ABSTRACT
Background
Dipeptidyl peptidase-4 inhibitors (DPP4is) and metformin are the most frequently prescribed first-line drugs for Japanese patients with type 2 diabetes (T2D). We investigated the risk of cardiovascular events by second-line treatment type in these patients.
Research design and methods
Patients with T2D, prescribed either metformin or DPP4i as a first-line drug, were identified in claims data from Japanese acute care hospitals. The primary and secondary outcomes were cumulative risks of MI or stroke and of death, respectively, from second-line treatment initiation.
Results
Patients prescribed first-line metformin or DPP4i was 16,736 and 74,464, respectively. In patients receiving first-line DPP4i, the death incidence was lower in those receiving second-line metformin than in those receiving second-line sulfonylurea (p < 0.001), whereas the primary outcome was not significantly different. No significant differences were observed for either outcome when DPP4is and metformin were used as first- and second-line drugs or vice versa.
Conclusions
Metformin was suggested to have larger effect to reduce death than sulfonylurea in patients receiving first-line DPP4i. The order of first- and second-line for the DPP4i and metformin combination did not affect the outcomes. Given the nature of the study design, certain limitations, including potential under-adjustment for confounders, should be considered.
1. Introduction
Dipeptidyl peptidase-4 inhibitors (DPP4is) and metformin are the most frequently prescribed first-line drugs for patients with type 2 diabetes in Japan [Citation1,Citation2]. Some Japanese studies, including our previous study, have suggested that biguanide/metformin as the first-line drug might reduce the risk of cardiovascular events more than DPP4is in Japanese patients with type 2 diabetes [Citation3,Citation4]. Our previous study also implied that not only the first-line drug but also other factors, including adherence to the first-line drug, ease of adding second-line drugs and subsequent drugs, and the type of drugs added might be involved in risk reduction [Citation3]. Thus, the treatment patterns with second-line drugs and the effect of treatment on the reduction of cardiovascular events in patients prescribed DPP4is or metformin as first-line drugs should be investigated.
Treatment effects, including those of second-line drugs, on cardiovascular events in patients with type 2 diabetes have not been completely investigated in Japan. Metformin is a recommended and the most frequently used first-line drug worldwide, other than Japan [Citation5], and various studies have examined the effects of second-line treatments after first-line metformin treatment [Citation6–12]. However, in Japan, metformin is not the only recommended first-line drug; several other drugs have also been used [Citation13]. Studies on the effects of treatments, including second-line prescribed drugs with metformin as the first-line drug, are scarce. Furthermore, although DPP4is are the most prescribed first-line drugs in Japan [Citation1], they are not the primary first-line treatments in other countries [Citation14]. Consequently, the effect of subsequently prescribed drugs, after DPP4is as the first-line intervention, is not well studied.
In this study, we analyzed data from a health insurance claims database to investigate the prescription pattern of second-line treatments in Japanese patients who were prescribed metformin or DPP4is as the first-line drug; which was analyzed in our previous study [Citation3]. We also compared the incidence of the observed cardiovascular events, after the initiation of the second-line treatment, between the combinations of first-line and second-line treatments that were frequently used. The events were also compared after the initiation of the first prescription of the first-line treatment. As multiple hypoglycemic agents are often used simultaneously and the prevention of cardiovascular events is the primary goal, evidence of treatment effects, including those of second-line drugs, on the reduction of cardiovascular events may provide valuable referential information for selecting drugs to treat type 2 diabetes.
2. Patients and methods
2.1. Study design and data source
This was a retrospective cohort study conducted using data from a Japanese health insurance claims database. In patients with type 2 diabetes who were on monotherapy with either metformin or a DPP4i as the first-line antidiabetic drug, the pattern of second-line treatment was examined and the combinations of first- and second-line treatments that were frequently used and suitable for comparing the incidence of cardiovascular events were selected. A database of the Medical Data Vision Co., Ltd. between April 2008 and November 2018 was used. The database included data of approximately 28 million patients from 385 Diagnosis Procedure Combination (DPC) hospitals, which included approximately 22% acute care hospitals (as of August 2019) [Citation15]. DPC hospitals are acute care hospitals applying the DPC/Per-Diem Payment System (DPC/PDPS) [Citation16]. The observation period was the duration from the first medical record until the last medical record for each patient in the database. The baseline period was the period that included the date of the first prescription of the first-line treatment and the preceding 30 days.
