1. Introduction
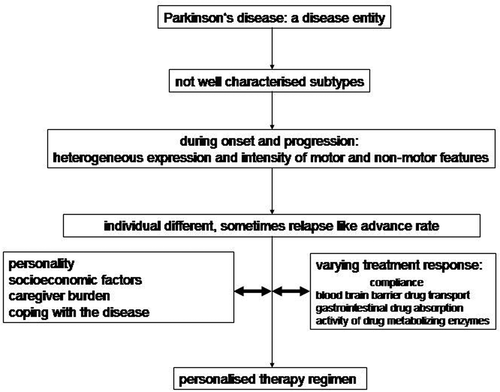
Idiopathic Parkinson’s disease (PD) is a chronic, heterogeneous neuropsychiatric disease entity. It consists of various not well characterized subtypes [Citation1,Citation2]. Symptomatic drug treatment of each other influencing onset of motor and non-motor PD features aims to improve impaired quality of life [Citation3]. The treating neurologist performs a patient-tailored PD drug therapy, which considers the individually varying symptom expression, their progression and living conditions of patients and their carers. Therefore a continuous, close interaction with both, the patient and the caregiver, is necessary in daily practice ().
Figure 1. Symptoms of idiopathic Parkinson’s disease ask for a patient-tailored treatment regimen.
1.1. The rising impact of unrealistic standards and health economics
In view of the above-mentioned heterogeneity of PD and its progression, one may crucially scrutinize the value of standardized treatment approaches. One exemplary tool is the implementation of treatment guidelines. Actually, they reflect a certain misconception for the real world of patient maintenance. On the one hand, esteemed authors publish these therapy recommendations in high-ranked journals. On the other hand, these publications have an impact on PD drug dosing and – labels, both of which are predominantly based on the design and outcomes of phase II and III trials. However, these preponderant pivotal studies were mostly performed under ideal ‘laboratory’ and thus experimental conditions with a well-selected patient cohort. One out of many drawbacks is the exclusion of patients with severe concomitant disorders from study participation. Drug approving authorities with no direct patient contact, i.e. the US Food and Drug Administration, increasingly influence the trial designs. In contrast, observational phase IV ‘real-world’ studies may better reflect acceptance, safety, tolerability of PD therapies in the various healthcare systems worldwide [Citation4]. However, the value of these phase IV results is underestimated in meta analyses and guidelines. Sometimes one may get the impression that these publications at least partially support the economic interests of payers, like insurances. They increasingly try to misuse guidelines in a justiciable fashion, i.e. in Germany, and limit the therapeutic freedom for a patient-tailored PD therapy. This is the background scenario for the prescribing neurologist, when performing considerations for the PD drug prescription in the individual patient.
1.2. Change of role behavior and patient relationship
Moreover physicians, involved in direct patient care, must take into account patients’ and caregivers’ coping strategies with PD and their personality features. All of them may also influence adherence to PD drug application. Unfiltered information, i.e. provided by social media in the internet, contributes to a growing knowledge of patients and their care partners on therapy in PD [Citation5]. The role of the PD specialist changes the demand rises for time-consuming discussions on pro’s and con’s of available PD treatments. Predominantly, young, early onset PD patients with a high educational level ask for more advice and have the tendency to influence PD drug regimen on their own. Such a more interactive relationship counteracts a more authoritarian and easier to perform behavior of the neurologist. Nevertheless treatment of PD patients asks physicians to establish a long-lasting relationship to patients, often combined with continuous counseling of care partners. Essential basic characteristics are respect, trust, accountability, and transparency [Citation5]. A good interpersonal level is the precondition for compliance, which is a problem of many PD patients. It may partially be improved by easy to perform and simple PD drug intake regimen [Citation6]. Additionally, slow and careful drug titration with concomitant, perpetual surveillance of the individual drug treatment tolerability and safety is essential for the long-term success.
