ABSTRACT
Introduction
Tourette syndrome (TS) is a chronic tic disorder characterized by both motor and vocal tics. The vast majority of patients present with co-morbid behavioral problems, especially tic-related obsessive-compulsive behaviors and attention-deficit and hyperactivity disorder. Evidence-based guidelines on the pharmacotherapy of TS have become available in recent years.
Areas covered
The main purpose of this paper is to provide an overview of the current and emerging pharmacotherapeutic strategies for TS. A comprehensive search for the literature on the pharmacotherapy of tics was conducted using multiple databases (MEDLINE, Scopus, Web of Science, and Google Scholar), without date limits.
Expert opinion
In consideration of the heterogeneity of the TS phenotypes, pharmacotherapy should be tailored to the individual patient. The choice of the pharmacological agent should take into account both the efficacy-to-tolerability ratio and the presence of co-morbid conditions. Evidence-based pharmacotherapy should aim at improving health-related quality life within a dynamic framework that typically requires active monitoring of the clinical presentation and reevaluation of the treatment intervention over time.
1. Introduction
Tics are defined as sudden, repetitive, nonrhythmic movements (motor tics) or sounds (vocal tics) [Citation1]. Tic expression has been described as the willful capitulation to an irresistible sensory urge, commonly referred to as premonitory urge [Citation2,Citation3]. Tic disorders are currently classified as both neurodevelopmental conditions and hyperkinetic movement disorders. Tourette syndrome (TS) is a chronic tic disorder characterized by the presence of multiple motor tics plus at least one vocal tic, with onset before 18 years of age [Citation1]. The prevalence of TS has been estimated to be around 0.5–0.8%, with males being three to four times more likely to be affected than females [Citation4–6]. Patients most commonly present with simple motor tics affecting their face and neck (e.g. eye blinking, mouth pulling, facial grimacing, neck tensing) and simple vocal tics such as sniffing, grunting, coughing, and throat clearing. Complex motor tics are movements involving multiple muscular districts, whereas complex vocal tics are entire words, including repeated words (palilalia), other people’s words (echolalia), and swear words (coprolalia) [Citation7].
It has been estimated that about 90% of patients with TS present with co-morbid behavioral problems, including obsessive-compulsive behaviors, attention-deficit, hyperactivity, anxiety, and affective symptoms [Citation8,Citation9]. Tic-related obsessive-compulsive behaviors, which are reported by up to 70% of patients with TS, encompass repetitive counting (arithmomania), ordering, checking, forced touching, concerns for symmetry and evening-up behaviors that are often driven by sensory urges and ‘just right’ perceptions [Citation10–13]. It is thought that the shared clinical features between tics and tic-related obsessive-compulsive behaviors reflect overlapping pathophysiological mechanisms, with treatment implications [Citation14].
Although the exact pathophysiology of TS is still unknown, alterations within the cortico-striato-thalamo-cortical circuits and disturbances in overall sensorimotor processing seem to play important roles [Citation15]. Multiple neurotransmission pathways are likely to be involved in addition to dopaminergic networks [Citation16]. The contribution of possible deficits in inhibitory control is controversial [Citation17]. In consideration of the natural history of tics, a proportion of young patients first diagnosed with tics/TS might not require active interventions and would instead benefit from psychoeducation and watchful monitoring. For the remaining ones, available treatment options encompass behavioral interventions – mainly habit reversal training, as part of the comprehensive behavioral intervention for tics (CBIT), and exposure and response prevention – and pharmacotherapy [Citation18–20]. Among non-pharmacological treatment strategies, CBIT is the intervention that has been investigated more thoroughly, with evidence based on large randomized controlled trials. More invasive procedures such as deep brain stimulation can be considered for severe and refractory cases [Citation21]. The pharmacotherapeutic armamentarium for the treatment of TS encompasses first- and second-generation antidopaminergic medications, alpha-2 agonists, plus a range of pharmacological agents with less established evidence [Citation22]. This latter group includes heterogenous compounds, ranging from presynaptic monoamine depletors to anticonvulsants, cannabinoids, and traditional Chinese therapies [Citation23].
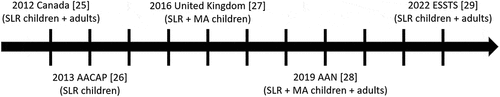
Evidence-based guidelines on the pharmacotherapy of TS have become available in recent years [Citation24–29]. The most recent guidelines have been published in 2019 by the American Academy of Neurology (AAN) and in 2022 by the European Society for the Study of Tourette Syndrome (ESSTS) (). The 2019 AAN guideline [Citation28] presents a set of practice recommendations, integrating findings from a comprehensive systematic review of the available evidence [Citation30] and a multidisciplinary panel of international experts. The 2022 ESSTS guideline [Citation29] provides clinicians with an update of the 2011 recommendations for the pharmacotherapy of TS in Europe [Citation24] using evidence from clinical trials and clinical expertise. While the AAN guidelines rate pharmacological agents based on their evidence level, the ESSTS guidelines present an updated ranking of recommended medications based on a pan-European expert survey.
Figure 1. Timeline of the guidelines on pharmacotherapy for patients with Tourette syndrome (most recent versions, 2012–2022).
The present paper provides an overview of current and emerging pharmacotherapeutic strategies for TS. A comprehensive search for the literature on the pharmacotherapy of tics was conducted using MEDLINE (via PubMed), Scopus, and Web of Science databases. The gray literature was searched using Google Scholar. The search terms used were ‘Tourette syndrome’ OR ‘tic disorder’ OR ‘tics’ in conjunction with ‘pharmacotherapy’ OR ‘pharmacology’ OR ‘medication’ OR ‘drug’ OR ‘treatment.’ Published recommendations and expert opinions were also reviewed. No date limits were used. In addition to the medications listed by the guidelines, pharmacological agents supported by the best available evidence were selected for inclusion in the present review. This work complements recently published reviews [Citation31–33] by integrating the best available evidence on the most commonly used anti-tic medications with ratings from the current guidelines and emerging pharmacological agents.
2. Antidopaminergic medications
2.1. First-generation antidopaminergic medications
Alterations in dopaminergic neurotransmission within cortico-striato-thalamo-cortical circuitries are thought to play a pivotal role in the pathophysiology of TS [Citation15]. Specifically, it has been suggested that tic expression might be linked to a hyperdopaminergic state due to receptor sensitivity, tonic-phasic dysfunction, and both pre- and post-synaptic dysfunction [Citation34]. Antidopaminergic medications are still considered the most effective pharmacotherapy for tics [Citation30,Citation35]. However, these pharmacotherapeutic agents have different degrees of dose-dependent adverse effects that often limit their use. The most commonly reported adverse effects include sedation, weight gain, hyperprolactinemia, extrapyramidal symptoms, and QT prolongation. Specifically, first generation antidopaminergic medications are characterized by a higher propensity to cause extrapyramidal and cardiac adverse effects compared to second-generation agents, which are more likely to be limited in their use by metabolic adverse effects [Citation36–38].
