ABSTRACT
Introduction
Digital ulcers (DUs) develop in approximately 50% of patients with systemic sclerosis (SSc). DUs are painful and disfiguring, with a major impact on hand function and quality of life. Although some pharmacological treatments have been shown to confer benefit, new treatments are badly needed: SSc-related DUs are an area of major unmet clinical need. This review focuses on advances in pharmacological management.
Areas covered
DU definition, types of DU, and clinical burden are briefly described and the general approach to multidisciplinary management, followed by a more detailed description of pharmacological management, with particular reference to blocking the endothelin pathway, and supplementing the nitric oxide and prostacyclin pathways. Other aspects of pharmacological management, including analgesia and botulinum toxin injections are also discussed. To inform the review, the MEDLINE database was searched for English-language papers published between 1946 and December 2022 using search terms: ‘systemic sclerosis (scleroderma)' and ‘digital ulcer’ or ‘finger ulcer’ or ‘digital vasculopathy.’
Expert opinion
The key challenges to preventing and treating DUs are to develop and validate reliable, sensitive outcome measures to facilitate clinical trials, and then to undertake trials of emerging new approaches to treatment, including topical therapies and (in early disease) vascular remodeling therapies.
1. Introduction
Digital ulcers (DUs: ulcers of the fingers and toes) are part of the spectrum of systemic sclerosis (SSc)-related digital vasculopathy. SSc is a multisystem connective tissue disease with a major vascular component, and almost all patients with SSc experience Raynaud’s phenomenon (discolouration of the fingers in response to cold or to emotional stress), which is often very severe and which progresses to digital ulceration in approximately 50% of the patients [Citation1], with a minority of patients developing critical ischemia and gangrene [Citation2]. DUs are a major source of pain and disability (including work disability) [Citation3,Citation4], and have a substantial impact on quality of life [Citation5,Citation6]. Amongst patients with SSc, those with DU use significantly more healthcare resources than those without [Citation7,Citation8].
Current treatments are often only partially effective, if at all. SSc-related DUs therefore represent a major unmet clinical need. The aim of this review article is to describe recent advances in pharmacological management. We begin with a brief description of the clinical problem (exemplified by two case histories), followed by the general approach to management, followed by a more detailed description of pharmacological management with a focus on recent advances. The subsequent ‘expert opinion’ section will include future challenges.
1.1. Case histories
1.1.1. Case 1 – Early disease
A 41-year-old male, nonsmoker, developed rapid onset of Raynaud’s phenomenon, initially with uniphasic (white) color change and within a few weeks progressing to bluish discolouration around the tips of both index fingers. He was admitted under the vascular surgical team who excluded large vessel occlusion and he received 5 days of intravenous (IV) iloprost infusions, but he went on to develop ulcers at the tips of both index fingers. After four weeks, he developed puffy fingers with the sensation of his skin being tight over them. Otherwise, he was well with no cardiovascular or gastrointestinal symptoms.
On examination, he had mild sclerodactyly of both index fingers with dry ulcers on the tip of each (). He had two chest wall telangiectases.
Figure 1. Mobile phone images of (a) the left index ulcer, with the dusky color change and (b) right index ulcers with (c) showing very abnormal nailfold capillaries with enlarged capillaries, avascularity and hemorrhage, consistent with the diagnosis of SSc.
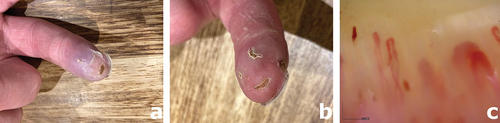
Full blood count and biochemical profile including C-reactive protein (CRP) were all normal. His immunology screen was positive for anti-PM-Scl100. Nailfold capillaroscopy was abnormal with dilated capillaries, areas of avascularity and hemorrhage ().
Diagnosis: SSc, probably limited cutaneous subtype, but with the short history close follow-up will be required to look for any progression.
Management: He had previously been commenced on nifedipine 5 mg short-acting three times daily. This was changed to sustained release nifedipine, titrated up to 40 mg twice daily, with subsequent addition of sildenafil 25 mg three times daily and low dose aspirin.
1.1.2. Case 2 – Established disease
A 65-year-old female, diagnosed as having limited cutaneous SSc with anti-ScL70 antibody positivity 13 years previously, was admitted with an exacerbation of digital ulceration.
Over the years she had had frequent episodes of digital ulceration. Initially, she was treated with yearly IV iloprost infusions combined with a calcium channel blocker. However, three years prior to this admission she started to experience postural hypotension along with presyncope. Different calcium channel blockers were tried but all were associated with similar side effects, and treatment was changed to naftidrofuryl oxalate at a dose of 200 mg three times a day and glyceryl trinitrate (Nitro-Dur) patches along with biannual iloprost infusions.