2.2. Patient identification
To identify the target patients, the inclusion/exclusion criteria applied were the same as those in our previous study [Citation3] (Table S1), except for the exclusion of patients without available acetylated hemoglobin (HbA1c) data during the baseline period. The eligible patients were those who had type 2 diabetes (defined by the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) code E11 or E14 [Citation17]) at least once, were aged≥18 years, were prescribed either metformin (defined by the generic name) or a DPP4i (defined by the Anatomical Classification of Pharmaceutical Products (ATC) code: A10N1) as the first-line antidiabetic drug (coded as A10 by ATC) for monotherapy, and whose medical records, from at least 6 months before the initiation of the first-line treatment, were available. Those who were diagnosed with type 1 diabetes (coded as E10 by ICD-10) during the observation period and/or were hospitalized with any diagnosis of myocardial infarction (MI; coded as I12× by ICD-10), or cerebrovascular disease (coded as I60×-I69× by ICD-10) before the first-line treatment were excluded from the study.
2.3. Outcomes
The outcomes were time-to-event data displayed using Kaplan-Meier curves from the first prescription of the second-line treatment. The primary endpoint was the incidence of MI or stroke, which was defined according to a three-point criterion for major adverse cardiovascular events as follows: a composite of death from cardiovascular causes, non-fatal MI, and non-fatal stroke [Citation18,Citation19]. The diagnosis of MI or stroke, irrespective of whether it was fatal or non-fatal, associated with hospitalization (recorded as the greatest resource-consuming condition, trigger-for-hospitalization condition, or the main condition) was used to define the primary endpoint because the database does not have death records, other than those recorded as the outcome of hospital discharge. The secondary endpoint was the incidence of death (recorded as total deaths) as the outcome of hospital discharge. Additionally, time-to-event based on the Kaplan-Meier curves was shown for these endpoints from the first prescription of the first-line treatment.
2.4. Statistical analysis
Sulfonylurea, sodium-glucose co-transporter-2 inhibitor (SGLT2is), and DPP4is or metformin, for patients on metformin or DPP4is as a first-line drug, were selected as the second-line drugs for examination based on a previous study in which these drugs were often used in the second-line treatment [Citation1,Citation2]. As the combination of DPP4is and SGLT2is (hereinafter referred to as DPP4is+SGLT2is) is available, the prescription for this combination was separately counted as a second-line treatment, in addition to prescription of each single agent. The number of patients, mean age, and percentage of patients of each sex, duration of treatment period (entire observation period from initiation of the first-line treatment divided by the period before and after commencement of the second-line treatment), and the gap from the end date of the first-line treatment until the starting date of the second-line treatment were analyzed based on descriptive statistics.
To investigate the treatment effect, Kaplan-Meier curves of the events were computed for each endpoint after adjusting for confounding factors of the first-line treatment, with metformin or DPP4i, using propensity scores. The propensity scores were calculated using a logistic regression model with first-line metformin prescription as the explained variable, and age, sex, Charlson Comorbidity Index [Citation20,Citation21], hypertension (defined by the prescription of antihypertensive agents coded as C03, C07, C08, or C09 by ATC), dyslipidemia (defined by the prescription of statins or other antihyperlipidemic agents coded as C10 by ATC), and prescription of antithrombotic drugs (such as aspirin, novel oral anticoagulants defined by generic name, or other antithrombotic agents coded as B01 by ATC) at baseline as the explanatory variables. Based on the propensity score, the patients were divided into five quintiles, and the weights were adjusted for each quintile [Citation22]. Statistical significance was defined as p < 0.05 using a log-rank test. The statistical analyses were performed using Microsoft Excel 2010 (Microsoft, Redmond, WA, U.S.A) and SAS version 9.4 (SAS Institute, Cary, NC, U.S.A).