Among the first-generation antidopaminergic medications, haloperidol was the first agent that proved effective for the treatment of tics back in the 1960s. As well as being a potent dopamine antagonist of the dopamine D2 receptors, haloperidol blocks both muscarinic receptors and adrenergic receptors. Its effectiveness in tic reduction is well established [Citation39]. However, haloperidol is also notoriously associated with dose-dependent adverse effects, particularly sedation, apathy, anhedonia, hyperprolactinemia, and extrapyramidal symptoms [Citation22]. As a consequence, the use of haloperidol for the treatment of TS has progressively decreased over the last few decades from being a first-line agent to being used only in selected cases who are characterized by severe tics that failed to respond to other interventions [Citation28,Citation29].
Pimozide is both a dopamine D2 receptor antagonist and a calcium channel blocker. Throughout the 1980s, pimozide was increasingly being used as an alternative anti-tic agent to haloperidol, as it was associated with fewer extrapyramidal adverse effects. However, pimozide can cause cardiac arrhythmias and prolongation of the QTc interval, in addition to sedation, weight gain and hyperprolactinemia as commonly reported tolerability issues. This medication is currently regarded as possibly more likely than placebo to reduce tic severity, and its use in the treatment of TS is recommended only for severe and treatment resistant cases [Citation39,Citation40]. Among first-generation antidopaminergic medications, comparatively less is known about the use of fluphenazine for the treatment of TS. Based on suggestions about a better tolerability profile compared to haloperidol (especially with regard to sedation and extrapyramidal symptoms), fluphenazine is listed in the AAN guidelines as an additional first-generation antidopaminergic agent for which evidence is promising but limited [Citation28].
2.2. Second-generation antidopaminergic medications
During the 1990s, the availability of new medications with better tolerability profiles resulted in decreased use of first-generation antidopaminergic medications, which were mainly replaced by second-generation antidopaminergic medications. Risperidone is a D2 dopamine receptor blocker as well as a 5-HT2 serotonin receptor antagonist at lower doses. Its spectrum of action also includes D3 and D4 dopaminergic, alpha-2 adrenergic, and H1 histaminergic receptors [Citation41,Citation42]. The effectiveness of risperidone in reducing tic severity has been thoroughly documented, together with its lower propensity to cause extrapyramidal adverse effects [Citation39]. Weight gain, dyslipidemia, hyperglycemia, and hyperprolactinemia are well known metabolic adverse effects of risperidone [Citation43]. Over the last decade, the availability of a better tolerated antidopaminergic medication such as aripiprazole has led to a change in prescribing preferences of the European experts: risperidone was rated as first choice in the 2011 survey, but lost its pole position to aripiprazole in the 2022 update [Citation24,Citation29].
Among second-generation antidopaminergic agents, ziprasidone is characterized by low affinity for D2 dopaminergic receptors, moderate affinity for M1 muscarinic and H1 histaminergic receptors, and high affinity for 5HT2A, 5HT1A, and 5HT2C serotonergic receptors [Citation20,Citation42]. According to the AAN guidelines, there is initial evidence that ziprasidone is possibly more likely than placebo to reduce tic severity [Citation28], but this agent is not included in the recommendations by the experts in the updated ESSTS guidelines [Citation29]. Other second-generation antidopaminergic medications, such as quetiapine, have shown promise in small open-label trials and case reports; however, there is a lack of rigorous studies assessing the place for these agents in the treatment of TS [Citation44]. Of note, among its multiple actions, quetiapine is characterized by moderate affinity for D2 dopaminergic receptors, from which it rapidly dissociates, thus decreasing the risk of adverse effects such as extrapyramidal symptoms and hyperprolactinemia [Citation42].
2.3. Other antidopaminergic medications
Substituted benzamides such as sulpiride and tiapride are selective antagonists of dopamine D2-D3 receptors that can be classed as second-generation antidopaminergic agents. Available data about these medications have a geographical distribution, as they are used mainly in Europe for the treatment of TS. Substituted benzamides seem to be characterized by fewer extrapyramidal adverse effects compared to other antidopaminergic agents [Citation42,Citation45], although their tolerability is often limited by dose-dependent sedation, weight gain, hyperprolactinemia and other metabolic adverse effects.
Aripiprazole is sometimes referred to as a third-generation antidopaminergic medication, as its overall effect of decreasing baseline D2 stimulation is achieved through receptor partial agonism, rather than antagonism. Specifically, aripiprazole functions as a partial agonist at the D2, D3, and D4 dopaminergic receptors, as well as 5-HT1A and 5-HT2C serotonergic receptors [Citation22]. The effectiveness of aripiprazole in reducing tics has been estimated to be at least comparable to other dopamine-modulating agents, such as haloperidol and risperidone [Citation32,Citation39].
According to a systematic literature review and meta-analysis of nine randomized controlled trials of augmentation therapy with antidopaminergic agents in treatment-refractory obsessive-compulsive disorder, patients with co-morbid tic-related obsessive-compulsive disorder could be two-to-three times more likely to respond to the dual pharmacotherapy, compared to those without tics [Citation46]. With regard to newer antidopaminergic medications, a more recent meta-analysis of randomized controlled trials found that only aripiprazole and risperidone were superior to placebo in decreasing obsessive-compulsive symptoms in the absence of tics [Citation47]. In children with TS and tic-related obsessive-compulsive disorder, there is evidence to support the use of antidopaminergic augmentation from case series of children treated with aripiprazole or risperidone [Citation48]. The results of a prospective uncontrolled open-label study in adults with TS showed that aripiprazole can significantly improve co-morbid conditions (especially obsessive-compulsive disorder), in addition to tics [Citation49]. Therefore, in patients with refractory tic-related obsessive-compulsive disorder, antidopaminergic augmentation can be recommended, keeping in mind the limited evidence base and the need for safety monitoring [Citation50–52]. Moreover, it has been suggested that aripiprazole may have positive effects on co-existing conditions, such as anxiety, affective symptoms and auto-aggression in adults with TS [Citation53].
The tolerability profile of aripiprazole is superior to the other antidopaminergic agents used for the treatment of TS [Citation54,Citation55]. Its propensity to cause sedation and weight gain seems to be less prominent, especially in adult patients [Citation56]. Moreover, aripiprazole has the unique property of potentially reducing serum prolactin concentration [Citation57]. Based on its favorable efficacy-to-tolerability ratio, aripiprazole is at present the most commonly used antidopaminergic medication for the treatment of both tics and co-morbid behavioral symptoms in patients with TS [Citation58,Citation59]. Specifically, over the last decade aripiprazole superseded risperidone as first choice anti-tic agent according to the European experts [Citation24,Citation29]. The available evidence on dosages (posology) of the antidopaminergic medications most commonly used for the treatment of TS, as well as their recommendations in the current American and European guidelines, are summarized in .
