ABSTRACT
Introduction
In this paper, we systematically review the efficacy and safety of thrombopoietin receptor agonists (TPORAs) for treatment of persistent and chronic immune thrombocytopenia (ITP) in children and adults.
Methods
We searched PubMed, MEDLINE, ScienceDirect, Scopus, EMbase and the Cochrane Library to collect randomized controlled trials (RCTs) of TPO-RAs which including avatrombopag hetrombopag eltrombopag and romiplostim treated persistent and chronic ITP from their earliest records to February 2022.
Results
We included 15 RCTs with a total of 1563 patients. There were ten trials of adults and five trials of children. The results of meta-analysis showed that in adult patients, patients treated with TPO-RAs had longer duration of platelet response, higher platelet response rate, lower use of rescue therapy, and lower incidence of bleeding events, and similar incidence of adverse events compared with placebo. Except for the incidence of any bleeding, the results in children were consistent with those in adults. The network meta-analysis of data on overall platelet response rates in adults showed that avatrombopag was more effective than eltrombopag and hetrombopag.
Conclusions
TPO-RAs has better efficacy and higher safety in the treatment of ITP. And the overall response rate of avatrombopag in adults was higher than that in eltrombopag and hetrombopag.
1. Introduction
Immune Thrombocytopenia (ITP) is an immune-mediated acquired disease in adults and children characterized by transient or persistent decrease of the platelet count (<100 × 109/L) and an increased risk of bleeding [Citation1]. Immune thrombocytopenia is a diagnosis of exclusion: patients who develop thrombocytopenia with no clear underlying cause are currently diagnosed with (isolated) primary ITP [Citation1,Citation2]. Depending on duration, ITP can be classified into newly diagnosed (≤3 months’ duration), persistent (3–12 months’ duration) and chronic (≥12 months’ duration) [Citation1]. Traditionally, immune thrombocytopenia (ITP) has been considered to occur in two distinct forms: childhood ITP and adult ITP. Adult ITP is usually insidious and follows a chronic course [Citation3], whereas pediatric ITP is usually transient and recovers spontaneously in at least two-thirds of cases within 6 months [Citation4–6]. ITP treatment guidelines [Citation7,Citation8] recommend intravenous immunoglobulin, prednisolone, or anti-D immunoglobulin as first-line treatment. These agents have a rapid onset of action but do not result in durable remissions in most patients. Rituximab, dexamethasone, TPO-RAs or splenectomy are second-line treatment options for persistent or chronic ITP. Both splenectomy and rituximab can potentially cure ITP, but the long-term remission rates are not entirely satisfactory (60% after splenectomy and only 20% at 2–5 years after rituximab) [Citation9,Citation10]. TPO-RAs are new agents that have good curative effect but long-term treatment may be required [Citation11]. The choice of subsequent therapy requires consideration of operative risk, risk of asplenia, drug side-effects, quality-of-life issues, and economic costs. Since TPO-RAs were licensed in the United States in 2008 for the treatment of ITP, their use has been increasing around the world [Citation12].
TPO-RAs, including eltrombopag, romiplostim, and recently developed avatrombopag and hetrombopag, bind to TPO receptors, cause conformational changes of TPO receptors, and stimulate the proliferation of megakaryocytic progenitors and platelets [Citation13–17]. Avatrombopag is a second-generation TPO-RAs approved in the U.S.A and the EU for the treatment of primary chronic ITP in adults who have not responded adequately to other treatments [Citation18]. Hetrombopag is an oral non-peptide TPO-RAs approved in China on 16 June 2021 for the second-line treatment of adult primary immune thrombocytopenia (ITP) [Citation19]. Some studies have shown that eltrombopag [Citation20–26], romiplostim [Citation27–31], avatrombopag [Citation32,Citation33] and hetrombopag [Citation34] were more effective than placebo in raising platelet counts and reducing the incidence of bleeding manifestations. However, the efficacy and safety of TPO-RAs have varied widely in related studies, and some studies have had inconsistent results. The efficacy and safety of TPO-RAs in children with ITP have not been fully evaluated. Thus, we performed this meta-analysis to comprehensively evaluate the efficacy and safety of TPO-RAs in the treatment of persistent or chronic ITP in adults and children.