1.3. The importance of optimum treatment and unmet needs
Nowadays, it is well accepted that an optimized PD drug treatment regimen shall be supplemented by continuous performance of physiotherapy and further activating therapies in a continuous fashion. This is particularly emphasized, i.e. in the Swedish guidelines, to counteract budget limitations by bureaucratic healthcare authorities with limited direct patient contact. Nevertheless repeat adaptations of the PD drug treatment regimen are continuously necessary due to the ongoing chronic neurodegenerative sometimes relapse like progression of PD. The times of the old fashioned attitude, to keep dopamine substitution as low as low as possible, are over. Necessary, additional application of antidepressants or neuroleptics often addresses the temporary onset and fluctuating severity of non-motor symptoms. The ultimate unmet needs are cure, respectively, reversal of deficits by repair, or delay of progression. All of them are not available yet. Generally, well-informed patients always ask for new treatment options, particularly for beneficial disease course modification. Exiting reports on successful experimental research outcomes exist and are also increasingly communicated by the media beyond scientific journals in an uncritical fashion. Researchers employed PD models, which mainly focus on motor deficits only. They do not mirror the heterogeneous spectrum of motor and non-motor symptoms in PD. When clinical trials were subsequently performed, they were negative. Examples are transplantation, gene therapy, stem-cell implantation. Studies with potential disease course modifying treatments, i.e. coenzyme Q, were also negative [Citation7]. The treating neurologist must know these new scientific, even only experimental developments and frequently dampen high expectations by patients. A well-informed neurologist emanates competence, when he introduces and performs a patient-tailored dopamine substitution with its pharmacological particularities in PD patients.
2. Dopamine replacement concepts
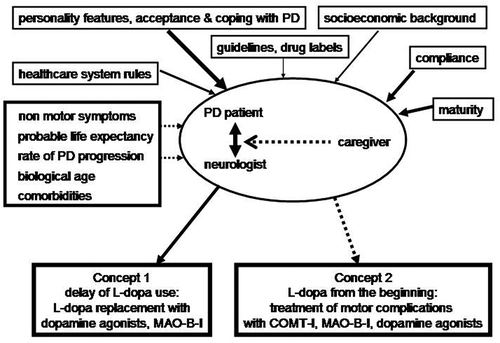
PD drug treatment is complex and its efficacy is still mainly associated with improvement of motor behavior. In this regard, a breakthrough was the introduction of the dopamine substitution concept. It also provides an amelioration of non-motor symptoms to a considerable extent [Citation8]. It was initiated with the implementation of levodopa (L-dopa), the blood-brain barrier trespassing metabolic precursor of dopamine, in the 1960s. A controversy is still ongoing on the long-term consequences of chronic L-dopa treatment in PD (as examples [Citation9,Citation10]). Experimental and clinical findings describe an acceleration of aging processes, impairment of the endogenous methylation potential with weakening of the detoxification capacity and generation of oxidative stress, all of which are associated with L-dopa (for review [Citation11]). These metabolic consequences support assumptions on negative effects of high L-dopa dosing in the long term. A further more popular problem is onset of oral L-dopa-associated motor complications. They mainly result from plasma fluctuations of L-dopa due to its peripheral short plasma half life and subsequently induced dopamine oscillations in the synaptic cleft of nigrostriatal neurons. This drawback of conventional oral L-dopa therapy counteracts the well-accepted concept of ‘continuous dopaminergic stimulation.’ Maintenance of a normal, physiological dopamine levels in the synaptic cleft is an essential precondition for nearly normal motor behavior in PD [Citation12]. When dopamine terminals degenerate in PD, a loss of their buffering capacity also occurs. However, intermittent dosing of oral L-dopa formulations causes fluctuations of striatal dopamine levels. Accordingly, a pulsatile stimulation of dopamine receptors takes place and ultimately induces onset of motor complications. As a result, motor and associated non-motor symptoms may switch between adequate motor behavior, recurrence of motor impairment, so-called ‘off’ states and involuntary movements, termed as dyskinesia, in advanced oral L-dopa-treated PD patients sooner or later. Thus, as an example, when PD patients are ‘off,’ they often complain as non-motor features apathy or anxiety [Citation13]. Due to this ‘double edged sword’ like character of L-dopa, other oral therapies, like monoamine oxidase-B inhibitors (MAO-B-I), dopamine agonists, the N-methyl-D-aspartate antagonist and dopamine reuptake blocker amantadine, or catechol-O-methyltransferase inhibitors (COMT-I), are additionally employed [Citation14]. There are two rough, but each other overlapping, concepts for these L-dopa co-therapies (). One is to focus on the initiation of oral L-dopa from the beginning following diagnosis, as L-dopa is well tolerated, highly efficacious, and cheap. Following the so-called ‘honeymoon’ period of L-dopa, characterized by an individual different interval without motor complications, L-dopa co-therapies are added to counteract oscillations of motor behavior. The other concept aims to delay oral L-dopa use as long as possible to achieve an amelioration of PD symptoms with MAO-B-I, dopamine agonists, and amantadine. L-dopa is introduced, when it inevitably becomes necessary due to the advance of PD, but it will be kept as low as possible during the further treatment course. Both concepts overlap and have in common that PD patients take a ‘tailored drug cocktail’ [Citation15].