2.5. Ethics approval
As the data had been anonymized in the database, ethical approval was not required according to Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare, Japan.
2.6. Patient and public involvement
Patients were not involved in this study.
3. Results
3.1. Prescription pattern of the second-line treatment
In total, 91200 patients were identified for analysis (Table S1), with 16,736 receiving metformin and 74,464 receiving a DPP4i as the first-line drug. The baseline characteristics of the patients by first-line treatment are shown in Table S2.
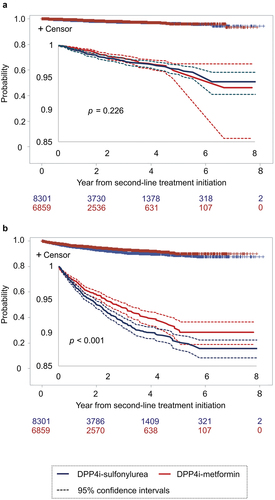
For patients who received a DPP4i as the first-line drug, metformin was the most frequently prescribed second-line drug (6,697 patients, hereinafter referred to as the DPP4i-metformin group), followed by sulfonylurea (4,393 patients, the DPP4i-SU group) ().
Table 1. Pattern of second-line treatments in Japanese patients administered metformin or DPP4i as the first-line drug using medical claims data.
For patients who received metformin as the first-line drug, DPP4is (4,676 patients, the metformin-DPP4i group) and SGLT2is (1,149 patients, the metformin-SGLT2i group) were the top two second-line drugs. The mean age of patients was the lowest in the metformin-SGLT2i group, at 51.1 ± 12.4 (mean ± standard deviation) years, and highest in the DPP4i-SU group, at 69.3 ± 12.2 years (). The percentage of women was lower (approximately 30%) among patients who received DPP4i as the first-line drug and DPP4i+SGLT2i as the second-line drug combination (the DPP4i-DPP4i+SGLT2i group) than in the other groups.
The mean observation period from the initiation of the first-line treatment to initiation of the second-line treatment was shorter in patients prescribed sulfonylurea (6.l ±10.3 months and 10.4 ± 13.8 months in the metformin-SU group and DPP4i-SU group, respectively; ) than in those administered other drugs as the second-line treatment. In those who received DPP4i+SGLT2i as the second-line drug combination, irrespective of the type of first-line treatments, the period was long, exceeding 21 months. The observation period after the initiation of the second-line drug was relatively short (approximately 13–14 months) in patients receiving SGLT2i as the second-line drug, and the corresponding period was the shortest (<5 months) in those receiving DPP4i+SGLT2i.
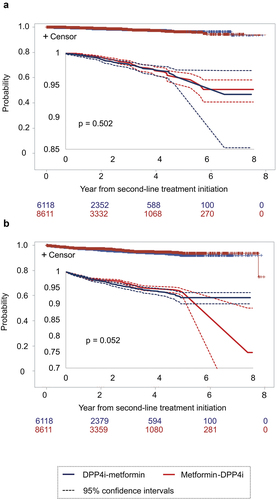
Based on these observations, we compared the treatment effect in those prescribed DPP4is as the first-line drugs and metformin or sulfonylurea as the second-line drug (the DPP4i-metformin group vs. the DPP4i-SU group). Next, we compared the combination of metformin and DPP4i as first- and second-line treatments in the reverse order (metformin-DPP4i group vs. DPP4i-metformin group). Other treatment patterns (e.g. metformin-SGLT2i was the second common treatment combination for patients on first-line metformin) were not included. This was due to the shorter observation period after the initiation of the second-line treatment, which was almost half of or shorter than that for the other treatments (other than metformin-SU); in addition, this group had a smaller sample. We noticed that the gap between the end of the first-line treatment and the initiation of the second-line treatment was 0 days in approximately 90% patients in the treatment groups (Table S3), and only those with a 0-day gap were included in the analysis. As a result, the number of patients analyzed was 4,095 in the DPP4i-SU group, 6,149 in the DPP4i-metformin group, and 4,011 in the metformin-DPP4i group.
