ABSTRACT
Background
Mirogabalin has been attracting attention for treating peripheral neuropathic pain. The package insert recommends that mirogabalin should be titrated depending on renal function. Here, we investigated the relationship between dose titration patterns and adherence, and persistence of mirogabalin treatment.
Research design and methods
Peripheral neuropathic pain patients who initiated mirogabalin between March 2020 and May 2021 were identified using an electronic medical record database. The dose titration pattern was described according to degrees of renal function. Regression analyses were performed to compare adherence and persistence between the patients with and without titration.
Results
Of the 4,138 identified patients, 1,696 (41.0%) titrated the dose within 45 days and were more adherent than those without titration (Adjusted odds ratio: 1.75, 95% CI 1.21, 2.54). Of the total 952 patients with renal function parameters, 229 (24.1%) titrated to the effective dose within 45 days and were less likely to discontinue than those without titration (Adjusted hazard ratio: 0.57, 95% CI 0.40, 0.81).
Conclusion
Mirogabalin dose titration was associated with better adherence and persistence. It is important for mirogabalin treatment to determine the initial prescription dose based on renal function and subsequent dose titration according to the package insert.
Trial registration
UMIN000047313
1. Introduction
Neuropathic pain is defined as ‘pain caused by a lesion or disease of the somatosensory nervous system’ [Citation1]. Neuropathic pain tends to result in a longer duration of illness and more persistent pain, causing distress to the patient [Citation2]. Initial treatment for neuropathic pain includes drug therapy. However, despite effective drug therapy being available, many patients may receive poor treatment and have a poor quality of life [Citation3–5]. Therefore, it is necessary to use existing drugs at appropriate doses or new drugs that can be easily titrated to appropriate doses.
Voltage-gated Ca2+ channel α2δ ligands, serotonin-noradrenaline reuptake inhibitors, and tricyclic antidepressants are used as first-line drugs for neuropathic pain [Citation6,Citation7]. Mirogabalin, a new voltage-gated Ca2+ channel α2δ ligand, was developed by Daiichi Sankyo Co., Ltd. (Tokyo, Japan) in 2019 and was approved for use in the treatment of neuropathic pain such as diabetic neuropathic pain and postherpetic neuralgia. Recent clinical trials showed that mirogabalin was effective in relieving pain and the side effects were minimal in Asian patients with diabetic neuropathic pain and postherpetic neuralgia [Citation8–11]. Mirogabalin’s side effects, such as weight increase, sleepiness, peripheral edema, or dizziness, can be suppressed by designing a regimen that increases the dose gradually from a low initial dose [Citation12,Citation13]. Mirogabalin is also used in patients with renal impairment. A recent clinical trial demonstrated that mirogabalin was tolerated and effective in patients with peripheral neuropathic pain and renal impairment when used at a fixed dose of 7.5 mg /day (severe impairment) or 15 mg /day (moderate impairment) [Citation14]. Therefore, in patients with reduced renal function, it is recommended to adjust the mirogabalin dose with reference to creatinine clearance (CrCL) values [Citation12].
Some studies have evaluated dose titration patterns and adherence using databases that reveal the clinical practice in patients with neuropathic pain [Citation15–21]. Previous studies have shown that the correct dose titration from the initial dose of pregabalin was associated with improved treatment adherence and persistence among patients [Citation21]. A recent study using prescription databases reported that most patients were prescribed mirogabalin at approximately 10 mg/day and only 30.9% of those were titrated to ≥ 20 mg/day 90 days after the first prescription [Citation22]. Although the dose titration pattern in clinical practice has already been reported, no study has examined the prescription pattern and its relationship with adherence to mirogabalin according to the degree of renal function.
Thus, this study aimed to investigate the relationship between mirogabalin dose titration patterns and adherence in patients with peripheral neuropathic pain using electronic medical records and a claims database.
2. Patients and methods
2.1. Data source and setting
We used the RWD database (RWD-DB), which is maintained by the Health, Clinic, and Education Information Evaluation Institute (HCEI, Kyoto, Japan) with support from Real World Data Co., Ltd (Kyoto, Japan). It includes a record about 20 million patients from more than 200 medical institutions across Japan as of 2021. Medical institutions include a wide range of hospital sizes. Large-scale institutions with over 500 beds were included mainly, but small institutions with less than 20 beds were also covered. The medical information stored in the database contains demographics, diagnoses, prescriptions, treatment procedures, and laboratory test results from both outpatient and inpatient services. The data were automatically extracted from electronic medical records at each medical institution. Patient records were maintained by allocating unique identifiers for each individual, valid within the same institution.
