ABSTRACT
Introduction
Oral cladribine is a highly effective pulsed selective immune reconstitution therapy licensed for relapsing multiple sclerosis (RMS) since 2017. A full treatment course comprises two treatment cycles given 1 year apart, followed by two treatment-free years. The management of cladribine-treated patients beyond year 4 needs to be addressed as patients have now passed the initial 4 years since European Medical Agency approval.
Areas covered
A panel of neurologists and a neuroradiologist experienced in MS treatment/monitoring evaluated clinical trial data and real-world evidence and proposed recommendations for the management of cladribine-treated patients beyond year 4.
Expert opinion
Continuous monitoring of disease activity during the treatment-free period is important. Subsequent management depends on the presence or absence of inflammatory disease activity, determined in the absence of consistent guidelines via practice-driven neurological decision criteria. Persisting or newly occurring inflammatory disease activity is an indication for further treatment, i.e. either re-initiation of cladribine or switching to another highly effective disease-modifying therapy. The decision to retreat or switch should be based on clinical and radiological evaluation considering disease course, treatment history, and safety aspects. In the absence of disease activity, either retreatment can be offered, or the treatment-free period can be extended under structured monitoring.
1. Introduction
Oral cladribine is a highly effective pulsed selective immune reconstitution therapy approved by the European Medical Agency (EMA) since August 2017 for the treatment of adult patients with highly active relapsing multiple sclerosis (MS) as defined by clinical or imaging features and by the US Food and Drug Administration (FDA) since March 2019 to treat relapsing-remitting MS and active secondary progressive disease in adults. The aim of pulsed selective immune reconstitution therapy with cladribine is a long-term response to treatment achieved by a full treatment course comprising two brief treatment phases administered with 1 year of intermission. The established cumulative dose over 2 years is 3.5 mg/kg body weight. Further dosing is not recommended in years 3 and 4 [Citation1].
The efficacy, safety, and tolerability of cladribine have been confirmed in an extensive clinical study program [Citation2,Citation3], including long-term follow-up in open-label extensions [Citation4,Citation5] and post-marketing studies [Citation6–8]. In the open-label study CLASSIC-MS, 93 patients were followed with a median time since the last Parent Study dose of 10.4 years (range 9.5–14.2) [Citation7]. A positive effect on treatment satisfaction and quality of life was demonstrated in the randomized controlled trial CLARITY [Citation9] and the non-interventional studies CLARIFY [Citation10], CLADQoL [Citation11] and CLEVER [Citation12]. The long-term effectiveness of cladribine tablets is being further investigated in several clinical studies including the non-interventional, prospective study CLADQoL (focus on quality of life), CLARIFY-MS Extension, CLARION, and the interventional 2-year extension study MAGNIFY-MS Extension.
Cladribine tablets exert their effect mainly via selective depletion of dividing and non-dividing T and B cells. The active metabolite of cladribine is 2-chlorodeoxyadenosine triphosphate (Cd-ATP). Its intracellular accumulation leads to the disruption of cellular metabolism, the inhibition of DNA synthesis and repair, and subsequent apoptosis [Citation13]. Cladribine preferentially affects lymphocytes due to their relatively high deoxycytidine kinase (DCK) to 5′-nucleotidase ratio and dependence on adenosine deaminase activity to maintain the equilibrium of cellular triphosphorylated nucleotide concentrations. The accumulation of the cladribine nucleotide produces rapid and sustained reductions in T and B cell subsets [Citation14,Citation15], whereby other immune subsets are relatively spared, consistently with the rapid onset of treatment effects [Citation16]. Additionally, cladribine has been shown to reduce levels of proinflammatory cytokines, serum, and cerebrospinal fluid chemokines, adhesion molecule expression, and mononuclear cell migration [Citation13,Citation17–21], and to penetrate the blood–brain barrier, leading to concentrations of up to 25% of plasma level in the CSF [Citation22]. The recovery of the immune system preserves long-term therapeutic options with existing or upcoming drugs. While cladribine is able to penetrate the blood–brain barrier, it is currently unknown whether the substance affects RMS directly via effects in the CNS [Citation23]. Cladribine tablets are further characterized by a fast onset of action shown in the interim analysis of the MAGNIFY-MS study (NCT03364036), with reported changes in combined unique active lesions during the first 2 months after initiating treatment [Citation16], a short half-life of 1 day enabling co-exposition with other drugs, and absence of a rebound effect [Citation3,Citation24].