This meta-analysis has been registered with PROSPERO under the registration number CRD42022323502.
2. Methods
2.1. Search strategy
We searched PubMed, MEDLINE, ScienceDirect, Scopus, EMbase and the Cochrane Library to collect papers which published in English of TPO-RAs treated persistent and chronic Immune ITP from their earliest records to February 2022. In addition, references and conference proceedings included in the study were searched to complement the acquisition of relevant information. Search terms included immune thrombocytopenia, idiopathic thrombocytopenic purpura, immune thrombocytopenic purpura or ITP; romiplostim, eltrombopag, hetrombopag, avatrombopag, thrombopoietin or TPO. Taking PubMed as an example, its specific retrieval strategy is shown in .
2.2. Inclusion and exclusion criteria
2.2.1. Study types
randomized controlled trials (RCTs)
2.2.2. Study subjects
Adult or pediatric patients with primary ITP with a duration of more than 6 or 12 months before randomization had a baseline platelet count of 30,000 μL or less and had failed at least one previous treatment for non-TPO-RAs ITP.
Doses received by patients who received maintenance immunosuppressive therapy, mainly glucocorticoids, were stable for at least 1 month and remained constant throughout the study. Splenectomy was performed at least 4 weeks before randomization. Other treatments for ITP were completed at least 2 weeks before enrollment.
2.2.3. Intervention
Patients in the experimental group received a certain dose of TPO-RAs, and the appropriate dose was adjusted according to platelet response during the trial. The control group received the same dose and form of placebo.
2.2.4. Outcome
①The time from the first dose to the first response; ②The cumulative weeks of platelet response during the trial; ③Overall platelet response: platelet count≥50,000 μL during the trial; ④Durable platelet response: the duration of platelet response (platelet count≥50,000 μL) ≥ 75% during the trial period; ⑤Rescue therapy: rescue medication was defined as any medication designed to increase platelet counts or prevent bleeding; ⑥Any bleeding: World Health Organization (WHO) bleeding scale [Citation35], grade 1–4 (grade 0, no bleeding; grade 1, petechiae; grade 2, mild blood loss; grade 3, gross blood loss; grade 4, debilitating blood loss); ⑦Significant bleeding: WHO bleeding scale, grade 2–4;⑧Any adverse event; ⑨Serious adverse event: according to Common Terminology Criteria for Adverse Events (CTCAE) [Citation36].
2.2.5. Exclusion standard
①Irrelavent or duplicate publications; ②Non-RCT studies; ③Non-original data (eg, reviews); ④Lab-based study only; ⑤Non-primary, persistent or chronic ITP; ⑥Non-English; ⑦The required data were not available and attempts to contact the authors were fruitless.
2.3. Literature selection and data extraction
Two investigators independently screened literature, extracted data, and then cross-checked. If there is a disagreement, it should be discussed or negotiated with a third person. During literature screening, titles were read first and, after excluding apparently irrelevant literature, abstracts and full texts were further read for inclusion. If necessary, contact the original study author by e-mail or telephone for undetermined but important information about the study. Data extraction includes:①Basic information of included studies: study title, first author, journal of publication, etc; ②The baseline characteristics and interventions of the subjects; ③Key elements of risk of bias assessment; ④Required outcome indicators.
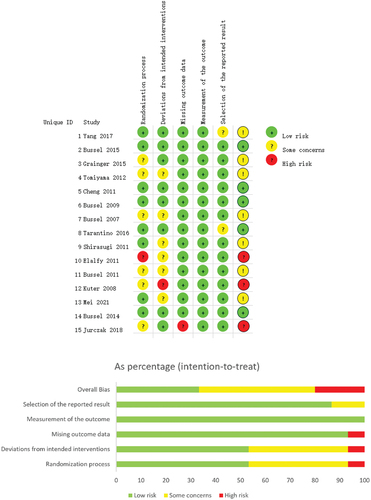
2.4. Risk of bias assessment of included studies
Two investigators independently assessed the risk of bias of the included studies and cross-checked the results. The revised Cochrane risk-of-bias tool for randomized trials (RoB 2) was used to assess the risk of bias. This tool assesses the risk of bias based on the following domains: 1) Bias arising from the randomization process; 2) Bias due to deviations from intended interventions; 3) Bias due to missing outcome data; 4) Bias in measurement of the outcome; 5) Bias in selection of the reported result.