The protocol of this study was approved by the Research Institute of Healthcare Data Science ethics committee (approval number: RI2021025). As this retrospective study was based on an electric medical records database and only anonymous data were processed in this study, it was unnecessary to obtain consent from each participant. This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (clinical trial registration number: UMIN000047313) and performed according to the guidelines of the Declaration of Helsinki.
2.2. Study design
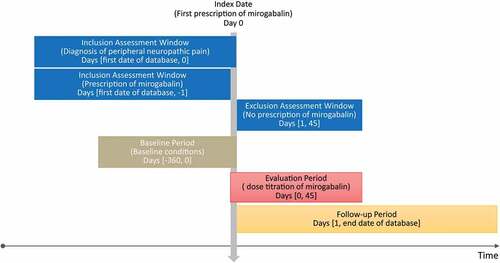
We conducted the retrospective cohort study in patients with peripheral neuropathic pain using the RWD-DB. shows the design diagram of this study. The inclusion criteria were as follows: (a) patients with at least one prescription date for mirogabalin between 1 March 2020, and 31 May 2021. The date of the first prescription during this period was defined as the index date (Day 0); (b) patients with a diagnosis of peripheral neuropathic pain on or before Day 0; and (c) patients who had at least one mirogabalin prescription between Day 1 and Day 45. The exclusion criteria were patients with at least one prescription for mirogabalin before Day 0. The International Classification of Diseases 10th Revision (ICD-10) code was used to identify peripheral neuropathic pain. The mirogabalin prescription was identified using the Anatomical Therapeutic Chemical Classification (ATC) code (N02BG11). The study period was defined as follows: the baseline period was from Day −360 to Day 0; the evaluation period for mirogabalin dose titration was from Day 0 to Day 45; the follow-up period was from Day 1 to the end date of the database. As for the sensitivity analysis, evaluation was performed at the different follow-up periods from Day 46 to the end date of the database.
2.3. Measurements
Baseline patient characteristics, including age, sex, number of hospital beds, height, weight, body mass index (BMI), drug name, number of prescribed days, prescribed doses, prior medications, and 24-h CrCL value, estimated glomerular filtration rate (eGFR) value and serum creatinine value were extracted from the database. The CrCL value was extracted from both the 24-h CrCL value and the estimated CrCL value calculated from the serum creatinine using the Cockcroft-Gault formula. If there were multiple laboratory test values, the value closest to Day 0 was used as the value at the baseline period. Comorbidities at baseline were defined based on the ICD-10 codes, and other pain medication drugs than mirogabalin were defined using the ATC codes.
2.4. Exposure
In the package insert, different initial and effective doses have been recommended for each patient having a CrCL value ≥60 mL/min, 30–<60 mL/min, <30 mL/min or under dialysis. The recommended initial doses are 10–30 mg, 5–15 mg, and 2.5–7.5 mg per day, and the recommended effective doses are 30 mg, 15 mg, and 7.5 mg per day in patients with each CrCL group, respectively. First, we examined whether the subsequent doses were titrated from the initial dose or not. In this case, patients were classified into the titrated group and the non-titrated group. Second, we examined whether the initial dose and the titrated dose followed the recommended regimen for each CrCL group. In this case, patients were classified into the titrated group, the non-titrated group, and the undefined group. The undefined group was defined as the patients whose initial dose had already reached the effective dose even if there was dose titration in them. Third, the initial dose pattern was classified as high group, low group, and regular group: regular group if the prescribed initial dose was within the recommended range; high group if it was higher; and low group if it was lower. We also evaluated the period pattern of the dose titration at various intervals: Day 1–45, Day 1–15, Day 16–30, Day 31–45, and on or after Day 46.