As of July 2021, cladribine tablets have been administered in 35,668 patients comprising 49,783.5 patient-years of exposure [Citation25]. As patients who started cladribine tablets immediately following the European marketing authorization in 2017 have now passed the initial 4 years, the management of cladribine-treated patients beyond year 4 needs to be addressed. For this purpose, a panel of neurologists and a neuroradiologist experienced in treating and monitoring MS patients evaluated clinical trial data and real-world evidence to propose recommendations for the management of cladribine-treated patients beyond year 4.
2. Methods
In 2020, a panel of 8 neurologists proposed a long-term management approach focused on the initial 4 years guided by responder type [Citation26]. The same panel, extended by the additional expertise of a neuroradiologist, reconvened in a virtual meeting in February 2022. The aim of the meeting was to reach consensus on a best practice approach for the management of cladribine-treated patients beyond year 4. Based on the currently available literature and guideline recommendations for MS, the panel proposed an algorithm that included consensus statements for decision criteria. The results of the meeting were recorded and then consolidated into a draft manuscript.
3. Evidence review of long-term efficacy and benefits of pulsed selective immune reconstitution therapy
Standard cladribine tablets therapy during the initial 4 years produced a durable and effective clinical response on clinical and magnetic resonance imaging (MRI) outcome measures in the majority of patients. Interim data of the CLASSIC-MS (NCT03961204) trial from a population of 147 patients with a median follow-up of 10 years suggest sustained efficacy of cladribine tablets following two annual treatment courses, with a substantial proportion of patients (63.3%) requiring no further treatment with disease-modifying therapies (DMTs) [Citation27]. Long-term follow-up of CLARITY participants in the PREMIERE registry revealed that 66% of 941 patients did not receive any DMT over 4.5 years after the last dose of cladribine tablets [Citation28]. Concurrently, real-world data from the Italian CLARINET-MS study indicated that 57.2% and 63.7% of the participants were relapse- and progression-free 60 months after the last cladribine dose, respectively [Citation29].
This treatment-free period within the pulsed selective immune reconstitution therapy concept provides several advantages compared to continuous therapy regimens, such as flexibility for women who want to become pregnant. While pregnancy must be ruled out during the treatment periods, women with optimized disease control can conceive 6 months after the last dose [Citation30]. The treatment-free period also offers a window for vaccinations, albeit first real-world data suggest a vaccination response following cladribine treatment regardless of the time of application and the lymphocyte count [Citation31,Citation32]. The concept of pulsed selective immune reconstitution therapy has a lower treatment burden, less cumulative risk, a favorable risk–benefit ratio, and attested high treatment convenience due to short courses of oral application. The brief pulsed treatment period facilitates patient adherence compared to long-term therapies [Citation33] and leads to improved quality of life [Citation9]. Apart from the structured clinical monitoring for evaluating disease activity, at least yearly brain MRI assessments are recommended [Citation26]. No additional monitoring measures during the treatment-free years are required according to the summary of product characteristics [Citation1], but have been implemented in routine clinical practice. Bearing these arguments in mind, extending the treatment-free period beyond year 4 in the case of stable disease is a potential option.
Nevertheless, some patients from the CLARITY Extension experienced relapses (24.4%) and/or disability progression (27.6%) during or after the formal core period of treatment [Citation5]. However, the CLARITY study population included only patients who were treatment-naive or previously treated with platform therapies. Upon marketing authorization, a sizable number of patients were pre-treated with other high-efficacy drugs. Increased relapse rates and a higher risk of disability progression in patients from CLARITY Extension, who received placebo in the core study, emphasize the rationale for earlier treatment initiation with oral cladribine [Citation3]. As a non-negligible proportion of patients experienced returning disease activity, consideration of redosing with oral cladribine beyond year 4 is a viable option and compatible with the licensed indication.