Two years prior to admission, she developed a necrotic area on the tip of the index finger, and she was commenced on sildenafil, which was titrated up to 50 mg three times a day.
Immediately prior to admission, she had worsening episodes of digital ulceration that became infected, with pus formation, and critical ischemia affecting the tip of her right middle finger and left index, middle and ring fingers.
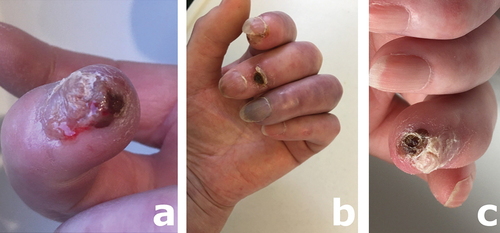
On examination, she had sclerodactyly and a wet-looking ulcer with pus formation at the tip of the right middle finger () and necrotic ulcers at the tips of the left index and ring fingers, and over the extensor aspect of the left middle distal interphalangeal joint ().
Figure 3. The principles of management of ‘uncomplicated’ RP, and of SSc-related digital vasculopathy, which has progressed to digital ulceration. Reproduced from Herrick AL [Citation20] with permission.
![Figure 3. The principles of management of ‘uncomplicated’ RP, and of SSc-related digital vasculopathy, which has progressed to digital ulceration. Reproduced from Herrick AL [Citation20] with permission.](/cms/asset/c20d7390-4d04-439f-9665-58f40997a135/ieop_a_2213434_f0002_oc.jpg)
On admission, hemoglobin was 113 gm/liter, white cell count was 7.3 × 109/l, CRP was slightly raised at 18 mg/l. Wound swabs grew Staphylococcus aureus. Neither plain radiography nor magnetic resonance (MR) imaging of the hands showed any evidence of underlying osteomyelitis.
Diagnosis: Exacerbation of SSc-related digital vasculopathy along with superimposed infection.
Management: She was admitted to hospital for IV iloprost infusions and received one dose of dalbavancin 1.5 g to cover infection after prolonged oral antibiotic courses in the community. Also she was commenced on bosentan. The pain from her finger ulcers was excruciating and she was reviewed by the acute and chronic pain team whilst in hospital.
1.2. Background
Defining DUs. The World Scleroderma Foundation definition of a DU [Citation9] is as follows: ‘Loss of epidermal covering with a break in the basement membrane (which separates dermis from epidermis). It appears clinically as visible blood vessels, fibrin, granulation tissue and/or underlying deeper structures (e.g. muscle, ligament, fat) or as it would appear on debridement.’ However, the definition of DUs has been the subject of much debate [Citation10] and the lack of agreement by experts on what is and what is not a DU has hampered drug development. This is because the number of DUs, ‘net DU burden’ and/or DU healing (or a combination thereof) is/are usually the primary or main secondary end-point(s) in clinical trials. The subjectivity inherent in clinician opinion poses challenges when assessing treatment efficacy.
Location/’types’ of DUs. As exemplified in the case histories () DUs occur mainly at the fingertips or over the extensor surfaces of the fingers, the latter especially in patients with contractures when the skin is stretched over (for example) the proximal or distal interphalangeal joints. DUs can also occur at other sites for example, at the nailbed, or on the radial or ulnar aspects of the finger. Although it was previously thought that fingertip ulcers were mainly ischemic, and extensor ulcers ‘mechanical,’ it is likely that all ulcers have an ischemic component [Citation11] and this is relevant to pharmacological management because increasing finger blood flow is a cornerstone of management. Most of the large-scale randomized controlled trials (RCTs) of SSc-related digital ulceration have confined themselves to DUs occurring at or distal to the proximal interphalangeal joints [Citation12–16], and in some studies there has been a further restriction to include only volar surface DUs [Citation13,Citation16]. Calcinosis-related ulcers (ulcers overlying deposits of calcium containing salts, which commonly occur in patients with SSc especially in the fingers) are often particularly slow to heal [Citation17], and tend to be excluded from clinical trials. However, there is an argument for allowing the inclusion of patients with all types of DUs into trials [Citation11].
Burden of DUs. The pain and disability associated with DUs have a major impact on hand function, and DUs are disfiguring [Citation5]. Also, DUs frequently become ‘complicated’ by infection (most often with Staphylococcus aureus (as in Case 1), including Methicillin-Resistant [MRSA] [Citation18]. This infection can spread to bone. Giuggioli et al [Citation19] reported osteomyelitis on plain radiographs in 19 of 248 patients with SSc (45 of whom had infected DUs). For all these reasons, DUs have a major impact on quality of life, and this burden of disease has become increasingly recognized over the last 10 years. Although there have been recent advances in treatment as described below, new effective treatments are badly needed.