Table 1. Antidopaminergic medications most commonly used for the treatment of Tourette syndrome: suggested posology and recommendations in the current guidelines.
3. Alpha-2 agonist medications
The pharmacological activity of alpha-2 agonists is directed against adrenergic neurotransmission, with clinically significant effects at the level of the sympathetic nervous system, which seems to be involved in the pathophysiology of TS [Citation22,Citation60,Citation61]. Both clonidine and guanfacine bind to alpha-2 adrenergic receptors at pre-synaptic level, resulting in decreased release of noradrenaline. The systemic effects of alpha-2 agonists are responsible for their known anti-hypertensive effects. Alpha-2 agonists have long been used for the treatment of both tics and behavioral symptoms, ranging from attention-deficit and hyperactivity disorder to irritability. Specifically, clonidine and guanfacine have been shown to be effective as non-stimulant pharmacotherapy for attention-deficit and hyperactivity disorder, and are therefore more commonly used in younger patients with TS and this common co-morbidity [Citation62]. There is also evidence that the effect size of alpha-2 agonists on tic reduction is considerably larger in patients who have both TS and co-morbid attention-deficit and hyperactivity disorder, as compared to patients with TS without attention-deficit and hyperactivity disorder [Citation63].
Despite the evidence that the effectiveness of clonidine as anti-tic agent is inferior to antidopaminergic medications such as aripiprazole [Citation32], its relatively safer adverse effect profile makes it a widely prescribed medication, especially in young patients with TS and co-morbid attention-deficit and hyperactivity disorder [Citation28]. The currently available evidence in favor of the efficacy of guanfacine, which was licensed in Europe a few years after North America, is less robust. Therefore, in the AAN guidelines guanfacine is recommended with a lower confidence in its evidence, compared to clonidine [Citation28]. Despite preliminary evidence that clonidine might be more effective in suppressing tics than guanfacine, which, in turn, might be less sedating, to date there has been no trial directly comparing the two alpha-2 agonists [Citation15].
The most commonly reported adverse effects in patients taking alpha-2 agonists are dose-dependent and include sedation, hypotension, and bradycardia (with similar tolerability profiles in both clonidine and guanfacine) [Citation62]. A few minor adverse events, such as dry mouth, typically emerge within the first couple of weeks of dosing and then generally remit. Slow titration is recommended, as abrupt withdrawal of higher doses of alpha-2 agonists may cause rebound hypertension [Citation22]. The suggested posology of alpha-2 agonist medications for the treatment of TS, as well as their recommendations in the current American and European guidelines, are summarized in .
Table 2. Alpha-2 agonist medications for the treatment of Tourette syndrome: suggested posology and recommendations in the current guidelines.
4. Other pharmacotherapeutic agents
There are a few medications that do not belong to either the antidopaminergic class or the alpha-2 agonist class, but have proven useful for the treatment of TS. Among other pharmacological classes, the medications that are most commonly prescribed off-label are topiramate and tetrabenazine [Citation22].
Topiramate is a broad-spectrum antiepileptic medication first marketed in the 1990s and characterized by multiple mechanisms of action. These include – but are not limited to – neuronal membrane stabilization and potentiation of GABAergic neurotransmission [Citation64]. The different patterns of activity of topiramate correspond to its widespread use across both neurological (e.g. migraine) and psychiatric (e.g. bipolar affective disorder) conditions. Over the last decade there has been increasingly more evidence that topiramate is a useful medication for the treatment of TS, in terms of both efficacy and tolerability [Citation65]. The most commonly reported adverse effects of topiramate seem to be dependent on both the target dose and titration speed, and include sedation, cognitive and language problems (especially word finding difficulties), irritability, paresthesia, nausea, sweating, and decreased appetite [Citation64,Citation66]. The observation that topiramate has been associated with weight loss makes it an appealing medication in polypharmacy, since most antidopaminergic agents used for treatment of TS can cause weight gain and metabolic adverse effects [Citation22]. The use of topiramate for the treatment of TS is currently on the rise, according to both the American [Citation28] and the European [Citation29] guidelines. Over the last few years, this medication has been prescribed, either as monotherapy or as add-on to other anti-tic agents, to a significant proportion of patients with TS in specialist clinics, where more complex and refractory cases tend to be seen [Citation67].
Tetrabenazine is a vesicular monoamine transporter-2 (VMAT-2) inhibitor that acts as a presynaptic monoamine depletor [Citation22]. Although this medication was known since the 1950s, in 2008 it became the first approved treatment for chorea associated with Huntington’s disease in the United States, as an orphan disease agent for the pharmacotherapy of chorea. In addition to Huntington’s disease, Tetrabenazine is currently licensed also for use in patients with tardive dyskinesia [Citation31,Citation68]. Evidence for the efficacy of tetrabenazine in the treatment of tics comes mainly from open label studies and in the recent AAN guidelines it has been deemed to be insufficient to establish a degree of certainty in the effect estimate [Citation28]. Clinically significant adverse effects such as depression and extrapyramidal symptoms are not rare, especially when higher doses are required. The relatively narrow efficacy-to-tolerability ratio of tetrabenazine in comparison to newer antidopaminergic agents such as aripiprazole has gradually decreased its use in patients with TS, at least as a first- or even second-line option [Citation69].
The suggested posology of topiramate and tetrabenazine for the treatment of TS, as well as their recommendations in the current American and European guidelines, are summarized in .
Table 3. Other pharmacotherapeutic agents most commonly used for the treatment of Tourette syndrome: suggested posology and recommendations in the current guidelines.
5. Emerging pharmacotherapeutic strategies
Most patients with TS improve with non-pharmacological interventions and/or established tic-suppressing medications, however a proportion of patients either fail to respond or develop problematic adverse effects. As new treatment modalities are being developed, novel pharmacological options are under investigation for the treatment of tics.
Lurasidone is a dopamine and serotonin receptor antagonist used for the treatment of schizophrenia and bipolar affective disorder. In a recently published small case series, this medication was added on to either risperidone or aripiprazole for the treatment of refractory TS with co-morbid obsessive symptoms and aggressive behavior, with encouraging results in terms of control of behavioral symptoms and tolerability (both motor and metabolic adverse effects) [Citation70]. Ecopipam is a first-in-class medication characterized by selective D1 receptor antagonism demonstrated significant tic reduction and good tolerability in both an open-label study [Citation71] and a follow-up randomized, placebo-controlled crossover study of young patients with TS [Citation72]. A phase IIb trial of ecopipam in young patients with TS (D1AMOND study) includes an ongoing open-label extension following the randomization period. The results of the randomized controlled trial component of the D1AMOND study were recently made available: these encouraging findings suggested a 30% reduction in tic severity [Citation73].