4. Proposed treatment management beyond year 4
Evidence from the CLARITY extension trial indicated a return of inflammatory disease activity in some patients already by the end of year 4 [Citation5]. Therefore, in line with the previously published expert opinion [Citation26], the expert panel underlined the importance of continuous monitoring for disease activity during the treatment-free period. The recommended monitoring procedures are summarized in Panel 1 of . Subsequent management beyond year 4 depends on the presence or absence of disease activity. The expert group proposed the clinical practice-driven clinical and neurological decision criteria compiled in Panel 2 of concerning disease activity: The major criteria indicative of disease activity are ≥1 relapse, EDSS worsening by ≥1 EDSS point confirmed over 3 months in patients with a baseline EDSS score ≤4.0, ≥0.5 EDSS points confirmed over 3 months in patients with a baseline EDSS >4.5, and emergence of ≥2 T2 lesions or ≥1 Gd+ T1 lesion within one year from a reference MRI. Of note, the stated definition for EDSS worsening is based on the consensus of the expert panel; however, the definition of sustained progression based on the use of EDSS in a clear and consistent manner is unlikely to be possible in the real-world clinical setting [Citation34]. MRI for monitoring purposes in order to detect (sub)clinical inflammatory disease activity should ideally be performed in a standardized way according to 2021 MAGNIMS-CMSC-NAIMS recommendations [Citation35], however, centers in certain countries worldwide may not be able to fully follow the standards suggested by MAGNIMS. Referring the patient to a specialized MRI center may be a feasible option in those cases. Relevant inflammatory disease activity can be detected either by brain or spinal cord MRI or both. Additional MRI applications such as optic nerve imaging should not be considered [Citation36]. For this purpose, brain MRI is the most relevant diagnostic procedure. In general, the use of gadolinium-based contrast for treatment monitoring is not strictly needed and can be considered as optional [Citation35]. However, in certain clinical situations (e.g. inconclusive relapse, suspected comorbidity), contrast-enhanced MRI should be considered. Image analysis should be performed by an experienced (neuro)radiologist. New lesions should be interpreted according to recent guidelines in order to exclude misinterpretations of comorbidities (e.g. lesion due to ischemic small vessel disease) as MS lesions [Citation37]. In addition, the expert panel formulated supportive parameters that may raise awareness to suboptimal control of disease activity. For instance, a deterioration in cognition assessed via the symbol digital modality test (SDMT), worsening of fatigue or decrease in a quality-of-life assessment may trigger an alert to perform an unscheduled brain (and/or optionally spinal cord) MRI of the patient. Therefore, it is important to assess these supportive parameters on a regular basis. Remote continuous monitoring of walking ability via digital apps may provide a more thorough assessment than performing the timed 25-foot walk test during the patient visit. As an example of digital tools, the Floodlight Proof-of-concept app has recently shown moderate-to-good test-retest reliability and significant correlations between the test features from the app and standard clinical and MRI measures [Citation38]. Additional explorative parameters can be obtained for further in-depth assessment but are not part of the clinical routine and therefore not suited for mandatory monitoring. Brain atrophy is recognized as an important biomarker for disease worsening but is difficult to implement in clinical routine. Assessing quality of life is an important patient-reported outcome that may provide useful additional information on the patient’s overall assessment. A variety of tools are available to assess quality of life, such as EuroQoL (EQ-5D), short-form questionnaire (SF-36), Multiple Sclerosis Quality of Life questionnaire (MSQoL-54), or Multiple Sclerosis International Questionnaire of Quality of Life (MusiQoL). The panel merely encourages the assessment of quality of life without favoring any specific tool, thus leaving the choice to the preference of the treating physician.
Figure 1. Proposed treatment algorithm.
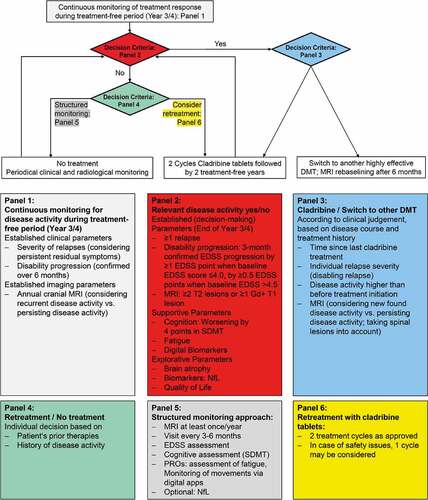