2.5. Statistical analysis
Stata 16, Review Manager 5.2 and ADDIS were used for statistical analysis according to the Mantel-Hanszel method. STD mean difference (SMD) was used as effect analysis statistic for continuous variables, and risk ratio (RR) was used as effect analysis statistic for dichotomous variables.
The Chi-square test was used to analyze the heterogeneity among the included studies, and P < 0.05 or I2>50% was used to quantitatively determine the heterogeneity. If there was statistical heterogeneity among the results, the source of heterogeneity was further analyzed, and the random effects model was used for meta-analysis after excluding the influence of obvious clinical heterogeneity. Subgroup analysis or sensitivity analysis, or descriptive analysis only, was performed for significant clinical heterogeneity. The level of meta-analysis was set as α = 0.05.Publication bias was assessed using Egger’s test.
3. Results
3.1. Literature selecting process and results
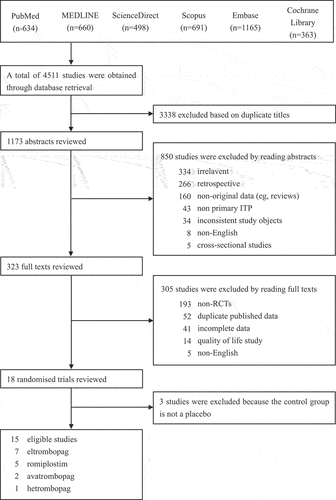
A total of 4511 articles were obtained from our literature search. After excluding 3338 articles with duplicate titles, we reviewed 1173 abstracts and 323 full texts, resulting in 18 RCT studies. Three of these studies were excluded because the control group of two studies was standard of care (SOC) and the control group of one study was recombinant human thrombopoietin (rhTPO). A total of 15 studies were finally determined. () Therefore, 10 randomized studies in adults (n = 1302) and 5 randomized studies in children (n = 261) were included in this analysis. There were 1 study on hetrombopag (n = 424), two studies on avatrombopag (n = 113), seven studies on eltrombopag (n = 765) and five studies on romiplostim (n = 261).
3.2. Basic characteristics and results of risk of bias assessment of included studies
The basic characteristics of the included studies are shown in . And the results of bias risk assessment are shown in .
Table 1. The basic characteristics of the included studies.
3.3. Results of meta-analysis
Due to the different dosage of TPO-RAs in patients of different ages, and the differences in disease characteristics and drug responses, this meta-analysis was performed in children and adults.
3.3.1. Efficacy
(1) The cumulative weeks of platelet response during the trial
Three trials have reported the mean cumulative weeks of platelet response in adult patients [Citation26,Citation28,Citation33]. One trial [Citation28]was invalid because the mean cumulative weeks of platelet response in the placebo group during the trial was 0. Since the follow-up time of the three trials was different, the cumulative number of weeks of platelet response was greatly affected by the follow-up time, so we chose SMD as the pooled statistic of this data. Results of meta-analysis showed that the difference in the cumulative weeks of platelet response [SMD = 1.28, 95%CI (0.72–1.84), P < 0.000 01] between the TPO-RAs and placebo groups was statistically significant. The duration of platelet response was significantly longer in the TPO-RAs than in the placebo groups. Three pediatric trials [Citation23,Citation27,Citation30] also showed statistically significant differences in the cumulative weeks of platelet response [SMD = 1.02, 95%CI (0.66–1.38), P < 0.000 01] between the TPO-RAs and control groups. The pooled results were homogenous (P = 0.770, I2 = 0.00%) (). And there was also one trial [Citation30] was invalid in children because the mean cumulative weeks of platelet response during the trial was 0 in the placebo group.
Table 2. Results of Meta-analysis.
