Patients
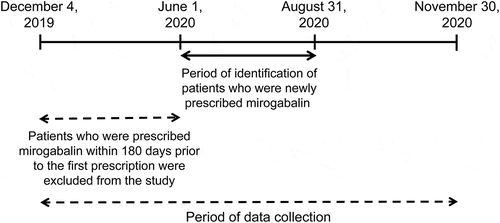
The inclusion criteria were: patients who were prescribed mirogabalin between 1 June and 31 August 2020. The exclusion criteria were: patients who had been prescribed mirogabalin within 180 days before the first prescription.
2.2. Ethics
This study (clinical trial registration number: UMIN000042592) was conducted in compliance with the Japanese ‘Act on the Protection of Personal Information’ [Citation18] and the Declaration of Helsinki [Citation19]. The ‘Guideline on Implementation of Pharmacoepidemiology Studies in Safety Assessment of Pharmaceuticals using Database of Medical Information’ was also referred [Citation20]. This study was conducted using a prescription database maintained at the INTAGE Real World Inc., Tokyo, Japan. As this was a retrospective study, new prescription data were not collected for it. As the prescription data provided by the INTAGE Real World Inc. were classified as anonymized/processed information and included only statistical information, patient consent and ethics committee reviews were not required.
2.3. Prescription database
The prescription database (INTAGE Real World Inc.) used in this study included approximately 30 million prescription data collected annually from approximately 1,450 nationwide dispensing pharmacies in Japan. This prescription database includes patient information on gender, age, clinical department from which patients obtained the prescriptions, and prescription information (drug names, numbers of prescription days, prescribed doses, and prior medications). This prescription database was previously used to conduct a large-scale prescription database study of oral analgesics in Japanese patients with chronic pain in 2017 [Citation4]. The prescription data were irreversibly encrypted by dispensing pharmacies using a hash function at the time of prescription data collection, and therefore did not include any patient identifying information.
2.4. Study design
The study design is shown in . The patients who were prescribed mirogabalin for 3 months between 1 June and 31 August 2020 were included, while those who were prescribed mirogabalin within 180 days prior to the first prescription were excluded from the study. The observation period was 90 days after the first prescription. The collection period of prescription data was 1 year (between 4 December 2019 and 30 November 2020).
2.5. Endpoints
2.5.1. Numbers/proportions of patients who were prescribed mirogabalin and number of prescription days
The numbers/proportions of patients who received continued prescription and pro re nata (PRN, as needed) prescriptions were tabulated. Prescription days were defined as the total number of days for which mirogabalin was prescribed during the study period.
2.5.2. Total prescription dose and daily prescription dose
The total prescription dose for patients who received continued prescription was tabulated as the total amount of mirogabalin prescribed during the study period. The daily prescription dose was calculated by dividing the total prescription dose of mirogabalin during the study period by the total number of prescription days.
2.5.3. Changes from the initial daily prescription dose
For patients who received continued prescription, the initial prescription dose was defined as the daily prescription dose on the day of the first prescription. Furthermore, the daily prescription doses on Days 7, 14, 30, 60, and 90 after the first mirogabalin prescription were tabulated. Changes of daily prescription doses from the day of first prescription dose to Day 90 (in the ranges < 2.5 mg, 2.5 to < 5 mg, 5 to < 7.5 mg, 7.5 to < 10 mg, 10 to < 15 mg, 15 to < 20 mg, 20 to < 25 mg, 25 to ≤ 30 mg, and > 30 mg) were also tabulated.
2.5.4. Maintenance dose and achieved dose
For patients who received continued prescription, the maintenance dose was defined as the daily prescription dose prescribed for the longest duration during the study period. The achieved dose was defined as the daily prescription dose on the day of the final prescription during the study period. The initial, maintenance, and achieved doses were tabulated in the ranges < 2.5 mg, 2.5 to < 5 mg, 5 to < 7.5 mg, 7.5 to < 10 mg, 10 to < 15 mg, 15 to < 20 mg, 20 to < 25 mg, 25 to ≤ 30 mg, and > 30 mg.
2.5.5. Co-prescription patterns
For patients who received continued and PRN prescriptions, the numbers/proportions of patients who received monotherapy (a single drug) and co-prescription patterns (either two drugs or three or more drugs) on the day of first prescription were tabulated separately.
2.5.6. Medication possession ratio (MPR)
The numbers of prescription days for patients who received continued prescription were tabulated between the first prescription day and the final prescription day (in the ranges ≤ 7, 8–14, 15–30, 31–60, 61–90, and > 90 days). The MPRs (in the ranges < 80%, 80 to ≤ 110%, and > 110%) were calculated by dividing the total number of prescription days by the number of days during the study period. Patients with MPR (80 to ≤ 110%) were regarded as having good drug adherence.