Presence of disease activity is an indication for further treatment, i.e. either retreatment with oral cladribine or another highly effective DMT. The decision should be aligned with clinical judgment based on disease course and treatment history. Panel 3 of comprises a list of factors to be considered in the decision process. The time interval since the last treatment should be taken into account along with the severity of disease activity compared to baseline activity before treatment was initiated. Recurrent radiological disease activity should also be considered in comparison to persisting disease activity [Citation26]. If the patient was stable during year 3 and 4, retreatment with oral cladribine may be an option. Furthermore, the extent of inflammatory disease activity is a deciding factor for switching treatment or maintaining cladribine therapy. In the case of breakthrough disease after year 4 with a severe flare requiring plasmapheresis, inflammatory activity on MRI such Gd+ lesions (e.g. ≥3), or a high number of new T2-lesions (e.g. ≥5) or relapse-related worsening, a switch of therapy is indicated. Based on the mode of action, i.e. selective and transient targeting of T and B cells, there are no restrictions expected regarding the choice of the subsequent DMT. To date, there are no data from clinical studies available investigating the superiority of one DMT over another after cladribine tablets. Long-term studies indicated no safety issues associated with switching to another highly effective DMT [Citation7,Citation28]. In case of atypical cessation of cladribine tablets during the initial 4 years, data from the German Multiple Sclerosis Registry indicated that most patients (60%) were switched to ocrelizumab in clinical practice [Citation39]. Studies investigating post year 4 scenarios considering disease activity prior to cladribine, previous immunotherapies, demographic factors, and comorbidities are currently ongoing. For mild-to-moderate disease activity with relapses without disability accumulation, a second course of oral cladribine can be administered. Prior therapies should also be considered in the decision process [Citation40]. In a real-world cohort from Finland, patients with two or more previous DMTs before switching to cladribine had a shorter time to first relapse when compared to patients with none or one previous DMT. This was driven mostly by early relapses experienced by patients switching from fingolimod, and likely represented rebound after fingolimod [Citation41]. As pointed out in the previously published expert opinion, for patients requiring a treatment switch, follow-up therapies should not be started earlier than 6 months after the last pulsed selective immune reconstitution therapy treatment cycle to allow repopulation of lymphocytes, while high disease activity may require a shorter interval in individual cases [Citation26,Citation42]. In the case of switch to another highly effective DMT, standard MRI re-baselining after 6 months according to the recent recommendation should be considered [Citation26,Citation42]. If the patient is retreated with cladribine, the experts favored a full treatment course comprising two treatment years in accordance with the summary of product characteristics of oral cladribine [Citation1]. Before starting the second course, a differential blood count must be obtained and safety aspects evaluated.
In the absence of inflammatory disease activity, either retreatment can be offered, or the treatment-free period can be extended under a structured monitoring approach, similar to the ‘as needed’ strategy currently applied after two cycles of alemtuzumab, another pulsed immune reconstitution therapy [Citation43]. The individual decision should be based on factors such as the patient’s prior therapies and history of disease activity (Panel 4 of ). Procedures as a part of the structured monitoring approach are listed in Panel 5 of and include periodical clinical and radiological assessments. Obtaining an annual brain MRI is highly recommended; optionally, additional spinal cord MRI may be considered. Clinical appointments should be scheduled every 3 to 6 months and include EDSS assessments, cognitive assessments (SDMT) as well as evaluation of patient-reported parameters, such as fatigue. Digital apps are suitable for monitoring of physical activity. As treatment is provided in an outpatient setting, it is important that also practice-based neurologists can perform the monitoring. Therefore, obtaining neurofilament light chain (NfL) levels has been included as an optional parameter. Since treatment with cladribine was initially selected based on high disease activity, the experts voted unanimously against de-escalation to a platform therapy, unless patient individual factors or safety issues oppose continuing treatment with cladribine tablets or another highly effective DMT. Patient age and the associated potential effects of immunosenescence on the effectiveness of DMTs, along with the risks mediated by these treatments, such as neoplasms and infections, in particular progressive multifocal leukoencephalopathy, should also be considered when determining the further treatment course. Independent of whether cladribine is resumed or the treatment-free period extended, the decision criteria listed in Panel 2 of provide guidance for further management.
5. Conclusion
The proposed algorithm for the management of cladribine-treated patients beyond year 4 takes various response scenarios into account and aims to provide guidance on an individual patient level. Dependent on the absence or presence of disease activity, options include an extension of the treatment-free period, retreatment with oral cladribine or switching to a different high-efficacy DMT.