Figure 2. Influencing factors on pharmacotherapy in idiopathic Parkinson’s disease.
2.1. Coping with available dopamine substituting compounds
MAO-B-I are generally well tolerated. They are less potent than dopamine agonists. They are applied with varying dosages even in a transdermal fashion [Citation16]. Introduction of dopamine agonists and amantadine is generally performed cautiously and slowly. This kind of drug titration avoids or reduces onset of leg edema, nausea, and vertigo particularly in the case of dopamine agonists. Supplementation with the peripheral dopamine receptor blocker domperidone, if available, for amelioration of nausea, or i.e. midodrine for compensation of dopamine replacement induced orthostatic blood pressure, if reimbursed, counteracts these common frequent side effects of dopamine replacement. Application of peripheral acting COMT-I’s only makes sense in combination with L-dopa/dopa decarboxylase inhibitor formulations.
2.2. Advanced therapies
Invasive techniques, such as deep brain stimulation or pump systems with application of apomorphine or L-dopa without and with entacapone, are mostly employed as a last therapeutic resort in more advanced, severely fluctuating PD patients [Citation17]. If available and reimbursed in a healthcare system at all, the use of these methods is based on the individual decision of patients and their caregivers [Citation5]. Invasiveness, reversibility of the procedure, device size and short, respectively, long-term side effects represent the most important selection criteria.
2.2.1. The most promising future concept for dopamine substitution
Implementation of continuous infusion of subcutaneous L-dopa, respectively, phospho-L-dopa, will enable a continuous brain delivery of L-dopa. It will improve treatment possibilities of motor and related non-motor fluctuations considerably, as already shown with the L-dopa/carbidopa intestinal gel (LCIG) application[Citation18–21]. A subcutaneous L-dopa administration with an easy-to-handle small pump system may be regulated by a still to be developed digital device. It will monitor motor behavior of the PD patient and L-dopa dosing similar to modern insulin device systems. Advanced therapies, like deep brain stimulation due to frequent onset of cognitive disturbances and personality changes or complex LCIG infusions, will probably be employed distinct less, when subcutaneous L-dopa infusion will become available [Citation22,Citation23]. In view of the now 60 years lasting experience of PD specialists with L-dopa, it is not comprehensible, that drug approving authorities enforce this new L-dopa delivery technique developing pharmaceutical manufacturers for performance of phase III trials again. As a result, the launch will be further postponed. Return of investments for trial performance will cause higher costs for this technique. This vicious circle will finally limit future prescription of subcutaneous L-dopa infusions due to the aforementioned health economic background scenario for the prescribing neurologist in the real world of patient maintenance. One can assume that further prescription hurdles will be implemented within bureaucratic price regulation scenarios. In this regard, one should consider that application of L-dopa/carbidopa/entacapone intestinal gel with a novel pump device was approved and launched after one pharmacokinetic trial on the L-dopa plasma behavior [Citation24]. This trial confirmed the therapeutic benefit of continuous plasma behavior and accordingly associated brain delivery of L-dopa on motor complications similar to more recent trial outcomes on the efficacy of subcutaneous L-dopa infusion [Citation19,Citation21,Citation25].
3. Expert opinion
In conclusion, when conducting a personalized treatment regimen, PD specialists must also consider further factors. These are presence of concomitant disorders, probable future life expectancy, possible compliance issues based on personality features, acceptance and coping with PD, safety and tolerability issues, convenience of drug application and the knowledge of PD patients and their caregivers (), both of whom more and more demand participation in therapeutic decisions.