2.5. Outcomes
The primary outcome was the medication possession ratio (MPR) for evaluating adherence to mirogabalin treatment. The MPR was defined as the total number of prescription days divided by the number of days from Day 1 to the end of the prescription period. The secondary outcomes were the proportion of days covered (PDC), the persistence of mirogabalin prescription, and switching to other pain medications. The PDC was defined as the total number of days with a covered prescription divided by the number of prescription days between Day 1 and the end date of the prescription period. The MPR would include the number of days in the overlapping period if the patient received the prescription early, whereas the PDC does not include the number of days in the overlapping period. Patients with MPR or PDC ≥80% were considered to have high adherence to mirogabalin treatment. The persistence of mirogabalin was defined as the number of days from Day 1 to the first date of discontinuation or censoring date. The censored date was defined as the last visit date on the database. Discontinuation was defined as a lapse of ≥30 days between the last date of the previous mirogabalin prescription and the first date of the prescription of other drugs for treating peripheral neuropathic pain. Switching was evaluated by calculating the days from Day 1 to discontinuation or censoring date, defined as a lapse of <30 days between the last day of the previous mirogabalin prescription and the first day of the new prescription of other neuropathic pain drugs.
2.6. Statistical analysis
In this study, patients were classified into the following groups according to the renal function depending on CrCL values or the presence of dialysis: CrCL ≥60 mL/min; 30–<60 mL/min; <30 mL/min or with dialysis; and missing CrCL.
We described the summary statistics of the baseline and clinical characteristics, including the dose of mirogabalin prescription, age, sex, height, weight, BMI, disease duration, serum creatinine levels, 24-h CrCL, eGFR, comorbidities, and concomitant medications. Categorical variables were described using the number and percentage of patients. Continuous variables were summarized as the mean, standard deviation (SD), median and interquartile range. Univariate and multivariable logistic regression were performed to explore the association between factors and adherence. The univariate logistic regression was performed with the proportion of patients with MPR or PDC ≥80% as the response variable and the mirogabalin dose titration pattern and pre-defined other factors as explanatory variables. The multivariable logistic regression used a stepwise method with all pre-defined factors input and only the mirogabalin dose titration pattern coercively left. Kaplan-Meier method, univariate and multivariable Cox regression were conducted to evaluate the associated factors with persistence and switching. Cox regression was performed in the same as the logistic regression manner. SAS version 9.4 (SAS Institute Inc.) was used for all statistical analyses.
3. Results
3.1. Study patients and characteristics
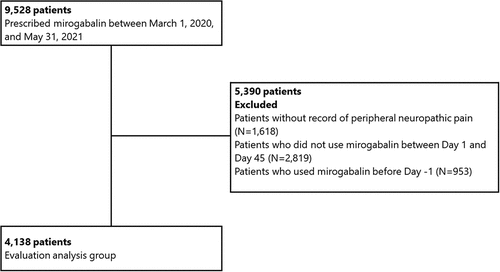
shows the composition of the study patients. A total of 4,138 patients met the criteria for the study cohort. shows the baseline and clinical characteristics. Of the 4138 patients initially prescribed mirogabalin, 952 (23.0%) patients had CrCL values or records of dialysis. The number of patients with CrCL value ≥60 mL/min, 30–<60 mL/min, <30 mL/min or dialysis, and missing CrCL were 543 (13.1%), 326 (7.9%), 83 (2.0%), and 3186 (77.0%), respectively. The mean (SD) of age was 67.1 (14.9) years, 49.1% were men, and patients with eGFR values were 2,512 (60.7%). Disease names extracted as peripheral neuropathic pain were peripheral neuropathy pain (94.3%), postherpetic neuralgia (8.4%), tumor-related (5.5%), entrapment neuropathy(5.3%), and diabetic neuropathy (3.8%). All pain disease names used in this study were included in peripheral neuropathic pain. We calculated the presence or absence of records of each pain disease while allowing duplicates. Common comorbidities were diabetes mellitus (33.2%) and malignancy (29.7%). As the degree of renal dysfunction decreased, the proportion of patients with diabetes, cerebral and cardiac disease increased. Regarding the size of the medical institution, most patients were treated in institutions with over 100 beds, and 2.6% and 1.4% of all patients were treated in the institution with 20–<100 beds and less than 20 beds, respectively. In the clinical department, the proportion of patients in orthopedics was 52.5%. During the baseline period, pregabalin was prescribed to 23.0% of patients, and some patients without reaching the effective dose indicated by the package insert were observed. Regarding the concomitant drugs prescribed on the index date, neurotropin (10.0%), tramadol (8.6%) and duloxetine (4.8%) were co-prescribed, and the co-prescription of pregabalin was only 0.7%.