2.5.7. Treatment discontinuation rate (TDR)
Treatment discontinuation in patients who received continued prescription was defined as a case in which the next prescription could not be confirmed after the day of the final prescription. The numbers/percentages of patients who discontinued mirogabalin use during the study period were tabulated. The TDRs during ≤ 7, 8–14, 15–30, 31–60, 61–90, and > 90 days were calculated by dividing the number of patients who discontinued treatment by the number of patients who were prescribed mirogabalin.
2.6. Subgroup analyses
The effects of gender, age, clinical department, and presence/absence of prior medications on each endpoint were evaluated.
2.7. Statistical analysis
The numbers/proportions of patients who were prescribed mirogabalin and the numbers of prescription days were tabulated for categorical variables, while descriptive statistics were calculated for continuous variables. In the subgroup analysis, the numbers/percentages of patients who were prescribed mirogabalin, numbers of prescription days, and prescription doses were tabulated by gender, age, clinical department, and presence/absence of prior medications.
No statistical hypothesis testing and 95% confidence interval analysis were performed.
SQL Server 2016 and Microsoft Excel for Office 365 (Microsoft Corporation, Redmond, WA, USA) and R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria), were used for statistical analysis.
3. Results
3.1. Patient composition
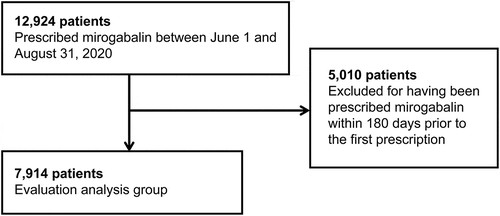
The composition of patients who were prescribed mirogabalin is shown in . Mirogabalin was prescribed to 12,924 patients during the 3-month period between 1 June and 31 August 2020. Of these, 5,010 patients who were prescribed mirogabalin within 180 days prior to the day of first prescription were excluded from the study, and the remaining 7,914 patients were included in the evaluation analysis.
3.2. Patient characteristics
The patient characteristics are shown in . The proportion of female patients tended to be higher (56.7%) than that of male patients. The mean age of the patients was 64.6 years. The proportion of patients aged 70–79 years was the highest (27.3%); the proportion of patients aged 60 years or older (64.3%) was higher than that of younger patients (aged ≤ 59 years; 35.7%). The proportion of patients who were prescribed in the orthopedic surgery department was the highest (56.5%). Prior medications were prescribed to 17.8% of patients. The most frequently prescribed prior medications were pregabalin (44.2%), followed by loxoprofen (non-steroidal anti-inflammatory drug [NSAID]; 14.1%), celecoxib (cyclooxygenase-2 inhibitor; 10.5%), and acetaminophen (8.3%).
Table 1. Patient characteristics
3.3. Endpoints
3.3.1. Numbers/proportions of patients prescribed and numbers of prescription days
shows the data regarding the prescription days and the numbers/proportions of patients who were prescribed mirogabalin. The total number of patients who were prescribed mirogabalin was 7,914, and the average number of prescription days was 52.3 days. The numbers/proportions of patients with continued and PRN prescriptions were 7,882 (99.6%) and 34 (0.4%), respectively, and 2 patients received continued prescription as well as PRN prescription. Therefore, most patients received continued prescriptions.
Table 2. Numbers/proportions of patients with continued and PRN prescriptions, and the prescription days
In subgroup analysis of continued prescription, the numbers/proportions of female patients tended to be higher than those of male patients, but no gender-specific difference in prescription days was observed. With regard to age, the numbers/proportions of patients and numbers of prescription days for elderly patients aged 60 years or older tended to be higher than in patients aged 59 years or younger. In particular, the numbers/proportions and numbers of prescription days for elderly patients aged 70–79 years were the highest. The proportion of patients in the orthopedic surgery department was the highest (56.6%), followed by those in the general internal medicine, anesthesiology/pain clinic, and dermatology departments. The number of prescription days was lowest for patients who obtained prescriptions in the dermatology department, but no large numerical difference was observed among the other clinical departments. The proportion of patients who had been prescribed prior medications was only 17.8%. The number of prescription days for patients with prior medications tended to be higher than that for patients without prior medications.
3.3.2. Total prescription dose and daily prescription dose
The total prescription dose and daily prescription dose of mirogabalin for patients who received continued prescription are shown in . The total prescription dose of mirogabalin was 618.2 mg. The daily prescription dose was 10.93 mg/day, which was lower than the maintenance doses (20–30 mg/day) specified in the mirogabalin package insert (MPI) [Citation17].