2. General approach to treatment
The current approach to management is illustrated in , a flow chart summarizing the treatment firstly of Raynaud’s phenomenon and secondly of digital ulceration [Citation20]. The two are combined deliberately because optimizing treatment of Raynaud’s phenomenon is a very important first step in the prevention and treatment of SSc-related digital ulceration. Best practice management of SSc-related Raynaud’s phenomenon includes life-style measures (including patient education) and vasoactive drug treatments (). The next steps in the prevention and treatment of SSc-related digital ulceration are:
Figure 2. Necrotic, infected ulcer at the tip of the right middle finger (a), and photograph of the left had showing an ulcer at the tip of the index finger, an ulcer overlying the middle finger distal interphalangeal joint, and a dusky colored, critically ischemic ring finger which had an ulcer at the fingertip not seen on this view but demonstrated (one month later) in (c).
Patient education, emphasizing to patients how they must seek medical advice as soon as a DU develops. Early intervention is likely to save digits by allowing prompt treatment before an ulcer enlarges and (very possibly) becomes infected.
Specialist nursing care, often including input from the tissue viability team [Citation21].
Drug treatment. This will be described in the next section and includes oral, IV and topically applied treatments.
Procedural and surgical treatments. These may be indicated for a minority of patients with DUs refractory to other treatments. Botulinum toxin injections will be described in the next section, as these come within the remit of ‘pharmacological therapies.’ Surgical intervention may be appropriate [Citation22], most commonly surgical debridement of necrotic tissue. The approach to debridement varies between centers [Citation23] and in some centers is undertaken by a skilled nursing team: prior application of lidocaine may lessen the pain of the procedure [Citation24]. Palmar (digital) sympathectomy has attracted considerable attention over the last 10–15 years but should only be undertaken in specialist centers [Citation22,Citation25]. Autologous fat grafting has been proposed as another therapeutic option [Citation26], and other procedural treatments have also been advocated or are under investigation, including hyperbaric oxygen [Citation27] and allogeneic mesenchymal stem cell therapy [Citation28]. The evidence base for most of these treatments is weak, at least in part reflecting the challenges of clinical trials of procedural therapies [Citation29].
2.1. Pharmacological management
In patients with SSc, structural as well as functional blood vessel changes occur. This is an important point in relation to current and future pharmacological management. The mechanism of action of most currently recommended drug treatments aims to redress the imbalance, which occurs in patients with SSc between vasoconstriction and vasodilation: in SSc, vasoconstriction predominates over vasodilation. Therefore, currently used drugs either block vasoconstriction or increase vasodilation. In other words, they treat the functional vascular abnormality. As discussed later, future drug development should also address the structural vascular abnormality, ideally at an early (preventative) stage.
Pharmacological management will be considered under the following subheadings:
Vasoactive therapies
Other pharmacological therapies (excluding procedural treatments) including antibiotic therapy and analgesia.
‘Procedural’ pharmacological therapies
Treatment of the acute DU (this can be a medical emergency).
2.1.1. Vasoactive therapies
The first step, as already highlighted, is to optimize treatment for Raynaud’s phenomenon. Several different vasoactive therapies have been advocated for the treatment of SSc-related Raynaud’s phenomenon (), namely calcium channel blockers [Citation30], phosphodiesterase type 5 (PDE5) inhibitors [Citation31], angiotensin II receptor blockers, alpha-adrenergic blockers, selective serotonin reuptake inhibitors (SSRIs), topical nitrate therapy, and IV prostanoid therapy. Yet despite the large number of vasoactive therapies, which have been advocated for secondary Raynaud’s phenomenon, a recent meta-analysis suggested that only calcium channel blockers and PDE5 inhibitors are likely to be helpful, and even for these the level of evidence was low [Citation32]. The 2017 European League Against Rheumatism (EULAR) recommendations for SSc-related Raynaud’s phenomenon [Citation33] suggest a calcium channel blocker as first line, and also recommend consideration of PDE5 inhibitors, IV iloprost, and fluoxetine, although this last did not have the same level of support. Although practice varies between centers and between countries [Citation34], SSc experts have for several years favored PDE5 inhibitors as second-line pharmacological treatment for both SSc-related Raynaud’s phenomenon and digital ulceration [Citation35,Citation36], after a calcium channel blocker as first-line. In the authors’ experience sustained release preparations of calcium channel blockers (e.g. sustained release nifedipine, or amlodipine) are better tolerated than shorter-acting forms, and because of the propensity for vasodilatory side effects our recommendation is to commence in low dose and gradually up-titrate to the maximum tolerated dose (as in Case 1).