Table 1. Baseline and clinical characteristics.
3.2. Dose titration patterns
describes the distribution of dose titration patterns. Of the 4,138 patients, 1,696 patients (41.0%) were titrated within 45 days, and 2,000 patients (48.3%) were titrated over the entire period. Most patients with dose titration had titrated within 45 days. Of the 952 patients with renal function severity based on CrCL values or a history of dialysis, 392 patients (41.2%) were titrated within 45 days, and 470 patients (49.4%) were titrated over the entire period. Considering the recommended initial and effective dose according to the package insert for 952 patients, 229 patients (24.1%) were titrated to the effective dose within 45 days, and 292 patients (30.7%) were titrated to the effective dose over the entire period. The proportion of patients whose initial dose was compliant (regular group) was 67.2%, 32.2%, and 32.5% for patients with the CrCL value ≥60 mL/min, 30–<60 mL/min, and <30 mL/min or dialysis, respectively. While those with lower was 23.8%, 5.5%, and 0%, and those with higher was 9.0%, 62.3% and 67.5%, respectively. The patients with CrCL values <60 mL/min tended to be prescribed a higher effective dose than recommended in the package insert in Japan.
Table 2. Distribution of dose titration pattern of mirogabalin.
3.3. The association between dose titration and adherence
describes their univariate logistic regression evaluating the association between dose titration and baseline factors, and adherence (MPR and PDC) to mirogabalin, where mirogabalin dose titration pattern and baseline characteristics were determined in detail. In this regression analysis, the non-titrated group was set as the reference. The odds ratio (OR) ≥1 indicated that the exposed group had better adherence with a higher proportion of MPR or PDC ≥80% compared to the non-titrated group. As a result of univariate logistic regression among 4,138 overall patients, the group with dose titration within 45 days was more likely to be adherent with an OR of 1.75 (95% CI 1.21, 2.54). The proportions of adherent patients were 97.6% and 95.9% in patients with the titrated and non-titrated groups, respectively.
Table 3. Univariate logistic regression for evaluating dose titration pattern and adherence of mirogabalin.
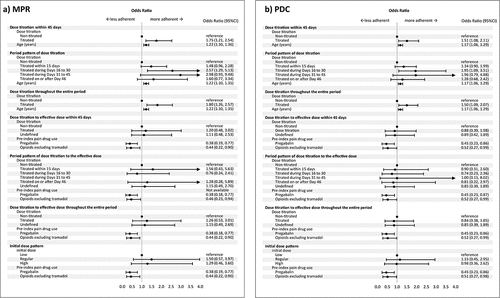
shows the results of the multivariable logistic regression for MPR and PDC. It used a stepwise selection method, including all the factors from . As a result of multivariable regression in 4,138 overall patients, the group with dose titration within 45 days had significantly improved adherence (adjusted OR: 1.75, 95% CI 1.21, 2.54). The univariate and multivariable logistic regression for PDC showed a similar tendency as in MPR. The sensitivity analysis investigated the relationship between dose titration and the MPR in which the start date of the follow-up period was changed from Day 1 to Day 46. Although there was no significant difference between patients with and without dose titration (Adjusted odds ratio: 1.14, 95% CI 0.78, 1.6), the tendency of point estimates was similar to the primary analysis (data were not shown).
Figure 3. Multivariable logistic regression for factors associated with mirogabalin adherence. Dose titration was evaluated as titrated and non-titrated using the prescribed initial and subsequent doses. Dose titration to effective dose was evaluated as titrated, non-titrated, and undefined: titrated if the dose titration follows recommended regimen; undefined if the prescribed initial dose were higher than the recommended initial dose; non-titrated otherwise. The initial dose pattern was classified as high, low and regular: regular if the prescribed initial dose was within the recommended range; high if it was higher; and low if it was lower. Arrow was used when values are outside the axis range.