In consideration of the need for safe and effective pharmacotherapy for TS and the key role of VMAT2 in modulating the activity of biogenic amines (including dopamine), other VMAT2 inhibitors in addition to tetrabenazine have been investigated as potential anti-tic agents. Deutetrabenazine is an isomer (deuterated form) of tetrabenazine with a longer half-life and reduced risk of adverse effects [Citation68]. Although open-label studies demonstrated that deutetrabenazine is a safe and effective pharmacotherapeutic option in patients with TS [Citation74], both the phase 2/3 ARTIST1 trial and the phase 3 ARTIST2 trial failed to show a significant benefit on tic reduction. Valbenazine is another VMAT2 inhibitor, specifically a purified parent drug of the (+)-alpha-isomer of tetrabenazine [Citation68]. The safety and tolerability of valbenazine for the treatment of tics were established in the T-Force study, but the primary endpoint of statistically significant tic reduction was not met in placebo-controlled trials in either adult or pediatric patients (fixed/optimized doses), including open-label extension studies [Citation75].
In addition to topiramate, other antiepileptic medications, such as Levetiracetam and Valproate, have been used for the treatment of TS [Citation76]. Levetiracetam is an anticonvulsant medication that acts as a neuromodulator by targeting synaptic vesicle glycoprotein SV2A and reducing neurotransmitter release [Citation77]. Its anti-tic efficacy had mixed results in placebo-controlled trials [Citation78]: one small study showed improvement [Citation79], whereas two others failed to show statistically significant results [Citation80,Citation81]. Like topiramate, valproate is an antiepileptic medication with known GABAergic effects, which are also believed to contribute toward its anti-manic properties. A randomized controlled trial compared intravenous valproate to aripiprazole, showing that both treatments led to significant tic reduction, although the intravenous valproate group responded to treatment faster [Citation82]. Benzodiazepines such as clonazepam are other GABAergic agents for which case reports/series and open-label studies showed potential for tic reduction, in the absence of randomized controlled trials [Citation15,Citation42]. The long-term use of benzodiazepines is limited by their potential to cause tolerance and addiction, as well as by their dose-dependent sedating properties. Baclofen is a centrally acting muscle relaxant that interacts with the GABA-B receptor subtype. This medication was found to improve impairment scores, but not tic severity, in one small randomized controlled trial [Citation83] and may be considered for the treatment of severe muscular tension.
According to a Cochrane review conducted over a decade ago, there was insufficient evidence to establish the efficacy of cannabinoids for the treatment of TS [Citation84]. However, scattered reports of improvement in tic severity following use of cannabinoids have prompted further systematic investigations of their effectiveness [Citation85]. A survey of patients with TS who have self-medicated with cannabinoids revealed that patients tend to favor cannabis (rich in psychoactive tetrahydrocannabinol) over dronabinol (a synthetic version of tetrahydrocannabinol) or nabiximols (a mix of tetrahydrocannabinol and non-psychoactive cannabidiol) [Citation86]. A few trials have been registered: these include an Australian study comparing a tetrahydrocannabinol and cannabidiol compound in a 1:1 ratio versus an inert oil (ACTRN12618000545268) and a Germany-based protocol aimed at testing nabiximol in comparison to placebo (CANNA-TICS) [Citation87]. Over the last few years, there have been attempts to modulate the endogenous endocannabinoid system with the aim of treating TS. Lu-AG06466 is a compound that prevents the breakdown of an endogenous ligand of the endocannabinoid system by selectively inhibiting the enzyme monoacylglycerol lipase [Citation85]. Positive findings indicating significant improvement in tic severity from an initial placebo-controlled crossover study evaluating Lu-AG06466 in patients with TS [Citation88] were not confirmed by a follow-up multicenter, double-blind, randomized, placebo-controlled trial [Citation89]. There are also ongoing trials evaluating palmitoylethanolamide, an endogenous fatty acid amide that mimics the properties of cannabinoids [Citation85]. Interestingly, palmitoylethanolamide might reduce the adverse effects associated with cannabinoids (mainly dry mouth, nausea, headache, fatigue, anxiety and confusion), making it an appealing compound to use in combination with traditional cannabinoids [Citation85]. A phase 2 open-label study evaluating a combination of palmitoylethanolamide and dronabinol reported an encouraging level of reduction in tic severity [Citation90], and a larger placebo-controlled trial has been registered. At present, there is agreement that cannabinoids should still be regarded as an experimental treatment in patients with TS, as indicated by the recent AAN [Citation28] and ESSTS [Citation29] guidelines [Citation91].
There are a few recent studies on emerging treatments for TS that modulate histaminergic, noradrenergic, and serotonergic pathways [Citation33]. A trial investigating atomoxetine, a noradrenaline reuptake inhibitor which might improve response inhibition parameters in patients with TS, has been registered. An H3-receptor antagonist called AZD5213 has been assessed for safety and tolerability in patients with TS, however it has not shown any significant difference compared to placebo. Pimavanserin is a serotonin receptor inverse agonist (without dopamine receptor antagonist properties) which is approved for the treatment of Parkinson’s disease psychosis. The results of a recent open-label phase 1 pilot study to evaluate pimavanserin in the treatment of motor and behavioral symptoms in adults with TS were encouraging and warranted further research by larger, placebo-controlled, trials [Citation92]. Of note, the adverse effects of pimavanserin were reported to be common but not severe (headache, bloating, dizziness, drowsiness, nausea, and dysgeusia. A significant degree of tic reduction was demonstrated when D-cycloserine, an antibiotic with learning enhancing properties, was administered to patients with TS undergoing habit reversal training (as compared to patients treated with habit reversal training and placebo) [Citation93]. A follow-up study of D-cycloserine augmented habit reversal training for youth with tic disorders has been registered.
Finally, complementary and alternative medicines have been reported for the treatment of TS, although the evidence is limited because of a lack of randomized control trials. These include dietary or nutritional supplements (mainly minerals and vitamins), as well as Chinese traditional medicine [Citation23]. For example, vitamin D supplementation was found to be associated with significant improvement in tic severity [Citation94]. Taurine, a naturally occurring sulfur-containing amino acid with GABA-receptor agonist properties, was found to significantly improve tic severity when used as add-on to tiapride, as compared to placebo [Citation95]. An ongoing trial is currently investigating the possible tic-modulating properties of Lactobacillus plantarum PS128, a probiotic that can affect brain neurotransmitter levels. Beyond Western medicine, there have been recent reports that traditional Chinese compounds might have clinically significant tic-reducing properties [Citation96,Citation97]. Polyherbal products called Ningdong granules, Choudongning capsules, 5-Ling granules, and Changma Xifeng tablets have been tested for the treatment of TS with promising preliminary results. The possible generalizability of these early findings in other populations of patients with TS still needs to be established. Moreover, the underlying mechanism(s) for the tic-reduction properties of Chinese herbal supplements is unknown.