(2) Overall platelet response
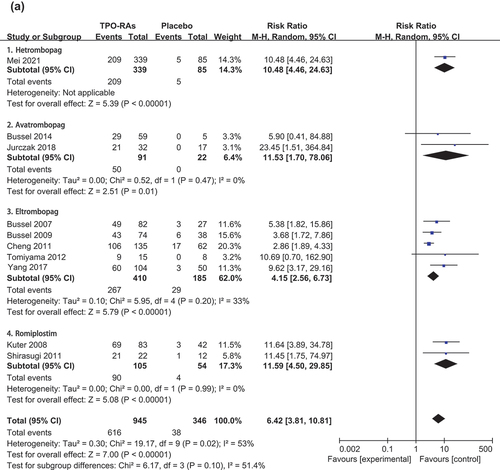
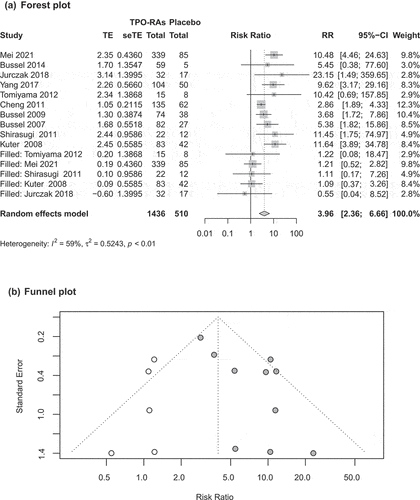
The meta-analysis of ten adult trials [Citation20,Citation21,Citation24–26,Citation28,Citation31–34] showed that compared with the placebo group, the TPO-RAs group had more patients with overall platelet response during follow-up [RR = 6.42, 95%CI (3.81–10.81), P < 0.000 01]. The pooled results were heterogeneity (P = 0.020, I2 = 53.00%). () The results of subgroup analysis () showed that the overall response rate of the four drugs was higher than that of the placebo group.
In addition, we conducted a network meta-analysis on adult data, and the results showed that the overall response rate of avatrombopag was better than that of eltrombopag and hetrombopag, and the difference was statistically significant. There was no significant difference in efficacy between avatrombopag and romiplostim. (S3) Overall platelet response in children was reported in five trials [Citation22,Citation23,Citation27,Citation29,Citation30]. The results showed that the use of TPO-RAs can improve the rate of overall platelet response [RR = 7.86, 95%CI (4.09–15.09), P < 0.000 01] in children. The pooled results were homogenous (P = 0.322, I2 = 14.40%). ()
(3) Durable platelet response
Five trials [Citation24,Citation26,Citation31,Citation33,Citation34] and two trials [Citation22,Citation27] have published data on durable platelet responses in adults and children. Platelet response rates were higher among adults who received TPO-RAs than among those who received placebo [RR = 12.00, 95%CI (6.09–23.36), P < 0.000 01]. The pooled results were homogenous (P = 0.583, I2 = 0.00%). There was no significant difference between the children group and the control group [RR = 7.46, 95%CI (2.21–25.19), P = 0.001] with homogeneity (P = 0.446, I2 = 0.00%). ()
(4) Rescue therapy
Six adult trials [Citation24,Citation26,Citation28,Citation31,Citation33,Citation34] and five pediatric trials [Citation22,Citation23,Citation27,Citation29,Citation30] reported the number of cases requiring rescue therapy. The results showed that compared with placebo, TPO-RAs decreased in adult patients [RR = 0.36, 95%CI (0.28–0.46), P < 0.000 01] and pediatric patients [RR = 0.60, 95%CI (0.41–0.88), P = 0.009]. The results were consistent in both adults (P = 0.462, I2 = 0.00%) and children (P = 0.260, I2 = 24.20%). ()
(5) Incidence of any bleeding
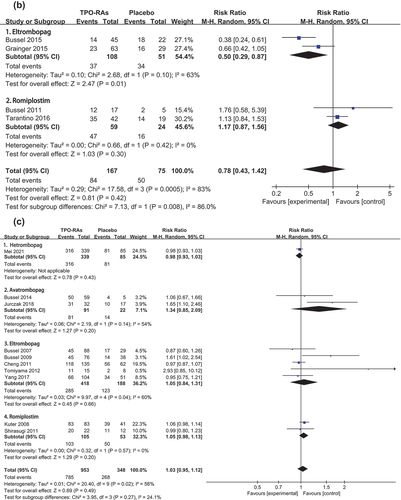
The incidence of any bleeding event in adults has been reported in seven trials [Citation21,Citation24–26,Citation28,Citation33,Citation34]. The pooled relative risks (RR) showed a lower rate of any bleeding event with TPO-RAs than with placebo [RR = 0.75, 95%CI (0.69–0.83), P < 0.000 01]. And the pooled results were homogenous (P = 0.089, I2 = 45.30%). Four trials reported the incidence of any bleeding event in children [Citation22,Citation23,Citation27,Citation30]. The results showed that in children, the incidence of any bleeding was similar between the TPO-RAs group and the placebo group [RR = 0.78, 95%CI (0.43–1.42), P = 0.416]. There was significant heterogeneity in the pooled results (P = 0.001, I2 = 82.90%). The results of subgroup analysis () showed that the eltrombopag group as a source of heterogeneity, and the results were consistent with the original results in eltrombopag group [RR = 0.50, 95%CI (0.29–0.87), P = 0.013]. But the results showed that the incidence of any bleeding event of romiplostim group and placebo group was similar [RR = 1.17, 95%CI (0.87–1.56), P = 0.300].