Table 3. Total prescription doses and daily prescription doses
In the subgroup analysis, no large gender-specific numerical difference in the total prescription dose and daily prescription dose of mirogabalin was observed. The daily prescription doses for elderly patients aged 70–90 years or older tended to be lower than those for patients aged 30–69 years (7.80–10.67 mg/day and 11.34–12.07 mg/day, respectively); no large numerical difference in total prescription dose was observed among other age groups, except in the ≥ 90 years and 20–29 years groups. The total prescription dose and daily prescription dose were the highest for patients in the neurology department; no large numerical difference was observed among the other clinical departments. The total prescription dose for patients with prior medications tended to be higher than that for patients without prior medications, but no difference in daily prescription dose was observed between these two groups.
3.3.3. Changes from the initial daily prescription doses
The changes from the initial daily prescription dose are shown in , , and Supplemental Table 1. Only small changes were observed in daily prescription dose between the first day (9.26 mg/day) to Days 7 and 14 (9.41 mg/day and 10.02 mg/day, respectively), but those on Days 30, 60, and 90 increased to 11.56, 12.61, and 13.37 mg/day, respectively (). The initial daily prescription dose and maintenance dose of mirogabalin specified in the MPI are 10 mg/day and 20–30 mg/day, respectively. The initial daily prescription dose of mirogabalin (9.26 mg/day) in our study was similar to the initial dose (10 mg/day) specified in the MPI, but the daily prescription dose of mirogabalin on Day 90 after the first prescription (13.37 mg/day) was lower than the maintenance dose (20–30 mg/day) specified in the MPI (). Additionally, the changes from the initial daily prescription dose were evaluated by comparing the initial daily prescription doses (in the ranges 2.5 to < 5 mg/day, 5 to < 7.5 mg/day, 7.5 to < 10 mg/day, 10 to <15 mg/day, 15 to < 20 mg/day, 20 to < 25 mg/day, 25 to ≤ 30 mg/day, and > 30 mg/day) with those on Days 7, 14, 30, 60, and 90. The proportion of patients who were prescribed the initial daily prescription doses of 10 to < 15 mg/day was the highest (53.6%), followed by that of patients with 5 to < 7.5 mg/day (30.1%), while 7.9% of patients prescribed at the initial doses of 20 to ≤ 30 mg/day increased to 30.7% on Day 90 after the first mirogabalin prescription ( and Supplemental Table 1).
Figure 3. Changes of the proportions of patients prescribed mirogabalin (2.5 to ≤ 30 mg/day) from the days of first prescription to Day 90. < 2.5 mg/day on the day of first prescription: only two patients (0%). > 30 mg/day on any prescription day: ≤ 0.3% (0.1% on Day 90 after the first prescription).
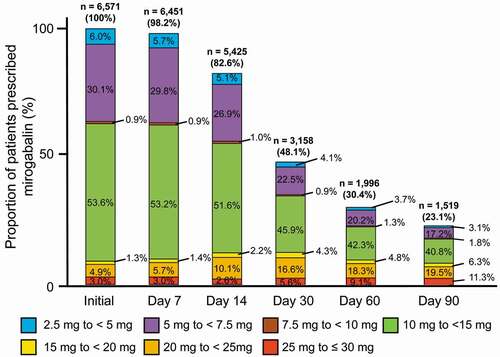
Table 4. Changes in the daily prescription doses from the first day of mirogabalin prescription to Day 90
In the subgroup analysis (), the daily prescription dose of mirogabalin for male patients tended to be higher than that for female patients. With regard to age, the daily prescription dose on Day 90 for patients aged 70 years or older tended to be lower than that for patients aged 69 years or younger. The daily prescription doses for patients in the neurology and dermatology departments on Day 90 tended to be higher than those for patients in other clinical departments, but there was no large numerical difference in the daily prescription doses among other clinical departments or with respect to the presence/absence of prior medications.
3.3.4. Maintenance doses and achieved doses
The maintenance and achieved doses of mirogabalin for patients who received continued prescription are shown in . The maintenance and achieved doses were 10.81 mg/day and 10.89 mg/day, respectively, both of which were lower than the maintenance dose (20–30 mg/day) specified in the MPI. The proportions of patients who were prescribed maintenance doses of 5 to < 7.5 mg/day and 10 to < 15 mg/day were 24.6% and 48.9%, respectively. Similar results were obtained for the achieved doses. The total proportions of patients prescribed at maintenance and achieved doses of 20 to ≤ 30 mg/day were 17.3% and 18.0%, respectively (Supplemental Table 2).