6. Conclusion
A wide range of pharmacotherapeutic strategies are currently in use or under investigation for the treatment of TS. However, only a few of them have received approval for use by pharmaceutical regulatory bodies. Haloperidol was the first antidopaminergic medication proven to be effective in the treatment of TS and is still the only medication with formal approval for TS in European countries [Citation29]. In the United States, the Food and Drug Administration granted approval to two first-generation antidopaminergic medications (haloperidol in 1969 and pimozide in 1984), as well as the newer antidopaminergic agent aripiprazole (in 2014). Specifically, haloperidol is licensed for patients with TS above the age of 3 years, pimozide for patients above the age of 12 years, and aripiprazole for patients between the ages of 6 and 18 years [Citation44].
The pharmacotherapeutic options for TS have gradually evolved over the last few decades, toward medications that are characterized by a more favorable efficacy-to-tolerability ratio, with increased chances of improving patients’ health-related quality of life. In spite of the widespread use of pharmacotherapy for the treatment of TS, there is a relative paucity of large, randomized controlled trials assessing the safety and efficacy profiles of individual medications. The most up-to-date guidelines from North America [Citation28] and Europe [Citation29] combine the best available evidence with expert recommendations, and converge on the use of antidopaminergic and alpha-2 agonist agents as anti-tic pharmacotherapy, followed by other medications such as topiramate and tetrabenazine. There is a trend for North American specialists favoring the use of clonidine, especially in younger patients, whereas the first choice of European experts has shifted from risperidone to aripiprazole over the last decade.
Due to the adverse effects associated with existing pharmacotherapy, there is increasing interest in using complementary and alternative medicine. Among emerging pharmacotherapeutic options, research is particularly active in the field of cannabinoids. Crucially, the psychoactive properties of certain cannabinoids (especially tetrahydrocannabinol) need to be accounted for when designing future placebo-controlled trials.
7. Expert opinion
The literature reporting international guidelines and expert recommendations on the pharmacotherapy of patients with TS has expanded considerable over the last decade. Treating clinicians are currently assisted by two comprehensive sets of up-to-date evidence-based guidelines recently developed in North America [Citation28] and in Europe [Citation29], respectively. While anti-dopaminergic agents and, to a lesser extent, alpha-2 agonists have been confirmed as the pharmacological classes with the largest amount of high-quality evidence, other medications have emerged as useful current or future alternative options. The Tic Disorders and Tourette syndrome Study Group of the Movement Disorder Society completed a survey of the complete society membership about clinical practice in patients with tic disorders [Citation98]. Of the medication classes used to treat tics, antidopaminergic agents were more commonly rated as ‘very or extremely effective’ than alpha-2 agonists or topiramate. For children, the preferred medication according to most clinicians was clonidine followed by aripiprazole. In the Americas, these were also the most commonly used medications for adults, whereas in Europe the most commonly used medication was aripiprazole, followed by risperidone. These results are in line with the available evidence from current AAN and ESSTS guidelines. The possibility that prescribing preferences could be driven by pharmaceutic companies’ outreach and advertising activities cannot be ruled out. However, the results of a recent international survey of health care services available to patients with TS showed that the treatment guidelines from North America and Europe are likely to drive differences in regional pharmacotherapeutic preferences [Citation99]. A pharmacoepidemiologic study examining recommendations for alpha-2 agonists and antidopaminergic medications in children and adolescents with tic disorders in Canada from 2012 to 2016 also revealed trends that were in line with guideline recommendations (decreasing use of risperidone and growing use of clonidine and guanfacine, as well as aripiprazole) [Citation100].
The application of evidence-based guidelines to everyday clinical practice can be challenging for a number of reasons. Firstly, evidence-based clinical guidelines are notoriously difficult to apply to conditions like TS, which are characterized by high phenotypic variability and a complex spectrum of co-morbidities. Secondly, statistically significant improvements in tic severity reported in randomized controlled trials of pharmacotherapy for TS may have a marginal impact on day-to-day practice. Finally, the possible role of the placebo effect should not be underestimated, as it has been shown that the placebo effect can account for a significant proportion of improvement in (30% of tic severity and 40% of overall impairment) in about one fifth of patients [Citation101–103]. The established role of placebos as tic suppressors highlights the importance of conducting placebo-controlled trials in order to determine the real efficacy of pharmacological options.
Standardization of care pathways and alignment with evidence-based treatment guidelines should complement, but cannot replace, skilled clinical observation and individualization of therapeutic approaches [Citation104]. The development of management plans for patients with TS should take into account a number of factors, including tic severity, overall clinical presentation, patient and family priorities/expectations, and resource availability. In consideration of the heterogeneity of the TS phenotypes, it is particularly important that pharmacotherapeutic interventions are tailored to the individual patient. Both medication- and patient-specific factors need to be considered. Specifically, the thought process that leads to the choice of the pharmacological agent should take into account both the efficacy-to-tolerability ratio and the presence of co-morbid conditions. Moreover, evidence-based pharmacotherapy should aim at improving patients’ health-related quality life within a dynamic framework that typically requires active monitoring of the clinical presentation and reevaluation of the treatment intervention over time.
Beyond psychoeducation and non-pharmacological interventions, pharmacotherapy can be recommended when the benefits for a patient are seen to outweigh the risks. In case of moderate-to-severe tic severity, the use of an alpha-2 agonist like clonidine could be supported by its favorable tolerability profile, although the magnitude of its treatment effect falls below that of the antidopaminergic agents. The presence of co-morbid attention-deficit and hyperactivity disorder would strengthen the case for choosing an alpha-2 agonist. In consideration of their relatively narrow efficacy-to-tolerability ratio, antidopaminergic medications could be administered when the tics are severe and/or refractory to more conservative measures. Aripiprazole could be recommended as first-choice antidopaminergic medication, as it has proven to be effective and overall better tolerated than both first- and second-generation antidopaminergic agents. If the TS phenotype includes tic-related obsessive-compulsive behaviors that prompt further pharmacotherapy in addition to behavioral treatment interventions and serotonergic medications, there is evidence that either aripiprazole or risperidone can be useful as add-on agents. When further pharmacotherapeutic options are required, topiramate would be a promising candidate. Topiramate has also that advantage that it can be used in polypharmacy without increased burden in terms of tolerability (especially extrapyramidal symptoms, weight gain, and metabolic effects).