Recent advances in the treatment of SSc-related DU have to some extent paralleled those in the treatment of pulmonary arterial hypertension (PAH), reflecting a shared pathophysiology underpinning the vascular abnormalities associated with PAH and with SSc-related digital vasculopathy. Drug development/treatment studies have focussed on three pathways: (blocking) the endothelin pathway, (supplementing) the nitric oxide (NO) pathway and (supplementing) the prostacyclin pathway (). A meta-analysis as far back as 2013 [Citation37] lent support for this approach: PDE5 inhibitors, bosentan and IV iloprost all conferred benefit. EULAR recommendations [Citation33] and the British Society for Rheumatology (BSR) guideline [Citation38] both recommend consideration of a PDE5 inhibitor, IV prostanoid therapy, and bosentan in the pharmacological treatment of SSc-related DUs (exemplified in Case 2, who is now on treatment with all of these). Bosentan is currently the only approved drug for the prevention of SSc-related DUs.
Table 1. Summary of current and possible future approaches to blocking the ET-1 pathway, supplementing the NO pathway and supplementing the prostacyclin pathway.
Treatment advances focussing on each of these three pathways will be considered in turn, always remembering that these treatments are additive to first-line treatment with a calcium channel blocker. Some treatments mentioned should still be considered experimental.
2.1.1.1. Blocking the endothelin pathway
Endothelin-1 (ET-1) is a powerful vasoconstrictor, and is overexpressed in the skin of patients with SSc. As well as being vasoconstrictive, ET-1 is profibrotic and therefore blocking its action has sound rationale in SSc. ET-1 receptor antagonists are now widely used in the treatment of PAH [Citation39]. Bosentan, a dual ET-1 receptor antagonist (‘dual’ because it blocks both the ET-1A and ET-1B receptors) is licensed in many countries, including the UK, for prevention of SSc-related DUs in patients with previous DUs. The main evidence underpinning its use comes from the RAPIDS-1 and RAPIDS-2 studies [Citation12,Citation13] which were both RCTs comparing bosentan to placebo. Both RAPIDs studies showed a benefit from bosentan over placebo in terms of prevention of new DUs, but not in terms of DU healing. Following on from the RAPIDs studies, the DUAL-1 and DUAL-2 studies [Citation15], in 289 and 265 patients, respectively, examined the effects of macitentan, another dual ET-1 receptor antagonist, in patients with SSc-related DUs. However, no beneficial effect was observed compared to placebo: macitentan in a dose of either 3 mg daily or 10 mg daily did not reduce the number of new DUs.
There have been no further large-scale RCTs examining ET-1 receptor antagonism in SSc-related digital ulceration. A recent open-label trial from Vietnam [Citation40] suggested benefit from 16 weeks’ bosentan compared to sustained-release nifedipine 20 mg bd, in terms of number of new DUs, although patient numbers were small. Now that bosentan is out of patent it will almost certainly be used more widely. The most recent NHS England commissioning policy for the treatment of SSc-related DUs [Citation41] recommends the use of bosentan after sildenafil but prior to IV prostanoid therapy. This policy has the advantage of being convenient for patients, reducing the need for hospitalization for IV prostanoids. There has been relatively little research comparing ET-1 receptor antagonists to PDE5 inhibitors. An observational study of 63 patients from Korea [Citation42] concluded that time to DU healing was similar between the 49 patients treated with a ET-1 receptor antagonist and the 11 treated with a PDE5 inhibitor, but that patients on an ET-1 receptor antagonist developed fewer new DU [Citation42]. The study design, and the small numbers of patients included, mean that results should be interpreted with caution. However, the study serves to emphasize the lack of RCTs comparing ET-1 receptor antagonists to PDE5 inhibitors.
2.1.1.2. Supplementing the nitric oxide pathway
NO is a powerful vasodilator. It increases the concentration of cyclic guanosine-5-monophosphate (cGMP) which causes vasodilation through vascular smooth muscle relaxation. The NO pathway can be supplemented in different ways: by prescribing nitrates (including topically), via PDE5 inhibition (PDE5 inhibitors inhibit degradation of cGMP), through stimulating soluble guanylate cyclase (e.g., with riociguat) which increases synthesis of cGMP, and via L-arginine, which is the substrate for the different NO synthase isoforms [Citation43]. Current and possible future approaches to supplementing the NO pathway are summarized in .