Table 5. Maintenance doses and achieved doses
In the subgroup analysis (), no large gender-specific numerical differences in the maintenance and achieved doses were observed. The maintenance and achieved doses for elderly patients aged 70 to 90 years or older tended to be lower than those for patients aged 30–69 years. The maintenance and achieved doses in the neurology department tended to be higher than those in other clinical departments, but no large numerical difference was observed among other clinical departments. The maintenance and achieved doses for patients with prior medications were similar to those for patients without prior medications.
3.3.5. Co-prescription patterns
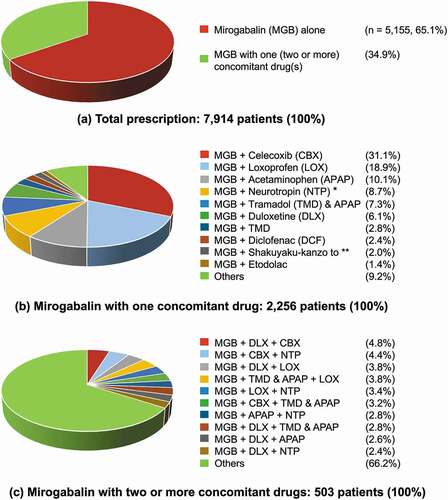
shows the co-prescription pattern of mirogabalin. Of the 7,914 patients with continued and PRN prescriptions, 65.1% were prescribed mirogabalin alone. The remaining 2,759 patients (34.9%) were prescribed a combination of two drugs (2,256 patients: 28.5%) or three or more drugs (503 patients: 6.4%). In the combination of two drugs, the most frequently prescribed concomitant drug was celecoxib (31.1%, 702/2,256 patients), followed by loxoprofen (18.9%), and acetaminophen (10.1%). The proportions of patients co-prescribed any three or more drugs were 4.8% or less.
Figure 4. Co-prescription patterns of mirogabalin. (a) Total prescriptions, (b) Mirogabalin with one concomitant drug, (c) Mirogabalin with two or more concomitant drugs.
In the subgroup analysis, no gender-specific differences in co-prescription patterns were observed. The most frequently co-prescribed two drug combinations were mirogabalin + celecoxib and mirogabalin + loxoprofen in patients aged 15 to 64 years, but mirogabalin was more frequently co-prescribed with celecoxib, acetaminophen, and loxoprofen in patients aged 65 years or older. Mirogabalin was frequently prescribed in combination with celecoxib or loxoprofen in the orthopedic surgery, general surgery, and neurology departments. Celecoxib or acetaminophen in general internal medicine, amitriptyline (tricyclic antidepressant) or duloxetine in the anesthesiology/pain clinic, loxoprofen or neurotropin (extract of cutaneous tissue of rabbits inoculated with vaccinia virus) in neurosurgery, and acetaminophen or neurotropin in the dermatology departments were frequently co-prescribed with mirogabalin.
3.3.6. MPR
The MPRs in patients who were prescribed mirogabalin are shown in Supplemental Table 3. The MPRs for 4,738 patients who received at least two continued prescriptions were evaluated. The proportions of patients with MPR < 80, 80 to 110%, and > 110% were 8.2%, 86.2%, and 5.6%, respectively, suggesting good drug adherence in most patients.
No numerical difference in MPR according to gender, age, or the presence/absence of prior medications was observed. The MPR in the 80–110% range was highest in the dermatology department (92.9%), while those in other clinical departments ranged from 77.4 to 88.0%.
3.3.7. TDRs
and Supplemental Table 4 show the TDRs and cumulative TDRs from the day of the first prescription of mirogabalin to Day 90 for patients with continued prescription. The cumulative TDR within ≤ Day 7 was 14.0%, while those during Days 31–60 and 61–90 reached 70.0% and 77.9%, respectively.
Figure 5. Cumulative TDRs after the first prescription of mirogabalin.
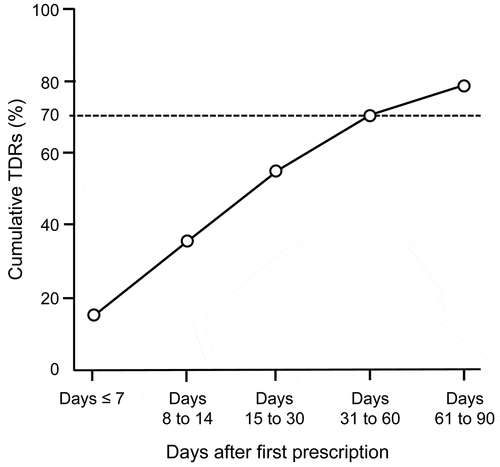
No gender-specific differences in the cumulative TDRs were observed. The cumulative TDRs in elderly patients aged 60 years or older tended to be lower than those in patients aged 59 years or younger. The cumulative TDR during Days 61–90 was lowest in the neurology department (60.5%), but no large numerical differences were observed among other clinical departments. The cumulative TDR for patients with prior medications tended to be higher than that for patients without prior medications.