On balance, there is good potential to continue improving the pharmacotherapeutic armamentarium currently available for TS. Unfortunately, within the class of presynaptic monoamine depletors, it has not been possible to expand the anti-tic options beyond tetrabenazine, as both deutetrabenazine and valbenazine have failed to confirm their initially positive results. Future well-powered placebo-controlled trials are needed to confirm promising findings about the safety and anti-tic efficacy of lurasidone, ecopipam, cannabinoids, and anticonvulsants, as well as selected complementary and alternative medications. Knowledge gaps could be addressed by developing head-to-head comparisons of different agents from the same pharmacological class or from different ones. Trials investigating the safety and efficacy of pharmacological combinations are also needed, as well as studies aimed at identifying the optimal treatment durations and dosages of established anti-tic medications. Finally, controlled studies in treatment resistant cases would be helpful in order to rationalize the widespread use of sequential pharmacotherapy. New avenues of investigation to better understand the pathophysiology of TS may in turn stimulate new approaches to developing better pharmacotherapy options and ultimately improve patients’ health-related quality of life.
Article highlights
Multiple evidence-based guidelines on the pharmacotherapy of Tourette syndrome have become available in recent years.
The two main classes of pharmacological agents currently used for the treatment of tics are antidopaminergic medications and alpha-2 agonists.
The available evidence indicates that antidopaminergic agents are characterized by higher efficacy, whereas alpha-2 agonists are associated with better tolerability.
In consideration of the heterogeneity of the Tourette syndrome phenotypes, pharmacotherapy should be tailored to the individual patient.
In addition to the efficacy-to-tolerability ratio, the choice of the pharmacological agent should take into account the presence of common co-morbidities (e.g. newer antidopaminergic agents can be useful as augmentation treatment for tic-related obsessive-compulsive behaviors, whereas alpha-2 agonists can be effective against co-morbid attention-deficit and hyperactivity disorder).
A broad-spectrum antiepileptic medication (Topiramate) was recently included in the evidence-based guidelines on the pharmacotherapy of tics, whereas a number of emerging agents are currently under investigation.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have been a consultant for Teva, Bracket and Nuvelution. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifthed. Washington (DC):American Psychiatric Publishing; 2022. Text Revision (DSM-5-TR).
- Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13(1):100–112.
- Cox JH, Seri S, Cavanna AE. Sensory aspects of Tourette syndrome. Neurosci Biobehav Rev. 2018;88:170–176.
- Knight T, Steeves T, Day L, et al. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47(2):77–90.
- Scharf JM, Miller LL, Gauvin CA, et al. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. 2015;30(2):221–228.
- Hartmann A, Szejko N, Mol Debes N, et al. Is Tourette syndrome a rare condition? F1000Res. 2021;10:434.
- Martino D, Madhusudan N, Zis P, et al. An introduction to the clinical phenomenology of Tourette syndrome. Int Rev Neurobiol. 2013;112:1–33.
- Hirschtritt M, Lee PC, Pauls DL, et al. Lifetime prevalence, age of risk, and etiology of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72(4):325–333.
- Cavanna AE. Gilles de la Tourette syndrome as a paradigmatic neuropsychiatric disorder. CNS Spectr. 2018;23(3):213–218.
- Worbe Y, Mallet L, Golmard JL, et al. Repetitive behaviours in patients with Gilles de la Tourette syndrome: tics, compulsions, or both? PLoS One. 2010;5(9):e12959.
- Neal M, Cavanna AE. Not just right experiences” in patients with Tourette syndrome: complex motor tics or compulsions? Psychiatry Res. 2013;210(2):559–563.
- Eddy CM, Cavanna AE. Tourette syndrome and obsessive compulsive disorder: compulsivity along the continuum. J Obsessive Compuls Relat Disord. 2014;3(4):363–371.
- Szejko N, Müller-Vahl KR. Challenges in the diagnosis and assessment in patients with Tourette syndrome and comorbid obsessive-compulsive disorder. Neuropsychiatr Dis Treat. 2021;17:1253–1266.
- Rothenberger A, Roessner V. Psychopharmacotherapy of obsessive-compulsive symptoms within the framework of Tourette syndrome. Curr Neuropharmacol. 2019;17(8):703–709.
- Ueda K, Black KJ. Recent progress on Tourette syndrome. Fac Rev. 2021;10:70.
- Maia TV, Conceição VA. Dopaminergic disturbances in Tourette syndrome: an integrative account. Biol Psychiatry. 2018;84(5):332–344.
- Morand-Beaulieu S, Grot S, Lavoie J, et al. The puzzling question of inhibitory control in Tourette syndrome: a meta-analysis. Neurosci Biobehav Rev. 2017;80:240–262.
- Yang C, Hao Z, Zhu C, et al. Interventions for tic disorders: an overview of systematic reviews and meta analyses. Neurosci Biobehav Rev. 2016;63:239–255.
- Essoe JK, Grados MA, Singer HS, et al. Evidence-based treatment of Tourette’s disorder and chronic tic disorders. Expert Rev Neurother. 2019;19(11):1103–1115.
- Billnitzer A, Jankovic J. Current management of tics and Tourette syndrome: behavioral, pharmacologic, and surgical treatments. Neurotherapeutics. 2020;17(4):1681–1693.
- Cavanna AE, Eddy CM, Mitchell R, et al. An approach to deep brain stimulation for severe treatment refractory Tourette syndrome: the UK perspective. Br J Neurosurg. 2011;25(1):38–44.
- Cavanna AE. Pharmacological treatment of tics. Cambridge: Cambridge University Press; 2020.
- Kumar A, Duda L, Mainali G, et al. A comprehensive review of Tourette syndrome and complementary alternative medicine. Curr Dev Disord Rep. 2018;5(2):95–100.
- Roessner V, Plessen KJ, Rothenberger A, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. 2011;20(4):173–196.
- Pringsheim T, Doja A, Gorman D, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry. 2012;57(3):133–143.
- Murphy TK, Lewin AB, Storch EA, et al. American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1341–1359.
- Hollis C, Pennant M, Cuenca J, et al. Clinical effectiveness and patient perspectives of different treatment strategies for tics in children and adolescents with Tourette syndrome: a systematic review and qualitative analysis. Health Technol Assess. 2016;20(4):1–450.
- Pringsheim T, Okun MS, Müller-Vahl K, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019;92(19):896–906.
- Roessner V, Eichele H, Stern JS, et al. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part III: pharmacological treatment. Eur Child Adolesc Psychiatry. 2022;31(3):425–441.
- Pringsheim T, Holler-Managan Y, Okun MS, et al. Comprehensive systematic review summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019;92(19):907–915.
- Cothros N, Medina A, Pringsheim T. Current pharmacotherapy for tic disorders. Expert Opin Pharmacother. 2020;21(5):567–580.
- Besag FM, Vasey MJ, Lao KS, et al. Pharmacological treatment for Tourette syndrome in children and adults: what is the quality of the evidence? A systematic review. J Psychopharmacol. 2021;35(9):1037–1061.