Nitrate therapy. Oral nitrates are not generally recommended for SSc-related digital vasculopathy. Although applying glyceryl trinitrate (GTN), an NO donor, by patch applied to the chest wall (i.e. for its systemic effect) was associated with improvement in Raynaud’s phenomenon [Citation44], this was at the expense of adverse effects. Systemic nitrate therapy has not been evaluated in patients with SSc-related DUs. Because DUs are a local problem (i.e. local to the finger or toe), topical administration is an attractive option, the rationale being that low doses applied to the digit might increase blood flow locally without causing systemic adverse effects. A local (as opposed to a systemic) vasodilatory effect was well demonstrated in a physiological study of topical GTN [Citation45], when blood flow increased in the treated digits in patients with SSc as well as in patients with primary (idiopathic) Raynaud’s phenomenon. A later physiological study showed that local application of GTN directly to DUs improved blood flow and was well tolerated [Citation46]. Topical nitrates are currently seldom prescribed for SSc-related digital vasculopathy but deserve further study.
PDE5 inhibitors. As stated earlier, PDE5 inhibitors have long been advocated in the treatment of SSc-related Raynaud’s phenomenon [Citation31]. PDE5 inhibitors are thought to confer specific benefit in those patients with SSc in whom digital vasculopathy has progressed to ulceration. The largest study to address this issue was the SEDUCE study [Citation14], an RCT of parallel group design, which compared sildenafil to placebo in 83 patients with 192 DUs. Although the primary endpoint (time to ulcer healing) was not met, there was some benefit from sildenafil: DU healing rate was greater in the sildenafil group compared to in the placebo group at week 8 (p = 0.01) and week 12 (p = 0.03). As already stated, the NHS England commissioning policy for SSc-related DU [Citation41] recommends adding a PDE5 inhibitor as the next step after ‘standard medical therapy for Raynaud’s phenomenon,’ which is usually with a calcium channel blocker. Sildenafil was added to nifedipine in Case 1.
One question is to what extent clinical practice has changed in response to these findings and recommendations, i.e., how much has the use of PDE5 inhibitors increased over the last 7 years? Results from a German survey, published in 2016 [Citation47], showed that prior to 2013 only a small minority of patients with SSc-related DUs were prescribed a PDE5 inhibitor (in the order of 6% after 2009), and a study from Greece [Citation48], conducted between 2016 and 2018, suggested that only a small proportion of patients with SSc was prescribed a PDE5 inhibitor. Analysis of data from patients with current or previous DUs, seen between March 2013 and November 2016 as part of the European multicentre DeSScipher study [Citation49] showed that 216 of 905 patients (23.9%) were on a PDE-5 inhibitor. It is likely that if similar surveys/studies were conducted now, then the numbers of patients on PDE5 inhibitors would be higher.
PDE5 inhibitors can be prescribed in combination with a calcium channel blocker, and with bosentan [Citation50,Citation51], although there have been no clinical trials of combination therapy. If Case 1 develops new ulcers, the plan is to commence bosentan. Analysis of data from the DeSScipher study [Citation49], reported that only 399 of 905 patients (44.1%) were on two or more vasoactive treatments, with 104 of 905 patients (11.5%) on three or more vasoactive agents. This may seem surprising, given the level of morbidity from DUs. Trials of combination therapy would be difficult to mount, although not impossible. Pending these, it seems sensible to learn from the experience of PAH and to try combination therapies, as advocated in the NHS England clinical commissioning policy [Citation41].
Soluble guanylate cyclase stimulation. The soluble guanylate cyclase stimulator riocuguat did not confer benefit over placebo in a small pilot study of 19 patients with SSc-related DUs [Citation52], treated for 16 weeks, in terms of ‘net ulcer burden.’ As discussed by the authors, there were a number of reasons for this lack of benefit, and a larger-scale study is required to examine whether longer-term treatment with riociguat might reduce DU burden.
L-arginine. There have been no clinical trials of L-arginine, but this is another possible approach to supplementing the NO pathway, which has been advocated in patients with Raynaud’s phenomenon and which warrants further study [Citation43].
2.1.1.3. Supplementing the prostacyclin pathway
IV prostanoid therapy is well established in the treatment of SSc-related DUs, and IV prostanoids ‘in particular iloprost’ are included in the EULAR recommendations for the treatment of active DU in patients with SSc [Citation33], as well as in the BSR guideline [Citation38]. Iloprost, a prostacylin analogue, is most widely prescribed, preventing DUs and promoting DU healing [Citation53,Citation54]. Iloprost is thought to exert beneficial effects not only through vasodilation but also through its anti-platelet effects, and there are probably other mechanisms of action that may explain the prolonged clinical benefit, which many patients experience, including stabilization of endothelial cell adherens junctions, normalization of angiogenesis, and inhibition of endothelial-to-mesenchymal transition [Citation55].