4. Discussion
The objective of this study was to clarify the prescription status of mirogabalin in daily clinical practice using a large prescription database. The results show that mirogabalin was prescribed to a considerable number of patients. Although the daily prescription dose, maintenance dose, and achieved dose of mirogabalin at 10 to < 15 mg/day were the highest compared with those in other dose ranges, and these doses were lower than the maintenance doses (20–30 mg/day) specified in the MPI, but approximately 30% of patients were prescribed 20 mg/day or more. The most frequently prescribed concomitant drug was celecoxib. Mirogabalin may be used for both short- and long-term treatments (in terms of cumulative TDRs) with good drug adherence. These results will be useful for prescribing mirogabalin to patients with PNeP.
With regard to patient characteristics, the proportion of patients who were prescribed mirogabalin in the orthopedic surgery department (56.5%) was higher than those in other clinical departments (1.8–8.0%). This high proportion was close to that (45.2%) reported in a previous large-scale prescription database study on oral analgesics, including pregabalin and duloxetine, that are frequently prescribed for patients with NeP [Citation4]. A s there is no general practitioner (GP) system in Japan and internal medicine practitioners play a role close to GPs in the United States and the EU [Citation4], Japanese patients are usually treated at individual clinical departments according to the patient’s condition/pain sites and/or primary diseases such as diabetes mellitus or herpes zoster. Therefore, the patients with NeP at sites such as the lower back, shoulders, neck, thighs, and knees are generally treated with orthopedic surgery in Japan. Prior medications were prescribed to 17.8% of patients, with pregabalin (44.2%) being the most frequent prior medication, followed by loxoprofen (14.1%) and celecoxib (10.5%). Therefore, it is necessary to carefully evaluate pain relief and safety in patients who switch from pregabalin to mirogabalin. A multicenter, open-label study (the MIROP study) was recently conducted on PNeP patients treated with maintenance doses of mirogabalin (10 or 15 mg twice daily [BID] for patients with normal renal function and 5 or 7.5 mg BID for patients with moderately impaired renal function) [Citation21]. These doses are the same as those specified in the package insert of mirogabalin. In the MIROP study, 16 out of 152 patients (10.5%) who switched from pregabalin to mirogabalin subsequently discontinued the mirogabalin treatment. No new safety concerns were observed, and pain symptoms improved in these patients with PNeP.
4.1. Numbers/proportions of patients who were prescribed mirogabalin
In a previous large-scale prescription database study on oral analgesics, NeP drugs such as pregabalin, tramadol & acetaminophen combination, and duloxetine were among the top 10 drugs frequently prescribed for Japanese patients with pain for 1 year in 2017 (n = 46,799, 12,780 and 11,883 patients, respectively) [Citation4]. In our study, mirogabalin was newly prescribed to 7,914 patients over a relatively short period of 3 months. Moreover, considering that the indication of mirogabalin was limited to PNeP and only approximately 1.5 year had passed until the implementation of this study since its approval in January 2019, it can be considered that mirogabalin was prescribed to a relatively large number of patients in daily clinical practice. In a prescription database study of pregabalin conducted between July and December 2013, the proportion of patients with chronic pain who were prescribed pregabalin was approximately 0.13% [Citation22]. Furthermore, in a previous large prescription database study on oral analgesics in 2017, the proportion of patients who were prescribed pregabalin was found to have substantially increased to 9.4% [Citation4]. A phase 3 study (the AMELA study, clinical trial registration number: NCT03901352) to evaluate the efficacy and safety of mirogabalin in Asian patients with post-spinal cord injury (SCI) NeP, which is a form of central NeP, was completed. The number of patients who are prescribed mirogabalin may change further if the application for additional indication related to central NeP is approved [Citation23].