(6) Incidence of significant bleeding
Five adult trials [Citation24,Citation26,Citation28,Citation31,Citation33] and three pediatric trials [Citation22,Citation23,Citation30] reported the number of cases in which significant bleeding events occurred. Results in both adults [RR = 0.65, 95% CI (0.47–0.90), P = 0.008] and children [RR = 0.41, 95% CI (0.17–0.98), P = 0.044] showed that the rate of significant bleeding event was significantly lower in the TPO-RAs group than in the placebo group. And the pooled results were homogenous between adults (P = 0.970, I2 = 0.00%) and children (P = 0.570, I2 = 0.00%). ()
3.3.2. Safety
(1) Any adverse event
The incidence of any adverse event was reported in 10 adult trials [Citation20,Citation21,Citation24–26,Citation28,Citation31–34] and 4 pediatric trials [Citation22,Citation23,Citation29,Citation30]. The pooled relative risk (RR) showed that the incidence of any adverse event was similar between TPO-RAs and placebo groups in adults [RR = 1.03, 95%CI (0.95–1.12), P = 0.487] and children [RR = 0.99, 95%CI (0.86–1.15), P = 0.933]. The pooled results were heterogeneity in adults (P = 0.016, I2 = 55.90%) and homogenous in children (P = 0.298, I2 = 18.40%) (). Subgroup analysis (Figure S4C) confirmed that avatrombopag and eltrombopag as sources of heterogeneity. Avatrombopag [RR = 1.34, 95%CI (0.85–2.09), P = 0.203], eltrombopag [RR = 1.05, 95%CI (0.84–1.31), P = 0.656] and romiplostim [RR = 1.05, 95%CI (0.98–1.13), P = 0.200] were consistent with the results of the original study.
(2) Serious adverse event
The incidence of serious adverse events was reported in seven adult trials [Citation21,Citation24–26,Citation30,Citation33,Citation34] and four pediatric trials [Citation22,Citation23,Citation27,Citation30]. There was no significant difference in the incidence of serious adverse events between the romiplostim group and the placebo group in adults [RR = 0.78, 95%CI (0.54–1.12), P = 0.182] and children [RR = 1.23, 95%CI (0.55–2.74), P = 0.608]. The pooled results were homogenous between adults (P = 0.159, I2 = 35.30%) and children (P = 0.359, I2 = 6.80%). ()
3.4. Publish bias assessments
The Egger test results showed no publication bias except for overall platelet reactivity in adults (). There were too few trials of cumulative weeks of platelet response to permit publication bias. There was publication bias in the overall platelet response in adults (P = 0.014). After further analysis by trim-and-fill method (), the pooled relative risk (RR) showed that the overall platelet response rate was improved with TPO-RAs [RR = 3.96, 95%CI (2.36–6.66), P < 0.000 01]. The result is the same as before the subtraction.
Table 3. The results of Publication Bias (Egger’s test).