- Frey J, Malaty IA. Tourette syndrome treatment updates: a review and discussion of the current and upcoming literature. Curr Neurol Neurosci Rep. 2022;22(2):123–142.
- Buse J, Schoenefeld K, Münchau A, et al. Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci Biobehav Rev. 2013;37(6):1069–1084.
- Mogwitz S, Buse J, Wolff N, et al. Update on the pharmacological treatment of tics with dopamine-modulating agents. ACS Chem Neurosci. 2018;9(4):651–672.
- Pringsheim T, Pearce M. Complications of antipsychotic therapy in children with Tourette syndrome. Pediatr Neurol. 2010;43(1):17–20.
- Gulisano M, Calì PV, Cavanna AE, et al. Cardiovascular safety of aripiprazole and pimozide in young patients with Tourette syndrome. Neurol Sci. 2011;32(6):1213–1217.
- Rizzo R, Eddy CM, Cali P, et al. Metabolic effects of aripiprazole and pimozide in children with Tourette syndrome. Pediatr Neurol. 2012;47(6):419–422.
- Yang C, Hao Z, Zhang LL, et al. Comparative efficacy and safety of antipsychotic drugs for tic disorders: a systematic review and Bayesian network meta-analysis. Pharmacopsychiatry. 2019;52(1):7–15.
- Pringsheim T, Marras C. Pimozide for tics in Tourette’s syndrome. Cochrane Database Syst Rev. 2009;2009(2):CD006996.
- Pandey S, Dash D. Progress in pharmacological and surgical management of Tourette syndrome and other chronic tic disorders. Neurologist. 2019;24(3):93–108.
- Quezada J, Coffman KA. Current approaches and new developments in the pharmacological management of Tourette syndrome. CNS Drugs. 2018;32(1):33–45.
- Barton BB, Segger F, Fischer K, et al. Update on weight gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295–314.
- Seideman MF, Seideman TA. A review of the current treatment of Tourette syndrome. J Pediatr Pharmacol Ther. 2020;25(5):401–412.
- Budman CL. The role of atypical antipsychotics for treatment of Tourette’s syndrome: an overview. Drugs. 2014;74(11):1177–1193.
- Bloch MH, Landeros-Weisenberger A, Kelmendi B, et al. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11(7):622–632.
- Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and meta-analysis. BMC Psychiatry. 2014;14(1):317.
- Masi G, Pfanner C, Brovedani P. Antipsychotic augmentation of selective serotonin reuptake inhibitors in resistant tic-related obsessive-compulsive disorder in children and adolescents: a naturalistic comparative study. J Psychiatr Res. 2013;47(8):1007–1012.
- Gerasch S, Kanaan AS, Jakubovski E, et al. Aripiprazole improves associated comorbid conditions in addition to tics in adult patients with Gilles de la Tourette syndrome. Front Neurosci. 2016;10:416.
- Miguel EC, Shavitt RG, Ferrão YA, et al. How to treat OCD in patients with Tourette syndrome. J Psychosom Res. 2003;55(1):49–57.
- Neri V, Cardona F. Clinical pharmacology of comorbid obsessive-compulsive disorder in Tourette syndrome. Int Rev Neurobiol. 2013;112:391–414.
- Pringsheim T, Piacentini J. Tic-related obsessive–compulsive disorder. J Psychiatry Neurosci. 2018;43(6):431–432.
- Wenzel C, Kleimann A, Bokemeyer S, et al. Aripiprazole for the treatment of Tourette syndrome: a case series of 100 patients. J Clin Psychopharmacol. 2012;32(4):548–550.
- Cox JH, Seri S, Cavanna AE. Safety and efficacy of aripiprazole for the treatment of pediatric Tourette syndrome and other chronic tic disorders. Pediatric Health Med Ther. 2016;7:57–64.
- Cox JH, Cavanna AE. Aripiprazole for the treatment of Tourette syndrome. Expert Rev Neurother. 2021;21(4):381–391.
- Solmi M, Fornaro M, Ostinelli EG, et al. Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry. 2020;19(2):214–232.
- Iasevoli F, Barone A, Buonaguro EF, et al. Safety and tolerability of antipsychotic agents in neurodevelopmental disorders: a systematic review. Expert Opin Drug Saf. 2020;19(11):1419–1444.
- Yang C, Yi Q, Zhang L, et al. Safety of aripiprazole for tics in children and adolescents: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(22):e15816.
- Yang CS, Huang H, Zhang LL, et al. Aripiprazole for the treatment of tic disorders in children: a systematic review and meta-analysis. BMC Psychiatry. 2015;15(1):179.
- Nagai Y, Cavanna A, Critchley HD. Influence of sympathetic autonomic arousal on tics: implications for a therapeutic behavioral intervention for Tourette syndrome. J Psychosom Res. 2009;67(6):599–605.
- Hawksley J, Cavanna AE, Nagai Y. The role of the autonomic nervous system in Tourette syndrome. Front Neurosci. 2015;9:117.
- Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry. 2014;53(2):153–173.
- Weisman H, Qureshi IA, Leckman JF, et al. Systematic review: pharmacological treatment of tic disorders - efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev. 2013;37(6):1162–1171.
- Mula M, Cavanna AE, Monaco F. Psychopharmacology of topiramate: from epilepsy to bipolar disorder. Neuropsychiatr Dis Treat. 2006;2(4):475–488.
- Yu L, Yan J, Wen F, et al. Revisiting the efficacy and tolerability of topiramate for tic disorders: a meta-analysis. J Child Adolesc Psychopharmacol. 2020;30(5):316–325.
- Yang CS, Zhang LL, Zeng LN, et al. Topiramate for Tourette’s syndrome in children: a meta-analysis. Pediatr Neurol. 2013;49(5):344–350.
- Badenoch J, Cavanna AE. Pharmacotherapy for tics in adult patients with Tourette syndrome and other tic disorders. Neurol Sci. 2020;41(7):1923–1926.
- Jankovic J. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. 2016;17(18):2461–2470.
- Porta M, Sassi M, Cavallazzi M, et al. Tourette’s syndrome and role of tetrabenazine: review and personal experience. Clin Drug Investig. 2008;28(7):443–459.
- Colizzi M, Bortoletto R, Zoccante L. The effectiveness of lurasidone add-on for residual aggressive behavior and obsessive symptoms in antipsychotic-treated children and adolescents with Tourette syndrome: preliminary evidence from a case series. Children. 2021;8(2):121.
- Gilbert DL, Budman CL, Singer HS, et al. A D1 receptor antagonist, ecopipam, for treatment of tics in Tourette syndrome. Clin Neuropharmacol. 2014;37(1):26–30.
- Gilbert DL, Murphy TK, Jankovic J, et al. Ecopipam, a D1 receptor antagonist, for treatment of Tourette syndrome in children: a randomized, placebo-controlled crossover study. Mov Disord. 2018;33(8):1272–1280.