Other IV prostanoids used for the treatment of SSc-related digital vasculopathy are epoprostenol, which is used preferentially in the US [Citation56,Citation57] and alprostadil [Citation58]. IV prostanoid therapy for SSc-related DU/digital vasculopathy tends to be used in two clinical situations: first in the acute setting when a patient presents with a very painful DU, sometimes with associated critical ischemia, requiring hospitalization as discussed below, and second in the more chronic setting when the DU (or DUs) is/are unresponsive to other therapies. IV prostanoids are also advocated by many clinicians as prophylaxis against the progression of digital vasculopathy/development of DUs in patients ‘at risk’ (i.e. with a history of DUs and very severe Raynaud’s phenomenon), especially at the onset of winter.
Disadvantages of IV prostanoid infusions are that they are frequently associated with systemic adverse events [Citation59–62] and their administration requires hospitalization, the latter disadvantage being particularly relevant in the ‘Covid era’ during which many patients are very reluctant to be admitted. For this reason, many clinicians try to avoid hospitalization/IV prostanoid therapy and therefore maximize other therapies, stressing to patients that they should seek medical advice as soon as a DU develops, in order to ensure early optimization of oral vasodilator therapy, often in combination with bosentan, and along with other aspects of management as required (e.g. antibiotic therapy, wound care, debridement).
In recent years, several papers have described and evaluated different IV prostanoid regimens, because practice varies considerably between countries and between clinicians [Citation60,Citation61,Citation63–65]. The 5-day regimen used in the double-blind RCT comparing IV iloprost to placebo [Citation53] is favored by many clinicians. However, longer durations of therapy have also been advocated [Citation65], as have monthly infusions [Citation64]. Ingegnoli et al [Citation64] provide a detailed description of previous studies of IV iloprost administration in patients with SSc-related DUs and an expert consensus opinion, which was for a 1–3-day monthly regime for DU healing, and a 1-day monthly regime for DU prevention. However, most of the small number of participating experts (8 of 10), were from mainland Europe [*64] and so the findings may not reflect international opinion and practice: the authors concluded that further research was required before firm recommendations could be made.
Different solutions have been proposed and investigated to obviate the need for hospitalization and the inconvenience to patients of having very restricted mobility during IV prostanoid infusions. Portable syringe pumps have been advocated, and administering iloprost in the patient’s own home [Citation66,Citation67], although many clinicians would have concerns about this approach given iloprost’s potential for adverse effects including cardiovascular events. Over the years, there have been several trials of oral prostanoids, but none has demonstrated convincing benefit. Most recently an RCT comparing oral treprostinil, a prostacyclin analogue, to placebo [Citation16] in a 20 week study of parallel group design in 147 patients, failed to meet its primary endpoint (change in net ulcer burden). However, there was a trend for improvement (−0.43 ulcers following treprostinil compared to −0.1 following placebo), and a subsequent retrospective analysis of data from 51 patients following discontinuation of treprostinil after an open label extension showed that patients deteriorated during the 12 months following discontinuation, suggesting that treprostinil had been beneficial [Citation68]. These findings raise the question as to whether trial endpoints were reliable and sensitive to change (discussed under ‘expert opinion’): if not, then a clinically relevant benefit could have been missed.
2.1.2. Other pharmacological therapies (excluding procedural treatments)
Antibiotics. As stated earlier, DUs often become infected, and so antibiotic therapy is an important aspect of management, whenever possible guided by microbiology results from ulcer swabs. If the underlying bone has become infected [Citation19], prolonged antibiotic treatment will be required.
Analgesics. The pain from DUs can be excruciating (as exemplified in Case 2), often keeping the patient awake at night. Adequate analgesia is an important aspect of management. Some patients will require opiates in the short-term. Very few studies have examined analgesia for DUs: two case reports suggest that a prolonged-release oxycodone/naloxone combination may be beneficial in some patients [Citation69] and highlight that this is a neglected area of research.
Statin therapy. There is a sound theoretical rationale for statins in SSc-related digital vasculopathy. However, these have been relatively little studied, with one trial comparing atorvastatin to placebo, published in 2008 [Citation70]. At present, there is insufficient evidence to routinely recommend statin therapy.
Antiplatelet agents. There is no good evidence base from clinical studies to support the use of antiplatelet therapy for SSc-related DU. However, platelet activation is well recognized in patients with SSc, and so there is good theoretical justification, always bearing in mind the risk of gastro-intestinal side effects in a patient cohort at high risk of upper gastro-intestinal disease. A recent EUSTAR (European Scleroderma Trials and Research Group) study reported that 90 of 3710 patients with SSc, studied between 2013 and 2019 and of whom 487 had DU, were on platelet inhibitors [Citation71], suggesting that only a minority of clinicians prescribe antiplatelet agents in patients with SSc.