4. Discussion
This meta-analysis showed that TPO-RAs was more effective than placebo in both adults and children. Patients in the TPO-RAs group had higher overall platelet response rate and durable platelet response, longer duration of platelet response, shorter onset time, and less rescue therapy. Besides, the overall response rate of avatrombopag was better than that of eltrombopag and hetrombopag in adults. The incidence of any bleeding or significant bleeding was significantly lower in adults. However, in children, it did not reduce the incidence of any bleeding.
As for the time from the first dose to the first response, the hetrombopag study by Heng et al. [Citation34] in adults showed the median time to platelet response was 21 days when the initial dose was 2.5 mg and 14 days when the initial dose was 5 mg. The study of eltrombopag in children by Bussel et al. [Citation22] showed the median time was 20 days for children aged 1 to 5, 12 days for children aged 6 to 11 and 19 days for children aged 12 to 17 which the time to platelet response with eltrombopag were similar in all three age cohorts. The study of romiplostim in children by Michael et al. [Citation27] showed a median time of 4.5 days in the romiplostim group and 0 days in the placebo group.
Regarding the safety of TPO-RAs, the results showed that the incidence of any adverse events and serious adverse events among adult patients who received TPO-RAs was the same as that in the placebo group. This result is consistent with the results of a meta-analysis by Ahmed et al. [Citation37] Trials in adults showed that major adverse events were mild to moderate in severity. The common treatment-related adverse events in the TPO-RAs group were liver function injury (ALT elevation, AST elevation and unconjugated blood bilirubin elevation), nasopharyngitis, nausea and vomiting, fatigue, headache and epistaxis. The reported serious adverse events were severe organ bleeding and thromboembolic events. Severe organ bleeding was mainly hematencephalon and gastrointestinal hemorrhage. A total of 16 drug-related thromboembolic events (1.23%) were reported in all adult patients, including deep venous thrombosis of the lower extremities, pulmonary embolism and renal venous thrombosis.
Among eltrombopag-treated patients, the annual incidence of venous thromboembolism (VTE) ranged from 0.41 to 0.67 and the annual incidence of arterial thrombosis (AT) ranged from 0.96 to 1.15 [Citation12]. The pathogenesis of the increased risk of thrombosis associated with TPO-RAs has not been determined [Citation38]. One study showed that ITP patients treated with TPO-RAs had elevated levels of plasminogen activator inhibitor-1 (PAI-1), leading to thrombosis that are more resistant to fibrinolysis and thus exhibit procoagulant features [Citation39]. Only one patient in the romiplostim group reported increased reticulin in bone marrow during treatment, and bone marrow reticulin returned to pre-treatment level 14 weeks after drug withdrawal.
For children, there was no significant difference between the TPO-RAs group and placebo group, regardless of the presence or absence of adverse events or serious adverse events. In included trials, common adverse events in children were contusion, epistaxis, headache, upper respiratory tract infection, upper abdominal pain, nausea and vomiting, etc. Serious adverse events were reported including epileptic seizure, severe infection and thrombocytosis. No thromboembolic events were reported. However, a retrospective study reported two cases of pulmonary embolism associated with TPO-RAs treatment in children [Citation40]. Alanine aminotransferase (ALT) concentrations elevation was the most common drug-related adverse in patients treated with eltrombopag. There were 8 (5%) patients had ALT concentrations three or more times the ULN [Citation22,Citation23]. TPO-RAs has become the first choice of second-line treatment for patients who have failed first-line treatment. And TPO-RAs play an important role in the maintenance of efficacy. At present, there are many studies of TPO-RAs in combination with frontline therapy as an initial treatment for ITP. Results of two trials [Citation41,Citation42] of high-dose dexamethasone in combination with TPO-RAs-eltrombopag in adults with ITP showed higher rates of durable 6-month platelet response with combination than with monotherapy. And the drug combination was well tolerated. More clinical data are needed to confirm the efficacy of TPO-RAs combination therapy in children with ITP. In addition, an observational study showed that eltrombopag was also effective and safe in the treatment of thrombocytopenia after hematopoietic stem cell transplantation [Citation43]. TPO-RAs also shows great promise in the treatment of thrombocytopenia after hematopoietic stem cell transplantation, and further clinical data are needed to confirm it.