- Mahableshwarkar A, Dubow J, Cuniff T, et al. Ecopipam in children and adolescents with Tourette syndrome: results from a randomized, double-blind, placebo-controlled phase 2b study. [cited 2022 Jul 01]. Available from: https://aanfiles.blob.core.windows.net/aanfiles/0c2f847f-591e-4267-98f9-0ad57ca3f5fe/EMBARGOED%202022%20AAN%20AM%20Abstract%20%231066%20-%20Ecopipam%20in%20Children%20and%20Adolescents%20with%20Tourette%20Syndrome%20-%20Mahableshwarkar%20titled%20(1).pdf
- Jankovic J, Jimenez-Shahed J, Budman C, et al. Deutetrabenazine in tics associated with Tourette syndrome. Tremor Other Hyperkinet Mov. 2016;6:422.
- Farber RH, Angelov A, Kim K, et al. Clinical development of valbenazine for tics associated with Tourette syndrome. Expert Rev Neurother. 2021;21(4):393–404.
- Cavanna AE, Nani A. Antiepileptic drugs and Tourette syndrome. Int Rev Neurobiol. 2013;112:373–389.
- Cavanna AE. Behavioural neurology of antiepileptic drugs: a practical guide. Oxford: Oxford University Press; 2018.
- Martínez-Granero MA, García-Pérez A, Montañes F. Levetiracetam as an alternative therapy for Tourette syndrome. Neuropsychiatr Dis Treat. 2010;6:309–316.
- Awaad YMA, Minarik S. Long-term use of levetiracetam to treat tics in children and adolescents with Tourette syndrome. J Ped Neurol. 2007;5(3):209–214.
- Smith-Hicks CL, Bridges DD, Paynter NP, et al. A double blind randomized placebo control trial of levetiracetam in Tourette syndrome. Mov Disord. 2007;22(12):1764–1770.
- Hedderick EF, Morris CM, Singer HS. Double-blind, crossover study of clonidine and levetiracetam in Tourette syndrome. Pediatr Neurol. 2009;40(6):420–425.
- Tao D, Zhong T, Ma S, et al. Randomized controlled clinical trial comparing the efficacy and tolerability of aripiprazole and sodium valproate in the treatment of Tourette syndrome. Ann Gen Psychiatry. 2019;18(1):24.
- Singer HS, Wendlandt J, Krieger M, et al. Baclofen treatment in Tourette syndrome: a double-blind, placebo-controlled, crossover trial. Neurology. 2001;56(5):599–604.
- Curtis A, Clarke CE, Rickards HE. Cannabinoids for Tourette’s syndrome. Cochrane Database Syst Rev. 2009;4:CD006565.
- Artukoglu BB, Bloch MH. The potential of cannabinoid-based treatments in Tourette syndrome. CNS Drugs. 2019;33(5):417–430.
- Milosev LM, Psathakis N, Szejko N, et al. Treatment of Gilles de la Tourette syndrome with cannabis-based medicine: results from a retrospective analysis and online survey. Cannabis Cannabinoid Res. 2019;4(4):265–274.
- Jakubovski E, Pisarenko A, Fremer C, et al. The CANNA-TICS study protocol: a randomized multi-center double-blind placebo controlled trial to demonstrate the efficacy and safety of nabiximols in the treatment of adults with chronic tic disorders. Front Psychiatry. 2020;11:575826.
- Müller-Vahl K. ABX-1431, a first-in-class endocannabinoid modulator, improves tics in adult patients with Tourette syndrome. Neurology. 2018;90:e2182–2183.
- Müller-Vahl KR, Fremer C, Beals C, et al. Monoacylglycerol lipase inhibition in Tourette syndrome: a 12-week, randomized, controlled study. Mov Disord. 2021;36(10):2413–2418.
- Bloch MH, Landeros-Weisenberger A, Johnson JA, et al. A phase-2 pilot study of a therapeutic combination of Δ 9-Tetrahydracannabinol and palmitoylethanolamide for adults with Tourette’s syndrome. J Neuropsychiatry Clin Neurosci. 2021;33(4):328–336.
- Szejko N, Saramak K, Lombroso A, et al. Cannabis-based medicine in treatment of patients with Gilles de la Tourette syndrome. Neurol Neurochir Pol. 2022;56(1):28–38.
- Billnitzer A, Jankovic J. Pilot study to evaluate pimavanserin for the treatment of motor and behavioral symptoms of Tourette syndrome. Mov Disord Clin Pract. 2021;8(5):694–700.
- McGuire JF, Ginder N, Ramsey K, et al. Optimizing behavior therapy for youth with Tourette’s disorder. Neuropsychopharmacology. 2020;45(12):2114–2119.
- Li HH, Xu ZD, Wang B, et al. Clinical improvement following vitamin D3 supplementation in children with chronic tic disorders. Neuropsychiatr Dis Treat. 2019;15:2443–2450.
- Ding L, Yang Z, Liu G, et al. Safety and efficacy of taurine as an add-on treatment for tics in youngsters. Eur J Neurol. 2020;27(3):490–497.
- Qi H, Liu R, Zheng W, et al. Efficacy and safety of traditional Chinese medicine for Tourette’s syndrome: a meta-analysis of randomized controlled trials. Asian J Psychiatr. 2020;47:101853.
- Wang N, Qin DD, Xie YH, et al. Traditional Chinese medicine strategy for patients with Tourette syndrome based on clinical efficacy and safety: a meta-analysis of 47 randomized controlled trials. Biomed Res Int. 2021;2021:6630598.
- Ganos C, Sarva H, Kurvits L, et al. Clinical practice patterns in tic disorders among Movement Disorder Society members. Tremor Other Hyperkinet Mov. 2021;11(1):43.
- Bhikram T, Elmaghraby R, Abi-Jaoude E, et al. An international survey of health care services available to patients with Tourette syndrome. Front Psychiatry. 2021;12:621874.
- Cothros N, Martino D, McMorris C, et al. Prescriptions for alpha agonists and antipsychotics in children and youth with tic disorders: a pharmacoepidemiologic study. Tremor Other Hyperkinet Mov. 2019;9.
- Cavanna AE, Strigaro G, Monaco F. Brain mechanisms underlying the placebo effect in neurological disorders. Funct Neurol. 2007;22(2):89–94.
- Cubo E, González M, Singer H, et al. Impact of placebo assignment in clinical trials of tic disorders. Mov Disord. 2013;28(9):1288–1292.
- Ferreira JJ, Trenkwalder C, Mestre TA. Placebo and nocebo responses in other movement disorders besides Parkinson’s disease: how much do we know? Mov Disord. 2018;33(8):1228–1235.
- Accad M, Francis D. Does evidence based medicine adversely affect clinical judgment? Br Med J. 2018;362:2799.