4.2. Maintenance dose, achieved dose, and changes in daily prescription dose
The recommended initial dose and maintenance dose of mirogabalin specified in the MPI were 10 mg/day and 20–30 mg/day, respectively. In our study, the proportion of patients who were prescribed the initial daily prescription doses of 10 to < 15 mg/day was the highest (53.6%) and mean initial dose (9.26 mg/day) was almost the same as that (10 mg/day) in the MPI. Maintenance dose, achieved dose, and the daily prescription dose of mirogabalin on Day 90 after the first prescription was 10.81, 10.89 and 13.37 mg/day, which were lower than the maintenance dose (20–30 mg/day) specified in the MPI. However, 30.9% of patients were prescribed a daily prescription dose of 20 to ≤ 30 mg/day on Day 90 after the first prescription, but the total proportions for patients prescribed at the maintenance and achieved doses of 20 to ≤ 30 mg/day were 17.3% and 18.0%, respectively. No large numerical difference was observed between the daily prescription dose, maintenance dose, and achieved dose. Although the reason why the daily prescription dose was lower than the maintenance dose of MPI is unclear, several possible reasons can be considered as follows: (1) ADRs such as somnolence, dizziness, and loss of consciousness induced by mirogabalin could lead to falls and fractures, particularly in elderly patients [Citation8,Citation17]. Therefore, physicians may be carefully prescribing mirogabalin at low doses at their discretion; (2) combination therapy for NeP is not only reported to result in stronger pain relief and lower ADR incidence than monotherapy, but is also expected to have the advantage of requiring the administration of lower doses [Citation24]. In our study, approximately 35% of patients were frequently prescribed mirogabalin in combination with NSAIDs or other NeP drugs with different mechanisms of analgesic action. Therefore, it is considered that the daily prescription doses of mirogabalin in these patients in daily clinical practice were likely to be lower than the maintenance doses strictly specified in the study protocol of clinical studies and in the MPI; (3) NeP patients with moderate and severe renal impairment are recommended to start with low doses of mirogabalin – 2.5 mg BID (5 mg/day) and 2.5 mg once daily [QD], respectively – and reduce the maintenance doses to 7.5 mg BID (15 mg/day) and 7.5 mg QD [Citation25], and these doses do not reach the maintenance dose (20–30 mg/kg) specified in the MPI. However, as patient data on renal function could not be collected in this study, the numbers/proportions of these patients were unclear. Further studies will be required for clarifying why the daily prescription doses were lower than the maintenance dose specified in the MPI.
4.3. Co-prescription patterns
The proportion of patients who were prescribed mirogabalin monotherapy was the highest (65.1%), suggesting that there was a prescription trend toward starting mirogabalin alone. The first and second most prescribed oral analgesics combined with mirogabalin were celecoxib (31.1%) and loxoprofen (18.9%), respectively. In the previous prescription database study on oral analgesics, the use of pregabalin combined with loxoprofen (13.3%) and celecoxib (11.9%) was more common than that of other oral analgesics [Citation4].The combination therapy of oral analgesics that have different mechanisms of analgesic action has been reported to provide stronger analgesic effects and fewer side effects than monotherapy [Citation24]; therefore, mirogabalin may be used not only for monotherapy but also for combination therapy with conventional NSAIDs or other NeP drugs.
4.4. MPR
The MPR in the range 80–110% was 86.2% in patients who were prescribed mirogabalin, indicating good drug adherence. This result was similar to the high MPR reported for pregabalin and duloxetine (82.3% and 90.0%, respectively) in the previous large-scale prescription database study of oral analgesics [Citation4]. As mirogabalin was used for the long-term treatment of PNeP with severe pain, the patients were considered compliant with continued treatment for PNeP.
4.5. TDR
The cumulative TDRs for mirogabalin were 14.0% (Days ≤ 7) and 70.0% and 77.9% (Days 31–60 and 61–90, respectively), and 22.1 to 30% of patients were continuously prescribed mirogabalin even when the prescription period was prolonged. The cumulative TDRs for pregabalin in the previous prescription database study of oral analgesics were 22.2% (Days 8–14) and 63.2% and 70.8% (Days 31–60 and 61–90, respectively) [Citation4], which were not very different from the results for mirogabalin obtained in our study. These results suggest that mirogabalin can be used for both short-term and long-term treatment of PNeP. The reasons for treatment discontinuation of mirogabalin are unclear, but the improvement/deterioration of NeP symptoms or occurrence of ADRs induced by mirogabalin may be related to treatment discontinuation.