Our work has some advantages, that we included RCT trials of avatrombopag and hetrombopag. Although the number of trials and sample sizes included were small, data in the avatrombopag and hetrombopag groups did not show heterogeneity in the meta-analysis, which strongly indicated that the efficacy and safety of avatrombopag and hetrombopag were consistent with romiplostim and eltrombopag. For avatrombopag and hetrombopag, limited by the number and quality of included trials, more high-quality trials are needed to verify the above conclusions.
5. Conclusions
TPO-RAs was more effective than placebo in the treatment of persistent and chronic ITP in both adults and children. Patients in the TPO-RAs group had higher overall platelet response rate and durable platelet response rate, longer duration of platelet response, shorter onset time, and less rescue therapy. Regarding differences in efficacy between the four agents, the overall response rate was relatively higher with avatrombopag in adult patients, besides romiplostim. The incidence of any bleeding or significant bleeding was significantly lower in adults. In addition, TPO-RAs also has a high safety profile. Among adults and children who received TPO-RAs, the rates of any adverse events and serious adverse events were the same as those in the placebo group. And the adverse effects were mainly mild to moderate, such as headache, nausea and vomiting, abdominal pain, etc. At present, TPO-RAs has become the first choice of second-line treatment for patients with ITP when frontline treatment fails. In addition, there are many studies on TPO-RAs combined with first-line therapy as initial treatment for ITP. TPO-RAs has a broad application prospect in the treatment of ITP.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author Contributions
Conception and design: A Zhang, T Li. Screening of the literature, writing and drafting of the paper: T Li. Data extraction and analysis: T Li, Q Liu, T Pu. Final approval of the version to be published: A Zhang, J Liu. All authors agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by Qilu Hospital of Shandong University.
Additional information
Funding
References
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in an international working group immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. DOI:10.1182/blood-2008-07-162503
- Michel M. Immune thrombocytopenia nomenclature, consensus reports, and guidelines: what are the consequences for daily practice and clinical research? Semin Hematol. 2013 Jan;50(1):S50–4.
- Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am j med. 1995 May;98(5):436–442.
- Kühne T, Imbach P, Bolton-Maggs PH, et al. Newly diagnosed idiopathic thrombocytopenic purpura in childhood: an observational study. Lancet. 2001 Dec 22-29;358(9299):2122–2125. DOI:10.1016/S0140-6736(01)07219-1
- Mathias SD, Li X, Eisen M, et al. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Determine the Effect of Romiplostim on Health-Related Quality of Life in Children with Primary Immune Thrombocytopenia and Associated Burden in Their Parents. Pediatr Blood Cancer. 2016 Jul;63(7):1232–1237.
- Burness CB, Keating GM, Kp GJ. Eltrombopag: a Review in Paediatric Chronic Immune Thrombocytopenia. Drugs. 2016 May;76(8):869–878.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019 Dec 10;3(23):3829–3866. DOI:10.1182/bloodadvances.2019000966
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010 Jan 14;115(2):168–186. DOI:10.1182/blood-2009-06-225565
- Rodeghiero F. A critical appraisal of the evidence for the role of splenectomy in adults and children with ITP. Br J Haematol. 2018 Apr;181(2):183–195.
- Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012 Jun 21;119(25):5989–5995. DOI:10.1182/blood-2011-11-393975
- Al-Samkari H, Kuter DJ. Immune Thrombocytopenia in Adults: modern Approaches to Diagnosis and Treatment. Semin Thromb Hemost. 2020 Apr;46(3):275–288.
- Ghanima W, Cooper N, Rodeghiero F, et al. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019 Jun;104(6):1112–1123.
- Erickson-Miller CL, Delorme E, Tian SS, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009 Feb;27(2):424–430.
- Broudy VC, Lin NL. AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl. Cytokine. 2004 Jan 21;25(2):52–60.
- Fukushima-Shintani M, Suzuki K, Iwatsuki Y, et al. AKR-501 (YM477) in combination with thrombopoietin enhances human megakaryocytopoiesis. Exp Hematol. 2008 Oct;36(10):1337–1342.
- Fukushima-Shintani M, Suzuki K, Iwatsuki Y, et al. AKR-501 (YM477) a novel orally-active thrombopoietin receptor agonist. Eur J Haematol. 2009 Apr;82(4):247–254.