3.4. The association between dose titration and persistence
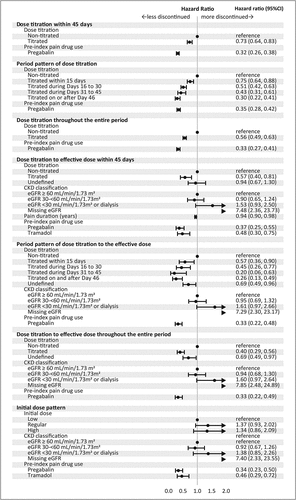
shows the Kaplan–Meier plot of the persistence of mirogabalin in overall patients and patients with renal function severity based on CrCL. The time to discontinuation was suggested to be longer in patients with dose titration than in those without dose titration. shows the results of the univariate Cox regression analysis for evaluating the relationship between dose titration and persistence and switching of mirogabalin. Hazard ratio (HR) <1 indicates discontinuation proportion of the exposed group was less than the non-titrated group. Among overall patients, the titrated group was significantly less likely to discontinue mirogabalin than the non-titrated group (HR: 0.71, 95% CI 0.62, 0.81), and HR decreased in the order of dose titration on or after Day 46, Day 31–45, Day 16–30, and Day 1–15. In patients with renal function, the titrated group to the effective dose had less likely to discontinue mirogabalin compared with the non-titrated group (HR: 0.55, 95% CI 0.39, 0.78), and no significant difference was observed between the undefined group and non-titrated group (HR: 0.77, 95% CI 0.57, 1.04). The period pattern of dose titration to effective dose was similar as shown in dose titration (HR for Day 46, Day 31–45, Day 16–30, Day 15: 0.25, 0.20, 0.44, 0.52, respectively). There was no association with the initial dose pattern.
Figure 4. Kaplan–Meier plot for non-persistence of mirogabalin. a) Dose titration within 45 days with overall patients, b) Period pattern of dose titration with overall patients, c) Dose titration throughout the entire period with overall patients, d) Dose titration to the effective dose within 45 days in patients with renal function, e) Period pattern of dose titration to the effective dose in patients with renal function, f) Dose titration to the effective dose throughout the entire period in patients with renal function.
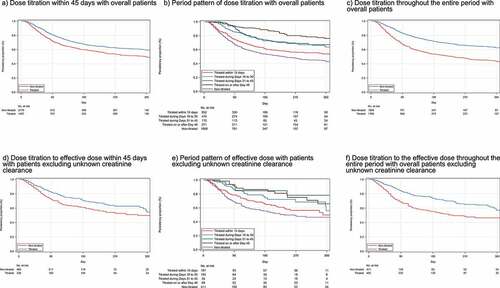
Table 4. Univariate COX regression for the association between dose titration and persistency and switching of mirogabalin.
As a result of multivariable Cox regression using the stepwise method (), the adjusted HR for the titrated group within 45 days was 0.73 (95% CI 0.64, 0.83) fold higher compared with the non-titrated group. The adjusted HRs for the titrated group to the effective dose within 45 days and undefined groups were 0.57 (95% CI 0.40, 0.81) and 0.94 (95% CI 0.67, 1.30) compared with the non-titrated group.
Figure 5. Multivariable COX regression for factors associated with mirogabalin persistence. Dose titration was evaluated as titrated and non-titrated using the prescribed initial and subsequent doses. Dose titration to effective dose was evaluated as titrated, non-titrated, and undefined: titrated the dose titration follows recommended regimen; undefined if the prescribed initial dose were higher than the recommended initial dose; non-titrated otherwise. The initial dose pattern was classified as high, low and regular: regular if the prescribed initial dose was within the recommended range; high if it was higher; and low if it was lower. Arrow was used when values are outside the axis range.
3.5. The association between dose titration and switching
For drug switching, the Kaplan–Meier estimate for the rate of not switching to other drugs was 95.8% (95% CI 93.9, 97.1%) in the titrated group and 96.1% (95% CI 94.6, 97.2%) in the non-titrated group. From the results of Cox regression for switching, there was no difference in switching proportion among dose titration patterns of mirogabalin ().
4. Discussion
This database study was designed to determine the association between dose titration patterns and adherence and persistence of mirogabalin in patients with peripheral neuropathic pain. Among the study cohort of 4,138 patients, 1,696 (1,696/4,138 = 41.0%) had titrated the dose within 45 days. Considering the degree of renal function of patients, in 952 patients with CrCL values, 229 (229/952 = 24.1%) were titrated to the effective dose within 45 days as recommended in the package insert. Regarding adherence, the proportion of adherent patients in the titrated group within 45 days was significantly higher than that in the non-titrated group (adjusted OR: 1.75, 95% CI 1.21, 2.54). Regarding persistence, patients in the titrated group to the effective dose within 45 days were less likely to discontinue than those in the non-titrated group (adjusted HR: 0.57, 95% CI 0.40, 0.81). This result suggests that prescribing the appropriate initial dose of mirogabalin based on renal function and the subsequent dose titration is important for improving adherence and persistence.