3.2. Comparison between the DPP4i-SU and DPP4i-metformin groups
For patients in the DPP4i-SU group, glimepiride was the most commonly prescribed drug (for 86.3% patients), followed by gliclazide (11.6%) and gliclazide (2.2%), and the mean daily dosages of these drugs for their first prescription were 0.9, 24.7, and 4.5 mg, respectively. For patients in the DPP4i-metformin group, the mean daily dosage of metformin for the first prescription was 581.0 mg. Significant differences in the incidence of MI or stroke were not observed between the DPP4i-SU and DPP4i-metformin groups as per the Kaplan-Meier curves plotted using the data from the log-rank test (p = 0.226, ) on the first prescription of the second-line treatment. A significant difference was observed between the groups (p = 0.022, Figure S1A) when the incidence of MI or stroke from the first prescription of the first-line treatment was compared, and its rate was lower in the DPP4i-metformin group than in the DPP4i-SU group for 7 years from the initiation of the first-line treatment. The incidence of death as the outcome of hospital discharge from the first prescription of the second-line treatment was lower in the DPP4i-metformin group than in the DPP4-SU group (p < 0.001, ).
3.3. Comparison between the DPP4i-metformin and metformin-DPP4i groups
A significant difference between the DPP4i-metformin group and metformin-DPP4i group (p = 0.502, and p = 0.052, respectively; ) was not observed, in terms of the incidence of MI or stroke and the incidence of death as the outcome of hospital discharge, in the first prescription of the second-line treatment. Similarly, no significant difference between the treatments was observed for either outcome from the initiation of the first-line treatment (Figure S2).
4. Discussion
In this study, we analyzed the prescription pattern of the second-line treatment for patients prescribed metformin or a DPP4i as the first-line drug, and compared the incidence of MI or stroke and the incidence of death between the combinations of first- and second-line treatments. To the best of our knowledge, this is the first study on the treatment effects, including second-line drugs, on these outcomes in patients with type 2 diabetes in Japan. No difference was observed in the incidence of MI or stroke when sulfonylurea and metformin were compared as the second-line drugs in patients prescribed DPP4is as the first-line drugs, whereas a lower incidence of total deaths as the outcome of hospital discharge was observed for metformin than for sulfonylurea. No significant difference in the outcomes was observed between DPP4is and metformin as the first- and second-line drugs and those in the reverse order.
The popularity of the DPP4i and metformin combination is reasonable because of the balance between their therapeutic effects and safety upon use [Citation23]. In addition, this combination is preferable because of the difference in their sites of action – DPP4i acts on promoting insulin secretion; whereas metformin primarily acts on reducing hepatic gluconeogenesis rather than enhancing insulin secretion [Citation13]. This combination is associated with a lower risk of inducing hypoglycemia than the combination of sulfonylurea and metformin [Citation24,Citation25]. A comparison of the outcomes between the combination of a DPP4i and metformin with that of the reverse order of initiation did not reveal any significant difference in either outcome. Although this comparison has not been reported to date, a similar reduction in HbA1c level with a combination of vildagliptin (a DPP4i) and metformin has been reported in patients with inadequately controlled diabetes using vildagliptin or metformin monotherapy [Citation26]. As the effect on the reduction in blood glucose level (shown as the reduction in HbA1c level) may be associated with the incidence of cardiovascular events, our results appear reasonable.