6. Expert opinion
The benefit of pulsed selective immune reconstitution therapy with oral cladribine is the occurrence of long-lasting modification of the immune system by brief periods of treatment followed by treatment-free years. As the original treatment concept covered 4 years, to date there is neither prospective clinical data nor guidance available regarding the outcome and treatment beyond the initial 4 years. Based on the absence or presence of disease activity, the experts proposed an algorithm, with the aim of providing guidance for the decision-making process regarding therapy management beyond year 4. The pros and cons of resuming cladribine treatment in year 5 were discussed extensively among the experts. The majority favored the option of extending the treatment-free period in the absence of disease activity. The advantages of the treatment-free period in year 3 and 4 have been outlined above. An extension would presumably further affect patients’ quality of life in a positive way. It is also important to identify patients at high risk of recurrent disease activity in order to offer them retreatment. Factors taken into consideration in the decision-making process include prior therapies [Citation40]. In the case of retreatment with cladribine, the experts recommended a full treatment course of 3.5 mg/kg as licensed. Whether an increased cumulative maintenance dose of oral cladribine would provide a superior benefit for RMS patients remains a subject of future research. In this context, a retrospective study using an off-label subcutaneous cladribine formulation indicated that treatment with increased cumulative maintenance dosing was associated with disease stability and a favorable safety profile over a prolonged follow-up period of up to 20 years [Citation44]. A switch to another highly effective DMT may be warranted in the case of breakthrough disease. Future study of treatment sequence is needed to provide evidence for recommendations regarding which highly effective DMT is best suited following cladribine. Preliminary data from the real-world study CLASSIC-MS indicated neither safety nor efficacy issues when switching to other DMTs [Citation7]. However, no conclusion can be drawn regarding the superiority of one DMT over another. In clinical practice ocrelizumab is used most frequently following cladribine tablets [Citation39]. In case of mild recurring disease activity, the experts do not recommend de-escalation to a platform therapy because the diagnosis of highly active MS remains, and there are no known risks associated with switching to another highly effective DMT [Citation7,Citation28].
The experts expect the implementation of the algorithm into clinical practice to be feasible as the assessments required for the decision criteria are already part of current clinical routine. Additional assessments termed explorative parameters, such as NfL and brain atrophy, which can be obtained for further in-depth evaluation, have not been integrated into mandatory monitoring because they are not yet routinely accessible in clinical practice for various reasons. Serum NfL is increasingly seen as a potential biomarker to be implemented in clinical routine [Citation45], but has not been investigated as a monitoring tool for subclinical disease activity in cladribine-treated patients yet. Barriers to employ the regular assessment of brain atrophy include lack of financial resources and insufficient experience among neurologists to interpret MRI data as a part of routine practice. Although patient-reported outcomes are an important tool in assessing disease activity, their value is limited because it is unclear which cutoffs are clinically meaningful in a heterogenous real-world population. However, on an individual patient basis, a deteriorating patient-reported outcome parameter can trigger an alert to watch more closely for recurring disease activity in this patient. In this context, the panel encourages the assessment of patient-reported outcomes as additional supportive parameters to trigger possible alerts in terms of worsening. Overall, the management approach outlined by the expert group still leaves the treating physician the freedom of individual decisions. In light of varied clinical response and the specific circumstances of individual patients, a range of available treatment options is an important step toward personalized medicine. Future prospective studies are required to evaluate the feasibility of the proposed algorithm in clinical practice. As of today, it should be kept in mind there is an absolute lack of data in the literature supporting a specific strategy after using cladribine tablets.
The experience gained within the next 4 years will dictate whether the proposed algorithm can be implemented as standard procedure into clinical practice. In 4 years from now, patients being retreated with a full course of oral cladribine followed by two treatment-free years will have to be reevaluated in order to assess if the proposed algorithm can be followed as guidance for years 9–12 or requires adaptation. In particular, safety data have to be analyzed in order to gain insight into the effect of cumulative cladribine dosing beyond the initial 3.5 mg/kg. In line with the expanding treatment landscape, drugs addressing the cause of the disease rather than controlling the symptoms may be the therapeutics of tomorrow, offering new options in case of recurrent disease activity. In addition, a shift in treatment goals is currently ongoing. Whereas reduction of relapse rate and attenuation of disease progression were the primary goals after the initial introduction of DMTs into the market, with the development of new and highly effective MS therapeutics, reduction of the relapse rate alone is no longer considered sufficient in daily practice. In consequence, composite endpoints such as NEDA (no evidence of disease activity) [Citation46], PIRA (progression independent of relapse activity) and RAW (relapse-associated worsening) [Citation47] have become additional outcome parameters. These all contribute to quality of life in patients, which supports the fact that patient-centered outcomes have gained more attention in recent years and will continue to be the focus of future studies. Furthermore, digital assessments enable continuous monitoring in everyday life and the tendency toward digital biomarkers is already reflected in clinical studies.
Article highlights
Pulsed oral cladribine is a highly effective and selective immune reconstitution therapy for relapsing multiple sclerosis.
Efficacy, safety, and tolerability of cladribine tablets have been confirmed in a clinical study program, complemented with long-term open-label studies. After completing two cycles (in years 1 and 2), further treatment with cladribine is not recommended in years 3 and 4.
We propose an algorithm for the management of cladribine-treated patients beyond year 4, based on persistent and/or new and/or absent inflammatory disease activity either clinically or on brain and/spinal cord magnetic resonance imaging (MRI) and individual patient history.