Among 4,138 overall patients, 41.0% were titrated within 45 days, 48.3% were titrated during the entire period, and most patients were titrated within 45 days. These results indicate that nearly 50% of all patients who initiated mirogabalin had at least one dose titration from the initial dose. One Japanese study showed a lower proportion of results than ours, with 30% of patients titrating the mirogabalin dose after initiation [Citation22]. It may be due to the additional inclusion criteria of patients prescribed one or more times after the index date were used in our study. In 952 patients with renal function parameters based on CrCL values, 24.1% were titrated to the effective dose within 45 days as recommended, 45.1% were not titrated, and 30.9% were undefined since they initiated from a higher than the recommended initial dose. The breakdown by renal function severity of the proportion of the dose titration to the effective dose was 14.5%, 14.1% and 31.5% in patients with CrCL value <30 mL/min or dialysis, 30–<60 mL/min, >60 mL/min, respectively. The recommended initial dose for patients with a CrCL value of <30 mL/min and 30–<60 mL/min were 2.5 mg and 5 mg; however, the median (IQR) initial doses in were 5 mg (2.5–10 mg) and 10 mg (5–10 mg), respectively. These results also indicate that nearly 25% of patients had titrated according to the recommended dose regimen for each renal function, and the appropriate dose titration was not achieved in other patients, especially in patients with impaired renal function. We must draw attention to the low compliance to the initial dose in patients with impaired renal function. One previous study reported the efficacy and safety of mirogabalin in patients with impaired renal function [Citation14]. In addition, other studies showed that dose titration according to the degree of renal function may relate to the improvement of pain score [Citation23,Citation24]. These studies suggested that the low compliance to package insert’s recommendation may lead to adverse reactions or insufficient efficacy. Therefore, more attention should be paid to the management of mirogabalin prescription in patients with reduced renal function although the safety of the prescription pattern of mirogabalin cannot be investigated in this study. Equally important is the result that a large number of patients (77.0%) did not have their baseline CrCL values (). It is possible that a large proportion of patients initiated mirogabalin without measuring the patient’s renal function, although further research would be necessary to support this interpretation since the reason for missing CrCL cannot be researched in this study. Therefore, it is desirable to measure the CrCL value at mirogabalin initiation and manage the dose titration according to the degree of renal function.
Study results showed that adherence to mirogabalin was associated with dose titration after initiation. In addition, mirogabalin treatment’s persistence was also associated with dose titration, titration to the effective dose, and dose titration patterns by period. The most likely explanation for this result is that patients with the dose titration may lead the optimal therapeutic effect in terms of efficacy and safety. Two previous studies found that patients who took mirogabalin from an initial dose and titrated gradually based on their renal function were more likely to continue the treatment and improve their pain scores [Citation23,Citation24]. Another database research on the dose titration of pregabalin in patients with neuropathic pain argued that the therapeutic optimal effect may affect the adherence and persistence of pain treatment; however, they only provide information on pregabalin treatment [Citation21]. Our findings also suggest that further persistence can be achieved if the physician adjusts the duration of dose titration even after adjusting the patient’s background factors related to the efficacy and safety of the drug.
The results of this study showed that the proportion of adherent patients with MPR ≥80% in the titrated and non-titrated groups was 97.6% and 95.9%, respectively. These results were higher than previously reported results: 86.2% for mirogabalin in one study [Citation22]; 82.3% and 90.0% for pregabalin and duloxetine [Citation4]. One possible explanation for the high adherence proportion is that the inclusion criteria are defined as patients with an additional prescription within 45 days from the initial prescription in this study. This means that patients who discontinued treatment immediately after the initial prescription would not be included in the study cohort. Thus, patients with high adherence might be estimated to be higher than in other studies. The results of multivariable logistic regression showed that age, history of prescriptions for opioids and pregabalin were selected as factors influencing poor adherence. One interpretation would be that the reason for switching from pregabalin may be due to a lack of efficiency or adverse effects of pregabalin treatment. The previous condition may affect the treatment even after initiation of mirogabalin treatment. It would be difficult to discuss it more due to the lack of information on the severity of pain and adverse events.