The second-most frequent prescription of sulfonylurea in patients on first-line DPP4i was probably because short-term hypoglycemic action was required. Our results showed that sulfonylurea was prescribed as the second-line drug for a shorter period from the first-line treatment than that with the other second-line treatments examined. This finding suggested that the prescription was made for patients with poor glycemic control with the first-line treatment and required treatment with immediate effect to improve the blood glucose level. Available evidence suggests that sulfonylurea, which has been used for treating diabetes for a long time, decreases the risk of microvascular complications and is considered effective in patients with type 2 diabetes irrespective of age and body weight [Citation27]. Although its effect on reducing the blood glucose level is preferable, it is reportedly associated with the risk of developing hypoglycemia [Citation28]. Prevention of hypoglycemia is one of the major goals to be considered when choosing hypoglycemic agents [Citation29]. An increase in the risk of developing hypoglycemia with sulfonylurea use has been suggested in not only monotherapy but also combination with other classes of antidiabetic agents. Although DPP4is are associated with a low risk of hypoglycemia [Citation30], the risk in combination with sulfonylurea reportedly varies [Citation31–34], and it included the development of severe hypoglycemia [Citation33]. The combination of metformin with sulfonylurea has been reported to be associated with a higher risk of developing hypoglycemia than metformin monotherapy or a combination of metformin with a thiazolidinedione [Citation28]. In addition, in treatments using metformin with sulfonylurea, a reduction in cardiovascular complications (shown for metformin monotherapy) is not observed, but mortality increases [Citation35,Citation36]. These results might be associated with the lower number of patients treated with first-line metformin who were prescribed sulfonylurea rather than SGLT2is. However, we found that sulfonylurea was prescribed for a certain number of patients on DPP4is as the first-line drugs, albeit with the risk of developing hypoglycemia. The popularity of sulfonylurea over SGLT2i for patients on first-line DPP4is might be due to the familiarity of physicians with the drug (because of its long period of availability), as well as its lower cost. DPP4is are relatively new and more expensive than metformin, and are expected to show short-term effect.
Regarding first-line DPP4i treatment, more preferable effects on the incidence of total deaths as the outcome of hospital discharge were observed in patients treated with second-line metformin than in patients treated with second-line sulfonylurea. Although such comparisons have not been made in the past, to the best of our knowledge, the result is consistent with those of previous studies comparing monotherapies with these drugs or with different treatments. For example, metformin monotherapy reduced the incidence of cardiovascular events more strongly than sulfonylurea in overweight patients [Citation35]. In addition, the risk of cardiovascular events or deaths was higher after treatment with metformin plus sulfonylurea than with metformin alone [Citation37,Citation38]. A lower risk of cardiovascular events or death has also been reported for treatment with a combination of metformin and DPP4is than with metformin and sulfonylurea [Citation24,Citation39].
SGLT2is, which have been available since 2014 in Japan, have been prescribed more often as the second-line drugs than sulfonylurea for patients who were prescribed metformin as the first-line drug. The young mean age of patients prescribed SGLT2is as the second-line drugs, particularly in those who were prescribed metformin as the first-line drug, might be associated with the safety profile of SGLT2i. It is often associated with some adverse events, including ketoacidosis, acute renal insufficiency, and events related to volume depletion [Citation40]. Consequently, careful prescription is advised, particularly for patients aged≥75 years or those who are prescribed diuretics [Citation40]. Nonetheless, SGLT2is have been shown to improve blood glucose levels independent of insulin, in addition to a reduction in body weight [Citation41–45]. We did not include groups treated with SGLT2is for comparing the outcomes because of the shorter observation period after the initiation of the second-line treatment in this study. Considering the relatively short term of SGLT2i availability and popularity in patients on first-line metformin compared with those in patients on sulfonylurea, changes in SGLT2i treatment status and evaluation of the therapeutic effect of the combination therapy, including SGLT2is, could be an important topic for future studies. In addition, we did not include glucagon-like peptide-1 receptor agonist as a second-line drug to be examined in this study because of small number of users in our claims database; the accessibility and popularity of this drug may have changed later and should be considered to be included in future studies.