2.1.3. ‘Procedural’ pharmacological therapies
There has been considerable recent interest in botulinum toxin injections. The last 10 years have seen a large number of anecdotal reports, small series, and review articles (recent reviews include [Citation72–74]) describing the use of botulinum toxin injections in patients with SSc and severe digital ichaemia, including with DUs, although many of the reports have focussed on severe Raynaud’s phenomenon as opposed to DUs. Most have reported on botulinum toxin A but botulinum B has also been studied [Citation75]. The mechanism of action of botulinum toxin injections is thought to be via blocking of sympathetic innervations [Citation76]. There have been no RCTs examining the use of botulinum toxin injections specifically for SSc-related DUs. A recent open label (non-randomized) study comparing botulinum toxin injections and prostanoid infusions [Citation77] suggested that ulcer healing occurred with both. Neither of two placebo-controlled RCTs examining botulinum toxin for SSc-related Raynaud’s phenomenon showed definite benefit. In the first RCT, which was in 40 patients [Citation78], and in which botulinum toxin A was injected into one hand and sterile saline into the other, the primary endpoint (change in blood flow from baseline to 1-month follow-up, assessed with laser Doppler imaging) was not reached, although there was some improvement in secondary endpoints. A more recent placebo-controlled trial [Citation79] in 90 patients (46 patients treated with botulinum toxin injections into both hands, 44 with placebo injections) also failed to demonstrate benefit.
Muscle weakness is a potential adverse effect of botulinum toxin injections [Citation74]. As summarized by Ennis et al [Citation74], the different injection protocols and outcome measures used by different clinicians complicate comparisons between studies, and make it difficult to make recommendations as to which patients are most likely to benefit. Interestingly, in the series of 20 patients reported by Goldberg et al [Citation80], which included 10 patients with SSc, the patients most likely to benefit were those with reversibility in their perfusion deficit (as assessed by photoplethysmography): this observation might help in selection of patients most likely to benefit.
2.1.4. Treatment of the acute DU
This is a medical emergency. Typically the patient presents with a very painful ulcer, which may be necrotic and/or infected. In this situation, there is no time to wait for the effects of maximizing oral vasodilatory therapy, and the patient may well already be on optimal oral therapy. In this situation, the patient should be admitted for IV prostanoid therapy, analgesia and (where appropriate) antibiotics and/or local debridement and/or (if other measures have failed, bearing in mind the weakness of the evidence base) botulinum toxin injections. Prior to discharge from hospital, the patient’s drug treatment should be reviewed with a view to optimizing oral vasodilator therapy and consideration of bosentan if the patient is not already on this.
3. Conclusion
Pharmacological therapies are a very important aspect of management of SSc-related DU. DUs all have an ischemic component, and at present the main aims of pharmacological therapy are first to try to prevent DUs by optimizing blood flow to the fingers in all patients with SSc, and second to identify and treat DUs as early as possible, to minimize tissue injury. An ‘open door’ policy for early review of finger lesions in patients with SSc will ensure timely treatment.
Recent advances directly relevant to the treatment have included increased awareness of the role of PDE5 inhibitors and of bosentan (and that these can be used in combination), and the recognition of the need to optimize and standardize regimes for IV prostanoid administration, which remains a cornerstone of treatment.
Perhaps disappointingly, there have been no studies in the last 2 years with major implications for pharmacological management, and a search on ClinicalTrials.gov suggests that there are very few studies of SSc-related DUs ‘in the pipeline.’ Again on a negative note, RCTs of botulinum toxin have not shown definite benefit in SSc-related Raynaud’s phenomenon, although this approach to treatment continues to attract considerable interest for SSc-related DUs.
4. Expert opinion
4.1. What are the key findings and weaknesses in the research done so far?
Key recent findings, from a meta-analysis, are that calcium channel blockers and PDE5 inhibitors improve symptoms of secondary Raynaud’s phenomenon, albeit conferring only modest benefit. Therefore by implication, calcium channel blockers and PDE5 inhibitors are likely to prevent DUs, by improving blood flow, although this suggestion is unproven. A ‘weakness’ of research so far is that there have been few recent RCTs in patients with SSc-related DUs. Reasons for this include the complexity of clinical trials of treatment for SSc-related DU. This complexity relates to the lack of reliable outcome measures for DUs, and the need for phase 3 studies to be multinational, and therefore expensive, given the rarity of SSc. The RCT of oral treprostinil [Citation16] epitomizes the complexities: many investigators (and patients) were convinced that oral treprostinil was effective, yet the primary endpoint was not met. It is highly likely that in this and other studies, negative findings could be due to inadequacy of current outcome measures, because these are subjective and/or insensitive to change.