Presence of disease activity by the end of year 4 is an indication for further treatment, i.e. either retreatment with cladribine or switching to a different disease-modifying therapy. In the absence of disease activity, either retreatment can be offered, or the treatment-free period can be extended under a structured monitoring approach.
The concept of pulsed selective immune reconstitution therapy with extended treatment-free periods without drug exposure is characterized by a low burden of treatment and monitoring and offers patients flexibility in family planning and vaccination.
Declaration of interest
A Bayas has received personal fees from Merck Serono, Biogen Idec, Novartis, Teva, Roche, Celgene/Brystol Myers Squibb (BMS), Sanofi/Genzyme and Janssen and grants for congress trips and participation from Biogen, Teva, Novartis, Sanofi/Genzyme, Celgene, and Merck Serono.
B Kallmann has received financial compensation for speaker’s fees and honoraria for attending advisory boards with Bayer, Biogen Idec, Biologix, Celgene/BMS, Hexal, Merck Serono, Novartis, Roche, Sanofi and Teva.
C Kleinschnitz has received speaker’s fees, honoraria for attending advisory boards, and financial support for conducting research projects from Merck Serono GmbH, Germany and Merck KGaA, Germany.
R Linker received compensation for activities with Biogen, Celgene/ Brystol Myers Squibb, Janssen Cilag, Genzyme, Merck Serono, Novartis, Roche as well as research support from Biogen, Merck Serono and Novartis.
S Meuth received honoraria for lecturing, and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Healthcare, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi, Chugai Pharma, Quintiles IMS and Teva. His research is funded by the German Ministry for Education and Research (BMBF), the Bundesinstitut für Risikobewertung (BfR), the Deutsche Forschungsgemeinschaft (DFG), the Else Kröner Fresenius Foundation, the Gemeinsamer Bundesausschuss (G-BA), the German Academic Exchange Service, the Hertie Foundation, the Interdisciplinary Center for Clinical Studies (IZKF) Muenster, the German Foundation Neurology and Alexion, Almirall, Amicus Therapeutics Germany, Biogen Idec, Diamed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva.
M Mäurer received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Almirall, Biogen Idec, BMS, GSK, Genzyme/ Sanofi, Merck Serono, Novartis, Roche, and Teva. He serves on a steering committee for Biogen and Novartis and as a consultant for Biogen, Genzyme and Roche.
P Rieckmann received honoraria for lectures from Almiral, Apple Healthcare, Baxter, Bayer, Biogen Idec, Bristol- Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Genpharm, Genzyme, Medtronic, Merck Serono, Novartis, Pfizer, Roche, Sanofi, Siemens AG, and Teva. He received research grants from Brainlight, German Neurology Foundation, h/p/Cosmos, Max Aicher Foundation, Oberfranken-Stiftung, Red Bull, and Teva. He served on advisory boards or steering committees for Aycan, Bayer, Biogen Idec, Canada Drug Review, the German Multiple Sclerosis Society, Novartis, Merck Serono, and Teva.
MP Wattjes received honoraria for lecturing and/or consultancy from Almirall, Bayer Healthcare, Biogen, Biologix, Bristol-Myers Squibb, Celgene, Genilac, Icometrix, Imcyse, IXICO, Medison, Merck-Serono, Novartis, Roche, Sanofi, Teva.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has received honoraria for presentations and the assembly of educational material from Alexion, Angelini, Bristol-Myers Squibb, Biogen, Boehringer, Immunic, Janssen, Merck, Novartis, Pfizer, Sandoz and Sanofi and is a member of scientific advisory boards of Alexion, Bristol-Myers Squibb, Biogen, Boehringer, Novartis, Sandoz and Sanofi. Another reviewer on this manuscript has received honoraria from Merck Serono and other companies in the multiple sclerosis space. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
Medical writing assistance was provided by Dr Petra Jöstingmeyer (med:unit GmbH, Germany) and was funded by Merck Healthcare Germany GmbH, Weiterstadt. The authors had full editorial control of the manuscript and provided their final approval.
Additional information
Funding
References
- EMA summary of product charateristics: mavenclad® (cladribine tablets) [cited 2022 Mar 7]. Available from: https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf.
- Cook S, Vermersch P, Comi G, et al. Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study. Mult Scler. 2011;17(5):578–593. Houndmills, Basingstoke, England.
- Giovannoni G, Comi G, and Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426.
- Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord. 2019;29(29):157–167.
- Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24(12):1594–1604. Houndmills Basingstoke England.
- Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91(6):660–668.
- Giovannoni G, Aydemir A, Di Cantogno EV, et al. CLASSIC-MS: long-term efficacy and real-world treatment patterns for patients with relapsing multiple sclerosis who received cladribine tablets in Phase III parent trials (1919). Neurology. 2021;96(15 Supplement):1919.
- Leist T, Cook S, Comi G, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord. 2020;46(46):102572.
- Afolabi D, Albor C, Zalewski L, et al. Positive impact of cladribine on quality of life in people with relapsing multiple sclerosis. Mult Scler. 2018;24(11):1461–1468. Houndmills Basingstoke England.
- Brochet B, Hupperts R, Langdon D, et al. Treatment satisfaction, safety, and tolerability of cladribine tablets in patients with highly active relapsing multiple sclerosis: CLARIFY-MS study 6-month interim analysis. Mult Scler Relat Disord. 2022;57(57):103385.
- Penner IK, Pul R, and Kallmann BA, et al. CLADQoL (CLADribine tablets – evaluation of quality of life) study: evaluating QoL 12 months after treatment initiation with cladribine tablets. Poster presentation P0849 presented at: MS Virtual Meeting of Americas Committee for Treatment and Research in Multiple Sclerosis and European Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS-ECTRIMS); Virtual; 2020.
- Ziemssen T, Cepek L, and Reifschneider G, et al. Evaluation of therapy satisfaction with cladribine tablets in RMS patients – final results of the non-interventional study CLEVER. Poster presentation P859 presented at: European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); Virtual; 2021.
- Beutler E. Cladribine (2-chlorodeoxyadenosine). Lancet. 1992;340(8825):952–956.
- Comi G, Hartung HP, Kurukulasuriya NC, et al. Cladribine tablets for the treatment of relapsing-remitting multiple sclerosis. Expert Opin Pharmacother. 2013;14(1):123–136.
- Wiendl H, Schmierer K, Hodgkinson S, et al. Characterization of peripheral immune cell dynamics and repopulation patterns in the first 12 months of cladribine tablets treatment: MAGNIFY-MS Study (2235). Neurology. 2021;96(15 Supplement):2235.
- de Stefano N, Barkhof F, and Montalban X, et al. Early reduction of MRI activity during 6 months of treatment with cladribine tablets for highly active relapsing multiple sclerosis: MAGNIFY-MS. Neurol Neuroimmunol Neuroinflamm. 2022;9(4):e1187.
- Bartosik-Psujek H, Belniak E, Mitosek-Szewczyk K, et al. Interleukin-8 and RANTES levels in patients with relapsing-remitting multiple sclerosis (RR-MS) treated with cladribine. Acta Neurol Scand. 2004;109(6):390–392.
- Janiec K, Wajgt A, Kondera-Anasz Z. Effect of immunosuppressive cladribine treatment on serum leucocytes system in two-year clinical trial in patients with chronic progressive multiple sclerosis. Med Sci Monit. 2001;7(1):93–98.
- Kopadze T, Döbert M, Leussink VI, et al. Cladribine impedes in vitro migration of mononuclear cells: a possible implication for treating multiple sclerosis. Eur J Neurol. 2009;16(3):409–412.
- Laugel B, Borlat F, Galibert L, et al. Cladribine inhibits cytokine secretion by T cells independently of deoxycytidine kinase activity. J Neuroimmunol. 2011;240-241:52–57.
- Niezgoda A, Losy J, Mehta PD. Effect of cladribine treatment on beta-2 microglobulin and soluble intercellular adhesion molecule 1 (ICAM-1) in patients with multiple sclerosis. Folia Morphol (Warsz). 2001;60(3):225–228.
- Strazielle N, Blondel S, Ghersi-Egea J-F. Cerebral bioavailability of cladribine: influence of metabolism and mechanism of transport across brain barriers (4347). Neurology. 2020;94(15 Supplement):4347.
- Meuth SG, Ruck T, Aktas O, et al. Cladribine tablets: oral immunotherapy of relapsing-remitting multiple sclerosis with short yearly treatment periods. Nervenarzt. 2018;89(8):895–907.
- Comi G, Cook SD, Giovannoni G, et al. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J Neurol. 2013;260(4):1136–1146.
- Giovannoni G, Berger J, Leist T, et al. Post-Approval safety of cladribine tablets with particular reference to COVID-19 outcomes: an update. ECTRIMS Vienna 2021.