4.1. Strengths and limitations
The novelty of this study is it investigated the effect of treatments including second-line drugs on cardiovascular events, which has not been thoroughly investigated in Japan. The effects of second-line drugs on the first-line DPP4is are not well studied in other countries as well. This study involved an analysis of nationwide claims data of a large number of patients from April 2008 to November 2018, which is appropriate in describing the current treatment status in Japan. Moreover, the data in the database were routinely collected for reimbursements; thus, the data might be more accurately recorded than those manually recorded by individual physicians, such as registry data.
There are several limitations that should be considered when interpreting the results. First, as this study involved the analysis of the claims data, the accuracy of the diagnoses and treatments recorded in the database influences the results of the study. In fact, as mentioned, the claims for the treatment are connected to the payment, and the diagnosis of the outcome is associated with hospitalization; consequently, the data used in this study are likely to be recorded correctly. Second, the database used in this study consisted of data from DPC hospitals, which are large acute hospitals. Thus, the patients might have had more severe diabetes or complications than general Japanese patients, which could affect the generalizability of the results. Moreover, as the database only included data on diagnoses and treatments within the same hospital, the events associated with diagnoses/deaths that occurred in other settings could not be counted. In addition, we could only collect records of death associated with hospitalization. Consequently, the incidence of the outcomes might have been underestimated; however, the influence might be independent of the type of treatment. Notably, although the causes of deaths should also be analyzed, here, we stated the incidence of death as the total death recorded as the outcome of hospital discharge because of the lack of sufficient data. Third, we adjusted for the confounding factors using propensity scores; however, the adjustments might not have been made for all important confounders. For example, as described earlier, sulfonylurea may be prescribed for patients with poor glycemic control along with DPP4is as the first-line treatment. In other words, the DPP4i-SU group may include more patients with poor glycemic control than the DPP4i-metformin group, and the difference in risk due to the characteristics of the groups may affect the outcomes even after adjustment of confounding factors. Notably, laboratory data of HbA1c level and estimated glomerular filtration rate available for some patients, included in the confounding factors for adjustments in a previous study, were not included in this study to obtain adequate sample size. Finally, we compared the effects of treatments based on the type of first- and second-line treatments. Treatment status after the initiation of second-line treatment, including the continuation of these treatments and availability and type of additional treatments, were not considered. Therefore, the differences in such statuses between treatment groups might have affected the outcomes. However, we consider that the differences in status are also caused by the characteristics of the treatments and thus should be included when comparing the treatment effects.
5. Conclusions
The two most common first-line drugs, DPP4is and metformin, were the most frequently prescribed second-line drugs in patients who received the alternate treatment as the first-line drug. Sulfonylurea was the second-most common second-line drug in patients with DPP4is as the first-line drugs. The difference in the incidence of MI or stroke was not observed between metformin and sulfonylurea as the second-line drugs for patients prescribed DPP4is as the first-line drugs; metformin was suggested to reduce total death as the outcome of hospital discharge more strongly than others. The incidence of MI or stroke and that of total death were similar between the regimens where DPP4is and metformin were used as the first- and second-line therapeutics and those with the reverse order of initiation. Certain limitations, including the accuracy of the recorded data, generalizability of the database, and the possibility of insufficient confounding factor adjustment, should be considered when interpreting the results because of the nature of the study design. Nevertheless, as multiple hypoglycemic agents are often prescribed for treating type 2 diabetes, we believe that these results will help physicians in deciding treatment regimens, including the use of second-line agents.
Declaration of interest
R Nishimura has received honoraria from Astellas Pharma Inc., Nippon Boehringer Ingelheim Co. Ltd, Eli Lilly Japan K.K., Kissei Pharmaceutical Co. Ltd, Medtronic Japan Co. Ltd, MSD, Novartis Pharma K.K., Novo Nordisk Pharma Ltd, Sanofi K.K., Sumitomo Pharma Co. Ltd., and Takeda Pharmaceutical Co. Ltd. R Nishimura has also received a grant from the Japan Diabetes Foundation and Nippon Boehringer Ingelheim Co. Ltd.