To our knowledge, this is the first study to evaluate the association between prescribing patterns, adherence and persistence to mirogabalin in patients with peripheral neuropathic pain, considering the renal function and the recommended dosage based on it. We firstly found that dose titration within 45 days and for the entire duration of the study after the initial prescription of mirogabalin is associated with both adherence and persistence. Therefore, it is worth noting that it is important to have a treatment strategy that considers renal function and includes plans for the initial dose and the dose titration to the effective dose at the initiation of mirogabalin treatment. We also found that 24% of patients had a dose titration according to the recommended regimen and only 14% of patients followed the recommended titration in patients with CrCL values <60 mL/min. Hence, more attention to side effects should be informed, and treatment should be carefully performed based on renal function.
This study has several limitations. First, some factors that may influence adherence that cannot be obtained from the database were not investigated. Adherence was influenced by various factors, such as trust between the prescribing physician and patient, perceived side effects, expected dependence, and patient knowledge about the efficacy and tolerability of mirogabalin. It was difficult to obtain these factors from medical information database studies. Second, patients treated in medical institutions (more than 100 beds) accounted for most patients. Therefore, the results of this study may not apply to patients treated in smaller clinics. Third, this study investigated peripheral neuropathic pain and did not include patients with central neuropathic pain. Therefore, caution should be exercised in applying the data from this study to patients with potentially central neuropathic pain. Additionally, patients enrolled in the medical information database included various types of neuropathic pain, not just the condition commonly referred to as peripheral neuropathic pain. Therefore, other neuropathic pain (e.g. diabetic neuropathic pain or cisplatin-induced neuropathic pain) may also be included in peripheral neuropathic pain, and these data should be interpreted with caution. Fourth, it was difficult to accurately determine from medical information according to prescription. In the case of drugs titrated up to the effective dose, such as mirogabalin, if patients feel their symptoms improve even at low doses, they may take lower doses. This study cannot include such patient-centered measures related to medication treatment. In addition, the RWD-DB did not contain the measurements of the drug efficacy (e.g. analgesia or neuroprotection effect). Future studies on mirogabalin should focus on examining the extent of pain improvement and combinations with other concomitant drugs.
5. Conclusion
This study investigated the pattern of dose titration after the initial prescription of mirogabalin in patients with peripheral neuropathic pain and evaluated the association between adherence and persistence. Considering renal function, 229 patients (229/952 = 24.1%) were prescribed the effective dose as recommended in the package insert for their level of renal function. Our findings provide evidence that the dose titration after the initial prescription of mirogabalin was associated with adherence and persistence. Prescribing the appropriate initial dose of mirogabalin based on renal function and the subsequent dose titration is important for the improvement of adherence and persistence.
Author contributions
K Kato contributed to the study design and writing of the manuscript. K Shiosakai, S Kodama contributed to the study design, conducted and performed the data collection and writing of the manuscript. T Kimura was involved in data analysis, contributed to the study design, conducted and performed the data collection and writing of the manuscript. All authors contributed to interpretation of data and reviewing the manuscript, and approved this manuscript for submission.
Declaration of interest
K Kato has received consulting fees for the performance of the study from Daiichi Sankyo Co., Ltd. S Kodama and K Shiosakai are full-time employees of Daiichi Sankyo Co., Ltd. T Kimura is a full-time employee of Real World Data, Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer dislcosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Pharmacotherapy for their review work, but have no other relevant financial relationships to disclose.
Ethics
The protocol of this study was approved by the Research Institute of Healthcare Data Science ethics committee (approval number: RI2021025). As this retrospective study was based on an electric medical records database and only anonymous data were processed in this study, it was unnecessary to obtain consent from each participant. This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (clinical trial registration number: UMIN000047313) and performed according to the guidelines of the Declaration of Helsinki.
Acknowledgments
The authors thank the Health, Clinic, and Education Information Evaluation Institute for developing the database for the study. Medical writing support was provided by Gentaro Mori and Sachiko Tanaka of Real World Data Co., Ltd.
Data availability statement
Data is not available due to ethical restrictions.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Jensen TS, Baron R, Haanpää M, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205.
- Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101:259–301.
- Sommer C, Cruccu G. Topical treatment of peripheral neuropathic pain: applying the evidence. J Pain Symptom Manage. 2017;53:614–629.