- Meuth SG, Bayas A, Kallmann B, et al. Long-term management of multiple sclerosis patients treated with cladribine tablets: an expert opinion. Expert Opin Pharmacother. 2020;21(16):1965–1969.
- Giovannoni G, Leist T, Aydemir A, et al. Classic-MS: long-term efficacy and real-world treatment patterns for patients receiving cladribine tablets - interim data with 8–14 years follow-up. Multiple Sclerosis Journal. 2020;Vol. 26:LB1229. S3.
- European Medicines Agency Assessment Report, cladribine tablets (Mavenclad), June 2017 [cited 2022 Mar 18]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/mavenclad-epar-public-assessment-report_en.pdf
- Patti F, Visconti A, Capacchione A, et al. Long-term effectiveness in patients previously treated with cladribine tablets: a real-world analysis of the Italian multiple sclerosis registry (CLARINET-MS). Ther Adv Neurol Disord. 2020;13(13):1756286420922685.
- Stangel M, Becker V, Elias-Hamp B, et al. Oral pulsed therapy of relapsing multiple sclerosis with cladribine tablets - expert opinion on issues in clinical practice. Mult Scler Relat Disord. 2021;54(54):103075.
- Grothe C, Steffen F, Bittner S. Humoral immune response and lymphocyte levels after complete vaccination against COVID-19 in a cohort of multiple sclerosis patients treated with cladribine tablets. J Cent Nerv Syst Dis. 2021;13(13):11795735211060118.
- Brill L, Rechtman A, Shifrin A, et al. Longitudinal humoral response in MS patients treated with cladribine tablets after receiving the second and third doses of SARS-CoV-2 mRNA vaccine. Mult Scler Relat Disord. 2022;63(63):103863.
- Lyons M, Morgan K, Lott N. Year 1 performance of adveva®, a patient support programme (PSP) for patients taking MAVENCLAD® (cladribine tablets) for highly active relapsing remitting nultiple sclerosis (RRMS) in United Kingdom (UK). Poster presentation EP1593 presented at: ECTRIMS; 2019, Stockholm, Sweden.
- Healy BC, Engler D, Glanz B, et al. Assessment of definitions of sustained disease progression in relapsing-remitting multiple sclerosis. Mult Scler Int. 2013;2013:189624.
- Wattjes MP, Ciccarelli O, Reich DS, et al. MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653–670.
- Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol. 2014;10(8):447–458.
- Filippi M, Preziosa P, Banwell BL, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain. 2019;142(7):1858–1875.
- Montalban X, Graves J, Midaglia L, et al. A smartphone sensor-based digital outcome assessment of multiple sclerosis. Mult Scler. 2022;28(4):654–664. Houndmills Basingstoke, England.
- Ellenberger D, Frahm N, Flachenecker P, et al. Treatment patterns prior to and post cladribine in patients with multiple sclerosis. Eur J Neurol. 2022;29(Suppl. 1):629–630.
- Pfeuffer S, Rolfes L, Hackert J, et al. Effectiveness and safety of cladribine in MS: real-world experience from two tertiary centres. Mult Scler. 2022;28(2):257–268. Houndmills Basingstoke, England.
- Rauma I, Viitala M, Kuusisto H, et al. Finnish multiple sclerosis patients treated with cladribine tablets: a nationwide registry study. Mult Scler Relat Disord. 2022;61(61):103755.
- KKNMS. Quality Handbook MS/NMOSD. Recommendations for the treatment of multiple sclerosis/neuromyelitis-optica-diseases for physicians. [Qualitätshandbuch MS/NMOSD. Empfehlungen zur Therapie der Multiple Sklerose/Neuromyelitis-optica-Spektrum-Erkrankungen für Ärzte]. 5. revised and updated edition January 2020 [cited 2021 May 3]. Available from: http://www.kompetenznetz-multiplesklerose.de.
- Giovannoni G, Mathews J. Cladribine tablets for relapsing-remitting multiple sclerosis: a clinician’s review. Neurol Ther. 2022;11(2):571–595.
- Rejdak K, Zasybska A, Pietruczuk A, et al. Long-term safety and efficacy of subcutaneous cladribine used in increased dosage in patients with relapsing multiple sclerosis: 20-Year Observational Study. J Clin Med. 2021;10(21):5207.
- Bittner S, Oh J, Havrdová EK, et al. The potential of serum neurofilament as biomarker for multiple sclerosis. Brain. 2021;144(10):2954–2963.
- Lublin FD. Disease activity free status in MS. Mult Scler Relat Disord. 2012;1(1):6–7.
- Kappos L, Wolinsky JS, and Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140.