S Aoi is an employee of Sumitomo Pharma Co., Ltd.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed receiving research support from Novo Nordisk, Poxel SA, Eli Lilly Japan, and The Cookie Testing Study Grou, as well as honoraria for lectures from Sumitomo Dainippon Pharma, Novo Nordisk, Eli Lilly Japan, AstraZeneca, Boehringer Ingelheim, and Mitsubishi Tanabe Pharma, and consulting and advisory board fees from Novo Nordisk and Eli Lilly Japan. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
R Nishimura and S Aoi contributed to the study conception. All the authors contributed to the study design and interpretation of data. Data was analyzed by K Iwasaki. The first draft of the manuscript was written by T Takeshima, and all the authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Acknowledgments
We thank Editage (www.editage.com) for English language editing, which was supported by Sumitomo Pharma Co., Ltd.
Data availability statement
The data are available from Medical Data Vision Co., Ltd., but restrictions apply to the availability of these data, which were used under license of the current study and are not available publicly. Data are available from the authors with the permission of Medical Data Vision Co., Ltd.
Additional information
Funding
References
- Morita Y, Murayama H, Odawara M, et al. Treatment patterns of drug-naive patients with type 2 diabetes mellitus: a retrospective cohort study using a Japanese hospital database. Diabetol Metab Syndr. 2019;11:90. doi:10.1186/s13098-019-0486-y.
- Nishimura R, Kato H, Kisanuki K, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9:e025806. doi:10.1136/bmjopen-2018-025806.
- Nishimura R, Takeshima T, Iwasaki K, et al. Comparison of the effects on cardiovascular events between use of metformin and dipeptidyl peptidase-4 inhibitors as the first-line hypoglycaemic agents in Japanese patients with type 2 diabetes mellitus: a claims database analysis. BMJ Open. 2022;12:e045966. doi:10.1136/bmjopen-2020-045966.
- Komamine M, Kajiyama K, Ishiguro C, et al. Cardiovascular risks associated with dipeptidyl peptidase-4 inhibitors monotherapy compared with other antidiabetes drugs in the Japanese population: a nationwide cohort study. Pharmacoepidemiol Drug Saf. 2019;28:1166–1174. doi:10.1002/pds.4847.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–2701.
- Bolinder J, Ljunggren O, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diab Obes Metab. 2014;16:159–169.
- Charpentier G, Fleury F, Kabir M, et al. Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med. 2001;18:828–834.
- Moses R, Slobodniuk R, Boyages S, et al. Effect of repaglinide addition to metformin monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 1999;22:119–124.
- Van Gaal L, Maislos M, Schernthaner G, et al. Miglitol combined with metformin improves glycaemic control in type 2 diabetes. Diab Obes Metab. 2001;3:326–331.
- Einhorn D, Rendell M, Rosenzweig J, et al. Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The pioglitazone 027 study group. Clin Ther. 2000;22:1395–1409.
- Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diab Obes Metab. 2011;13:65–74.
- DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100.
- The Japan Diabetes Society. Treatment Guide for Diabetes 2020-2021. Tokyo: Bunkodo; 2020. Japanese.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S98–110.
- Pharmacoepidemiology, Taskforce D, Japanese Society for Pharmacoepidemiology. Survey of Japanese databases in Japan available for clinical/pharmacoepidemiology. 2019. Available from: http://www.jspe.jp/committee/020/0210/. Accessed on Mar 7, 2023.
- Ishii M. DRG/PPS and DPC/PDPS as prospective payment systems. Japan Med Assoc J. 2012;55:279–291.
- Ministry of Health, Labour and Welfare. [Statistical classification of diseases and cause of death]. 2013. Available from: http://www.mhlw.go.jp/toukei/sippei/. Accessed on Mar 7, 2023. Japanese.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128.
- Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534.