This is the first large-scale prescription database study of mirogabalin, and it provides useful information regarding mirogabalin prescription for doctors who treat PNeP in daily clinical practice. However, there are some study limitations. The prescription data of mirogabalin in the database do not represent the information about the drugs that were actually administered to the patients. Although the prescription data included drug names, prescription doses, number of prescription days, gender, age, clinical department, and prior medications, they did not include other patient information regarding disease names, renal function, efficacy, and safety. Therefore, patient condition and disease recovery could not be analyzed; however, as the objective of this study was to evaluate the prescription status of mirogabalin, the lack of information about disease and outcomes is not expected to have a direct effect on the study results. As no follow-up evaluation was performed for patients when drugs were prescribed at pharmacies other than those participating in this study, the patients who changed pharmacies were considered among those who discontinued the treatment. We also cannot exclude the possibility of coronavirus disease 2019 affecting the prescription trend of mirogabalin. Furthermore, the indication for mirogabalin is currently limited to PNeP. In an overseas study (the ALDAY trial) that evaluated the efficacy of mirogabalin in the treatment of fibromyalgia, the primary endpoint of significant pain reduction in patients on mirogabalin compared with patients on placebo was not achieved [Citation26]. In Japan, clinical study of mirogabalin for fibromyalgia has not been conducted. An Asian phase 3 study to evaluate the efficacy and safety of mirogabalin for patients with SCI NeP was completed [Citation23], but the results of this study have not yet been published as a full article. As the prescription status of mirogabalin in patients, including most of the PNeP patients in this study, the prescription information of mirogabalin may change further if the indication changes in the future. Finally, phase 3 studies for PNeP and pharmacoepidemiological studies of mirogabalin have not been conducted worldwide. The further international, independent phase 3 trials and pharmacoepidemiological studies worldwide in the future are necessary for the better assessment of clinical value of mirogabalin in daily clinical practice.
5. Conclusions
A large-scale prescription database study was conducted to clarify the prescription status of mirogabalin in Japan. The results provide an overview of mirogabalin prescription, including the numbers/proportions of patients prescribed, prescription days, prescription doses, dose changes, co-prescription patterns, drug adherence, and cumulative TDR. Mirogabalin was prescribed to a considerable number of patients; however, the daily prescription doses, maintenance doses, and achieved doses of mirogabalin during the study period were found not to have increased to the maintenance dose (20–30 mg/day) specified in the MPI. The reason why most patients were prescribed mirogabalin at approximately 10 mg/day is being investigated. Finally, the results obtained may be useful for optimizing the use of mirogabalin for patients with PNeP in daily clinical practice.
Author contributions
T Ushida contributed to the study design and writing of the manuscript. M Yokoyama, K Shiosakai, and K Okuizumi contributed to the study design, conducted and performed the data collection and writing of the manuscript. S Ibe was involved in data analysis, contributed to the study design, conducted and performed the data collection and writing of the manuscript. K Saito contributed to the writing of the manuscript. All authors contributed to interpretation of data and reviewing the manuscript, and approved this manuscript for submission.
Declaration of Interest
T Ushida has received consulting fees for the performance of the study from Daiichi Sankyo Co., Ltd. M Yokoyama, K Shiosakai, K Saito, and K Okuizumi are full-time employees of Daiichi Sankyo Co., Ltd. S Ibe is a full-time employee of INTAGE Real World Inc. Daiichi Sankyo Co., Ltd. participated in study design, decision to publish, and preparation of the manuscript while INTAGE Real World Inc. participated in data collection and analysis, the preparation of the manuscript, study design, and decision to publish. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing assistance was provided by Honyaku Center Inc. during the preparation of this manuscript and funded by Daiichi Sankyo Co., Ltd.
Additional information
Funding
References
- Japan Society of Pain Clinicians, eds. Guidelines for the pharmacological management of neuropathic pain. 2nd ed. Tokyo Japan: Shinko Trading Co., Ltd.; 2016. [cited 3 Aug 2021]. Japanese/English. Japanese/English: http://minds4.jcqhc.or.jp/minds/Pharmacologic-management-of-neuropathic-pain/Pharmacologic-management-of-neuropathic-pain.pdf
- Sommer C, Cruccu G. Topical treatment of peripheral neuropathic pain: applying the evidence. J Pain Symptom Manage. 2017;53:614–629.
- de Leon-casasola O. New developments in the treatment algorithm for peripheral neuropathic pain. Pain Med. 2011;12(Suppl 3):S100–S108.
- Ushida T, Matsui D, Inoue T, et al. Recent prescription status of oral analgesics in Japan in real-world clinical settings: retrospective study using a large-scale prescription database. Expert Opin Pharmacother. 2019;20:2041–2052.
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14:162–173.
- Yang M, Qian C, Liu Y. Suboptimal treatment of diabetic peripheral neuropathic pain in the United States. Pain Med. 2015;16:2075–2083.