- Xie C, Zhao H, Bao X, et al. Pharmacological characterization of hetrombopag, a novel orally active human thrombopoietin receptor agonist. J Cell Mol Med. 2018 Nov;22(11):5367–5377.
- Markham A. Avatrombopag: a Review in Thrombocytopenia. Drugs. 2021 Nov;81(16):1905–1913.
- Yy S. Hetrombopag: first Approval. Drugs. 2021 Sep;81(13):1581–1585.
- Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012 May;10(5):799–806.
- Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007 Nov 29;357(22):2237–2247. DOI:10.1056/NEJMoa073275
- Bussel JB, de Miguel PG, Despotovic JM, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015 Aug;2(8):e315–25.
- Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015 Oct 24;386(10004):1649–1658. DOI:10.1016/S0140-6736(15)61107-2
- Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011 Jan 29;377(9763):393–402. DOI:10.1016/S0140-6736(10)60959-2
- Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009 Feb 21;373(9664):641–648. DOI:10.1016/S0140-6736(09)60402-5
- Yang R, Li J, Jin J, et al. Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Br J Haematol. 2017 Jan;176(1):101–110.
- Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016 Jul 2;388(10039):45–54. DOI:10.1016/S0140-6736(16)00279-8
- Shirasugi Y, Ando K, Miyazaki K, et al. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized Phase III clinical trial. Int J Hematol. 2011 Jul;94(1):71–80.
- Elalfy MS, Abdelmaksoud AA, Eltonbary KY. Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 2011 Nov;90(11):1341–1344.
- Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011 Jul 7;118(1):28–36. DOI:10.1182/blood-2010-10-313908
- Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008 Feb 2;371(9610):395–403. DOI:10.1016/S0140-6736(08)60203-2
- Bussel JB, Kuter DJ, Aledort LM, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood. 2014 Jun 19;123(25):3887–3894. DOI:10.1182/blood-2013-07-514398
- Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018 Nov;183(3):479–490.
- Mei H, Liu X, Li Y, et al. A multicenter, randomized phase III trial of hetrombopag: a novel thrombopoietin receptor agonist for the treatment of immune thrombocytopenia. J Hematol Oncol. 2021 Feb 25;14(1):37. DOI:10.1186/s13045-021-01047-9
- Fogarty PF, Tarantino MD, Brainsky A, et al. Selective validation of the WHO Bleeding Scale in patients with chronic immune thrombocytopenia. Curr Med Res Opin. 2012 Jan;28(1):79–87.
- Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014 Sep;106(9):dju244.
- Ahmed HAW, Masoud AT, Han J, et al. Eltrombopag Effectiveness and Tolerability in Chronic Immune Thrombocytopenia: a Meta-Analysis. Clin Appl Thromb Hemost. 2021 Jan;27:10760296211005555.
- Rodeghiero F. ITP and thrombosis: an intriguing association. Blood Adv. 2017 Nov 14;1(24):2280.
- Justo Sanz R, Monzón Manzano E, Fernández Bello I, et al. Platelet Apoptosis and PAI-1 are Involved in the Pro-Coagulant State of Immune Thrombocytopaenia Patients Treated with Thrombopoietin Receptor Agonists. Thromb Haemost. 2019 Apr;119(4):645–659.
- Ramaswamy K, Hsieh L, Leven E, et al. Thrombopoietic agents for the treatment of persistent and chronic immune thrombocytopenia in children. J Pediatr. 2014 Sep;165(3):600–5.e4.
- Gómez-Almaguer D, Herrera-Rojas MA, Jaime-Pérez JC, et al. Eltrombopag and high-dose dexamethasone as frontline treatment of newly diagnosed immune thrombocytopenia in adults. Blood. 2014 Jun 19;123(25):3906–3908. DOI:10.1182/blood-2014-01-549360
- Zhang L, Zhang M, Du X, et al. Safety and efficacy of eltrombopag plus pulsed dexamethasone as first-line therapy for immune thrombocytopenia. Br J Haematol. 2020 Apr;189(2):369–378.
- Karataş A, Göker H, Demiroğlu H, et al. Efficacy of eltrombopag in thrombocytopenia after hematopoietic stem celltransplantation. Turk J Med Sci. 2022 Apr;52(2):413–419.