- Ushida T, Matsui D, Inoue T, et al. Recent prescription status of oral analgesics in Japan in real-world clinical settings: retrospective study using a large-scale prescription database. Expert Opin Pharmacother. 2019;20:2041–2052.
- Taylor RS. Epidemiology of refractory neuropathic pain. Pain Pract. 2006;6:22–26.
- Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17:1010–1018.
- The committee for the guidelines for the pharmacologic management of neuropathic pain of JSPC. Guidelines for the pharmacologic management of neuropathic pain second edition. cited 2022 Sep 22]. Available from 2022 Sep 22: https://minds.jcqhc.or.jp/docs/minds/Pharmacologic-management-of-neuropathic-pain/Pharmacologic-management-of-neuropathic-pain.pdf#view=FitV.
- Kato J, Matsui N, Kakehi Y, et al. Mirogabalin for the management of postherpetic neuralgia: a randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain. 2019;160:1175–1185.
- Baba M, Matsui N, Kuroha M, et al. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig. 2019;10:1299–1306.
- Kato J, Matsui N, Kakehi Y, et al. Long-term safety and efficacy of mirogabalin in Asian patients with postherpetic neuralgia. Medicine (Baltimore). 2020;99:e21976.
- Baba M, Matsui N, Kuroha M, et al. Long-term safety and efficacy of mirogabalin in Asian patients with diabetic peripheral neuropathic pain. J Diabetes Investig. 2020;11:693–698.
- Kato J, Inoue T, Yokoyama M, et al. A review of a new voltage-gated Ca2+ channel α2δ ligand, mirogabalin, for the treatment of peripheral neuropathic pain. Expert Opin Pharmacother. 2021;22:2311–2322.
- Daiichi Sankyo Co., Ltd. Tarlige® (mirogabalin) Tablets, 2.5 mg, 5 mg, 10 mg, 15 mg, package insert. Japanese. [cited 2022 Sep 22]. Available from 2022 Sep 22: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/430574_1190026F1028_1_07
- Baba M, Takatsuna H, Matsui N, et al. Mirogabalin in Japanese patients with renal impairment and pain associated with diabetic peripheral neuropathy or post-herpetic neuralgia: a phase III, open-label, 14-week study. J Pain Res. 2020;13:1811–1821.
- Johnson P, Becker L, Halpern R, et al. Real-world treatment of post-herpetic neuralgia with gabapentin or pregabalin. Clin Drug Investig. 2013;33:35–44.
- Hoffman EM, Staff NP, Robb JM, et al. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology. 2015;84:1644–1651.
- Callaghan BC, Reynolds E, Banerjee M, et al. Longitudinal pattern of pain medication utilization in peripheral neuropathy patients. Pain. 2019;160:592–599.
- Christensen DH, Knudsen ST, Nicolaisen SK, et al. Can diabetic polyneuropathy and foot ulcers in patients with type 2 diabetes be accurately identified based on ICD-10 hospital diagnoses and drug prescriptions? Clin Epidemiol. 2019;11:311–321.
- Gudin J, Fudin J, Wang E, et al. Treatment patterns and medication use in patients with postherpetic neuralgia. J Manag Care Spec Pharm. 2019;25:1387–1396.
- Udall M, Harnett J, Mardekian J. Costs of pregabalin or gabapentin for painful diabetic peripheral neuropathy. J Med Econ. 2012;15:361–370.
- Yeh YC, Cappelleri JC, Marston XL, et al. Effects of dose titration on adherence and treatment duration of pregabalin among patients with neuropathic pain: a MarketScan database study. PLoS One. 2021;16:e0242467.
- Ushida T, Yokoyama M, Shiosakai K, et al. A large-scale database study for the prescription status of a new voltage-gated Ca 2+ channel α 2 δ ligand, mirogabalin, in Japan. Expert Opin Pharmacother. 2022;23:273–283.
- Kimura Y, Yamaguchi S, Suzuki T, et al. Switching from pregabalin to mirogabalin in patients with peripheral neuropathic pain: a Multi-Center, Prospective, Single-Arm, Open-Label Study (MIROP Study). Pain Ther. 2021;10:711–727.
- Nikaido T, Takatsuna H, Tabata S, et al. Efficacy and safety of add-on mirogabalin to NSAIDs in lumbar spinal stenosis with peripheral neuropathic pain: a randomized, open-label study. Pain Ther. 2022;20:1–20.