- Ushida T, Inoue T, Matsui D, et al. Cross-sectional study of patient satisfaction with oral analgesics in patients with chronic pain in Japan. Expert Opin Pharmacother. 2020;21:983–991.
- Daiichi Sankyo Co., Ltd. Tarlige® (mirogabalin) Tablets 2.5 mg ∙ 5 mg ∙ 10 mg ∙ 15 mg, pharmaceutical product interview form. Japanese. [cited 28 Apr 2021]. Available from: https://www.info.pmda.go.jp/go/interview/1/430574_1190026F1028_1_TL6_1F.pdf
- Taiwan Food and Drug Administration. Assessment report. TARLIGE® F.C. Tablets 15 mg. [cited 22 Oct 2021]. Available from: https://www.fda.gov.tw/tc/includes/GetFile.ashx?id=f637368791343691781
- Domon Y, Arakawa N, Kubota K, et al. Analgesic effect of mirogabalin via the α2δ-1 subunit of voltage-gated calcium channels – evidence from α2δ-1 (R217A) and α2δ-2 (R282A) mutant mice –. Japanese. Jpn Pharmacol Ther. 2019;47:585–591.
- Kato J, Inoue T, and Yokoyama M, et al. A review of a new voltage-gated Ca2+ channel α2δ ligand, mirogabalin, for the treatment of peripheral neuropathic pain. Expert Opin Pharmacother. 2021;1–12. DOI:https://doi.org/10.1080/14656566.2021.1958780.
- Domon Y, Arakawa N, Inoue T, et al. Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels. J Pharmacol Exp Ther. 2018;365:573–582.
- Baba M, Matsui N, Kuroha M, et al. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig. 2019;10:1299–1306.
- Kato J, Matsui N, Kakehi Y, et al. Mirogabalin for the management of postherpetic neuralgia: a randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain. 2019;160:1175–1185.
- Kato J, Matsui N, Kakehi Y, et al. Long-term safety and efficacy of mirogabalin in Asian patients with postherpetic neuralgia: results from an open-label extension of a multicenter randomized, double-blind, placebo-controlled trial. Medicine (Baltimore). 2020;99:e21976.
- Baba M, Matsui N, Kuroha M, et al. Long-term safety and efficacy of mirogabalin in Asian patients with diabetic peripheral neuropathic pain. J Diabetes Investig. 2020;11:693–698.
- Daiichi Sankyo Co., Ltd. Tarlige® (mirogabalin) Tablets, 2.5 mg, 5 mg, 10 mg, 15 mg, package insert. [cited 28 Apr 2021]. Japanese. Japanese: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/430574_1190026F1028_1_05
- Act on the protection of personal information (Amendment of Act No. 65 of 2015). [cited 28 Apr 2021]. Japanese. Japanese: https://www.ppc.go.jp/files/pdf/151112_kaiseian.pdf
- World Medical Association (WMA). WMA declaration of Helsinki - ethical principles for medical research involving human subjects. [cited 28 Apr 2021]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Guideline on implementation of pharmacoepidemiology studies in safety assessment of pharmaceuticals using database of medical information; 2014. [cited 3 Aug 2021]. Japanese. Japanese: https://www.pmda.go.jp/files/000147250.pdf
- Kimura Y, Yamaguchi S, Suzuki T, et al. Switching from pregabalin to mirogabalin in patients with peripheral neuropathic pain: a multi-center, prospective, single-arm, open-label study (MIROP Study). Pain Ther. 2021;10:711–727.
- Hirakata M, Yoshida S, Tanaka-Mizuno S, et al. Pregabalin prescription for neuropathic pain and fibromyalgia: a descriptive study using administrative database in Japan. Pain Res Manag. 2018;2018:2786151.
- Daiichi Sankyo Co., Ltd. An Asian, multicenter, randomized, double-blind, placebo-controlled, 14-week study of mirogabalin in participants with central neuropathic pain followed by a 52-week, open-label extension (NCT03901352). 2019. [cited 22 Oct 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03901352
- Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and future directions. Expert Rev Neurother. 2005;5:823–830.
- Baba M, Takatsuna H, Matsui N, et al. Mirogabalin in Japanese patients with renal impairment and pain associated with diabetic peripheral neuropathy or post-herpetic neuralgia: a phase III, open-Label, 14-week study. J Pain Res. 2020;13:1811–1821.
- Arnold LM, Whitaker S, Hsu C, et al. Efficacy and safety of mirogabalin for the treatment of fibromyalgia: results from three 13-week randomized, double-blind, placebo- and active-controlled, parallel-group studies and a 52-week open-label extension study. Curr Med Res Opin. 2019;35:1825–1835.