3.1. The impact of health economics
The influence of healthcare authorities, politicians, and insurances rises in terms of drug cost reduction. Different kinds of price regulation scenarios of the country specific healthcare system increasingly complicate drug selection and limit therapeutic options. This system favors prescription of cheaper, generic available drugs. These compounds considerably differ in terms of pharmacokinetic behavior. Bio-equivalence may differ between the range of 80% and 125% of the original formulation, so the dispersion width of the various available generic formulations may vary up to 45%. If they are introduced by the pharmacist only, like in Germany, the efficacy of a personalized PD drug treatment cocktail may decline. Particularly, effects of oral L-dopa treatment are particularly influenced by even minimal differences between original and the various generic oral L-dopa formulations. These country-specific, sometimes even regional different, not transparent drug price reduction and discount scenarios are supplemented by direct or hidden modes of budget limitations for prescribing neurologists, i.e. in Germany. One long-term consequence of this tendency for this ‘cheap as possible treatment paradigm’ is a decline of interest for research in PD pharmacotherapy by pharmaceutical manufacturers. Compounds like istradefylline, carbidopa/levodopa extended release capsules (Rytary®), extended release amantadine, apomorphine hydrochloride sublingual film, levodopa/carbidopa inhalation powder, pimavanserin or droxidopa are not marketed in the EU, but in the US due to these various price regulation scenarios. Regulatory authorities, i.e. in Germany, often accept an adequate pricing of an innovation only, when the drug has a new mode of action. Therefore, return of investment for the still enforced cumbersome expensive clinical trials with an old drug with a well-known mode of action but with better pharmacokinetic and thus pharmacodynamic effects will be complex, if authorities only offer the price of the generic compound. A typical example is Rytary®, which is not marketed in Europe. Thus, the drug approval system has more and more become complex due to the heterogeneity of country-specific bureaucratic regulations and hurdles worldwide. Consequently, the marketing of drugs with well-known mode of actions but with innovative pharmacokinetic delivery features only is more and more limited to regions with a better pricing- and easier approval environment [26]. In contrast, developing countries frequently enable a drug treatment with L-dopa formulations only for the average population only [27]. However in regions with a higher standard of the healthcare systems, well-informed PD patients or engaged patient groups ask for innovation, continuously seek for therapies, which will provide a better quality of life. The neurologist must be able to inform PD patients on existing complex, country-specific, mostly not transparent regulations and show motivation to overcome prescription hurdles. Chronic ill patients often accept risks of side effects for the hope of cure, repair or stop of progression, when they ask for new therapies.
3.2. The hysteria on side effects and dosing regulation
To a certain extent, this attitude also counteracts concerns by regulatory authorities, who establish detailed safety and tolerability reports in medication leaflets. This mandatory information even on rare side effects may induce uncertainty or even so-called ‘nocebo’ effects in patients [28]. They complain these listed symptoms, which they may get following a specific drug intake. Sometimes they even loose confidence in the competence of the prescribing physicians. This is particularly the case, when pharmacists are enforced to discuss potential drug interactions based on standardized, unfiltered data bases with patients without consideration of the individual circumstances for the drug prescription, like in Germany. As a result, the relationship between the treating neurologist and the patient is weakened and the competence of the prescribing physician is scrutinized. Most neurologists more or less rely on their own experience with the prescribed drug or drug cocktail, their so-called ‘gut feeling,’ and not on complex guidelines, approval and label scenarios. In the real world of PD drug therapy, dosing of compounds, i.e. L-dopa or a dopamine agonist, is generally done following an individual risk–benefit calculation. It takes into account potential side effects of a specific dose. Generally, drug efficacy depends on individual sets of circumstances. As an example, the pharmacodynamic effects of enzyme blockers in PD, such as MAO-B-I or COMT-I, will be lower, if the enzyme activity is high in the individual subject. If the enzyme activity is low, an enzyme blocker will provide better symptomatic effects. However, endogenous enzyme activity may influence the likelihood for the onset of side effects as worst-case scenario. An example is tyramine-induced hypertension during MAO-B-I use [29]. Therefore, tight dosing restrictions, i.e. for COMT-I or MAO-B-I in particular, are counterproductive, particularly when study data are available on the safety, tolerability, and efficacy of drugs, such as dosing of rasagiline with 2 mg or safinamide with 200 mg in PD patients. Moreover some drug labels, i.e. the reversible MAO-B-I safinamide must only be used in combination with L-dopa but not alone or in combination with dopamine agonists, are not comprehensible. It is in contrast to the indication of the irreversible MAO-B-I’s, selegiline or rasagiline [30].
3.3. Concluding Remarks
PD patients require an individual dosing of compounds with repeated adaptation of a patient tailored combination drug regimen throughout the whole course of the disease [31]. The know-how of the treating physician is an essential component to achieve an optimum therapeutic outcome for the patient.
Declaration of Interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Weiner WJ. There is no Parkinson disease. Arch Neurol. 2008;65:705–708.
- Przuntek H, Müller T, and Riederer P. Diagnostic staging of Parkinson?s disease: conceptual aspects. J Neural Transm (Vienna). 2004;111(2):201–216.