- Kimura T, Sugitani T, Nishimura T, et al. Validation and recalibration of charlson and elixhauser comorbidity indices based on data from a Japanese insurance claims database. Jpn J Pharmacoepidemiol. 2019;24:53–64.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med care. 2005;43:1130–1139.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225.
- Murayama H, Imai K, Odawara M. Factors influencing the prescribing preferences of physicians for drug-naive patients with type 2 diabetes mellitus in the real-world setting in Japan: insight from a web survey. Diabetes Ther. 2018;9:1185–1199.
- Eriksson JW, Bodegard J, Nathanson D, et al. Sulphonylurea compared to DPP-4 inhibitors in combination with metformin carries increased risk of severe hypoglycemia, cardiovascular events, and all-cause mortality. Diabet Res Clin Pract. 2016;117:39–47.
- Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475–483.
- Odawara M, Yoshiki M, Sano M, et al. Efficacy and safety of a single-pill combination of vildagliptin and metformin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Diabetes Ther. 2015;6(1):17–27. DOI:10.1007/s13300-015-0099-x
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352(9131):837–853. DOI:10.1016/S0140-6736(98)07019-6
- Bennett WL, Wilson LM, Bolen S, et al.Oral Diabetes Medications for Adults with Type 2 Diabetes: an Update Comparative Effectiveness Reviews. Vol. 27 Prepared by Johns Hopkins University Evidence-based Practice Center under Contract No. 290-02-0018AHRQ PublicationAHRQ Publicationp. 11–EHC038Rockville MD2011
- The Japan Diabetes Society. Clinical Practice Guideline for Diabetes 2019. Japanese. Tokyo: Nankodo; 2019.
- The Japan Diabetes Society. Treatment Guide for Diabetes 2018-2019. Tokyo: Bunkodo; 2018. Japanese.
- Tajima N, Kadowaki T, Odawara M, et al. Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetol Int. 2011;2(1):32–44. DOI:10.1007/s13340-011-0022-2
- Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diab Obes Metab. 2014;16(5):418–425.
- Iwakura T, Fujimoto K, Tahara Y , et al. A case of severe hypoglycemia induced by sitagliptin added to ongoing glimepiride therapy in patients with type 2 diabetes. J Jpn Diabetes Soc. 2010;53:505–508.
- Kashiwagi A, Kadowaki T, Tajima N, et al. Sitagliptin added to treatment with ongoing pioglitazone for up to 52 weeks improves glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011;2(5):381–390. DOI:10.1111/j.2040-1124.2011.00120.x
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. DOI:10.1016/S0140-6736(98)07037-8.
- Evans JM, Ogston SA, Emslie-Smith A, et al. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia. 2006;49:930–936.
- Douros A, Dell’aniello S, OHY Y, et al. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: population based cohort study. BMJ. 2018;362:k2693.
- Shimoda M, Kaku K. Controversy about the relationship between sulfonylurea use and cardiovascular events and mortality. J Diabetes Investig. 2016;7:674–676.
- Morgan CL, Mukherjee J, Jenkins-Jones S, et al. Combination therapy with metformin plus sulphonylureas versus metformin plus DPP-4 inhibitors: association with major adverse cardiovascular events and all-cause mortality. Diab Obes Metab. 2014;16(10):977–983. DOI:10.1111/dom.12306
- The Japan Diabetes Society. [Recommendations for the use appropriate use of SGLT2 inhibitors]. 2019. Available from: http://www.fa.kyorin.co.jp/jds/uploads/recommendation_SGLT2.pdf. Accessed on Mar 7, 2023. Japanese.
- Kaku K, Kiyosue A, Inoue S, et al. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diab Obes Metab. 2014;16:1102–1110.
- Kashiwagi A, Kazuta K, Yoshida S, et al. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(4):382–391. DOI:10.1111/jdi.12156
- Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled, Phase III study. Expert Opin Pharmacother. 2014;15:1501–1515.
- Seino Y, Sasaki T, Fukatsu A, et al. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30:1245–1255.
- Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65.