- Müller T, Öhm G, Eilert K, et al. Benefit on motor and non-motor behavior in a specialized unit for Parkinson’s disease. J Neural Transm (Vienna). 2017;124(6):715–720.
- Müller T, Tolosa E, Badea L, et al. An observational study of rotigotine transdermal patch and other currently prescribed therapies in patients with Parkinson’s disease. J Neural Transm (Vienna). 2018;125(6):953–963.
- Nijhuis FAP, van den HL, Bloem BR, et al. The Patient’s Perspective on Shared Decision-Making in Advanced Parkinson’s Disease: a Cross-Sectional Survey Study. Front Neurol. 2019;10:896.
- Grosset D. There Is No Parkinson Disease. J Neurol Sci. 2010;289(6):115–118.
- Müller T, Mueller BK, Riederer P. Perspective: treatment for Disease Modification in Chronic Neurodegeneration. Cells. 2021;10(4):873.
- de Bie RMA, Clarke CE, Espay AJ, et al. Initiation of pharmacological therapy in Parkinson’s disease: when, why, and how. Lancet Neurol. 2020;19:452–461.
- Leal RM, Rascol O, Ferreira JJ. The “long and winding road” of the disease-modifying effects of levodopa has not ended yet. Mov Disord. 2020;35:397–399.
- Müller T. Pharmacokinetics and pharmacodynamics of levodopa/carbidopa cotherapies for Parkinson’s disease. Expert Opin Drug Metab Toxicol. 2020;16:403–414.
- Olanow CW, Calabresi P, Obeso JA. Continuous Dopaminergic Stimulation as a Treatment for Parkinson’s Disease: current Status and Future Opportunities. Mov Disord. 2020;35:1731–1744.
- Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;59:408–413.
- Titova N, Chaudhuri KR. Personalized medicine in Parkinson’s disease: time to be precise. Mov Disord. 2017;32:1147–1154.
- Aydemir ST, Kumcu MK, Ulukan C, et al. Patient preference of device-based treatment of Parkinson’s disease. Int J Neurosci. 2021:1–5. https://doi.org/10.1080/00207454.2020.1853723.
- Chaudhuri KR, Antonini A, and Robieson WZ, et al. Burden of non-motor symptoms in Parkinson’s disease patients predicts improvement in quality of life during treatment with levodopa-carbidopa intestinal gel. Eur J Neurol. 2019;26:581–e43.
- Olanow CW, Espay AJ, Stocchi F, et al. Continuous Subcutaneous Levodopa Delivery for Parkinson’s Disease: a Randomized Study. J Parkinsons Dis. 2021;11(1):177–186.
- Poewe W, Stocchi F, Arkadir D, et al. Subcutaneous Levodopa Infusion for Parkinson’s Disease: 1 -Year Data from the Open-Label BeyoND Study. Mov Disord. 2021;36(11):2687–2692.
- Rosebraugh M, Liu W, Neenan M, et al. Foslevodopa/Foscarbidopa Is Well Tolerated and Maintains Stable Levodopa and Carbidopa Exposure Following Subcutaneous Infusion. J Parkinsons Dis. 2021;11(4):1695–1702.
- Klostermann F, Jugel C, Müller T, et al. Malnutritional neuropathy under intestinal levodopa infusion. J Neural Transm (Vienna). 2012;119(3):369–372.
- Harati A, Müller T. Neuropsychological effects of deep brain stimulation for Parkinson′s disease. Surg Neurol Int. 2013;4(7):S443–S447.
- Senek M, Nielsen EI, Nyholm D. Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: a randomized crossover study. Mov Disord. 2017;32(2):283–286.
- Hauser RA, Walsh RR, Pahwa R, et al. Amantadine ER (Gocovri®) Significantly Increases ON Time Without Any Dyskinesia: pooled Analyses From Pivotal Trials in Parkinson’s Disease. Front Neurol. 2021;12:645706.
- Cilia R, Cereda E, Akpalu A, et al. Natural history of motor symptoms in Parkinson’s disease and the long-duration response to levodopa. Brain. 2020;143(8):2490–2501.
- Leal RM, Duarte GS, and Ferreira AN, et al. Nocebo response in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2019;65:13–19.
- Riederer P, Müller T. Monoamine oxidase-B inhibitors in the treatment of Parkinson’s disease: clinical–pharmacological aspects. J Neural Transm (Vienna). 2018;125(11):1751–1757.