4.2. What is the ultimate goal?
The ultimate goal is to develop vascular remodeling therapies, which can be prescribed at an early stage in the SSc disease process, and which will prevent DUs. A more immediate goal is to develop treatments, which are more effective than those currently available to reduce DU burden, including the healing of existing DUs.
4.3. What is needed to achieve this goal and what is the biggest challenge in this goal being achieved?
There are two main challenges/hurdles to be overcome, namely the development of:
Better outcome measures for DUs.
New approaches to treatment (including preventative therapies). These approaches could include repurposing of therapies, and revisiting previously suggested lines of treatment, the benefits of which were perhaps not proven because of inadequacy of outcome measures.
4.4. What are the most likely developments in the coming years?
4.4.1. Outcome measures
Work is ongoing to develop these, including composite scores [Citation81]. Patient reported outcome (PRO) measures are gaining favor, and capture the ‘feels’ and ‘functions’ of the US Food and Drug Administration requirements in assessing outcome. The Hand Disability in Systemic Sclerosis-Digital Ulcers (HDISS-DU) PRO instrument [Citation82], which is both reliable and sensitive to change, is likely to become widely adopted in future clinical trials, especially in large-scale, later phase studies examining treatment response over time. A disadvantage of PRO measures is that they are subjective. DUs are highly visible, and so it could be argued that they should be easy to measure, although measurement is less simple than it sounds, and researchers have grappled with this problem for years, with attempts for example to measure the surface area of single and multiple DUs in a study reported in 1985 [Citation83]. Clinical photography offers a way forward here [Citation84] although this too has levels of complexity because two dimensional size will be dependent on various factors including the angle at which the photograph is taken. Mobile phone photography of DUs [Citation85] has been shown to be feasible and would allow patients to take frequent photographs in their own home, bringing the potential of capturing change much more precisely than previously possible. For example, in the SEDUCE study of sildenafil [Citation14], patients were assessed 4-weekly and therefore time to ulcer healing would be the same for a patient who ‘healed’ at 29 days (28 plus 1 day) as a patient who healed at 55 days (56 minus 1 day). Mobile phone photography would have allowed (say) twice weekly assessment throughout the study, potentially allowing much more accurate timing of DU healing.
Several other methods of measuring DUs have been proposed, including with ultrasound [Citation86,Citation87], with laser speckle contrast imaging and with laser Doppler imaging [Citation88,Citation89]. If found to be sensitive and reliable, these measures should be applicable in small proof-of-concept studies, allowing these to be adequately powered with smaller patient numbers than hitherto possible, and therefore making Phase 2 trials more feasible.
4.4.2. New approaches to treatment and areas of special interest
In our opinion, future clinical trials of drug treatments should focus on:
Topical treatments for DU healing. DUs are a local problem, and local treatments applied directly to the fingers would seem much preferable to systemic therapies, which bring the problem of systemic vasodilatory (and other) adverse effects. Although some recent small studies have examined local treatments, including the topical application of tadalafil cream (a PDE5 inhibitor) [Citation90], and administration by iontophoresis of treprostinil hydrogel [Citation91], much more investment in researching topical/local treatments is required. A recent retrospective analysis of patients with SSc and DUs refractory to standard treatments (including opiates) suggested that topical cannabidiol conferred benefit in terms of pain reduction and DU healing [Citation92]: a randomized controlled is now required to investigate this further. Other exciting areas of research include development of topically applied small molecular weight NO-releasing PDE5 inhibitors [Citation93].
Investigating new treatments. As our understanding of SSc pathophysiology increases, so too does the potential for new targeted treatments. Potential new approaches to treatment, for example with JAK inhibitors [Citation94,Citation95] need to be followed up with Phase 2 and then (if appropriate) Phase 3 trials. Admittedly (as discussed above) RCTs of DUs are challenging, but on a positive note these are now feasible due to increased international networking, demonstrated by the DUAL studies of macitentan [Citation15]. Numbers of patients available to participate in studies are finite, and so it is imperative (as discussed above) to refine our ability to undertake high-quality Phase 2 trials so that only the most promising candidates are taken forward to Phase 3. ‘New treatments’ should also include revisiting previously suggested approaches, using more robust clinical trial design than was possible several decades ago. For example, platelet inhibitors and drugs blocking the action of serotonin [Citation96], deserve to be revisited. Studies of combination therapies e.g. the combination of a PDE5 inhibitor and an ET-1 antagonist, learning from experience from PAH trials, would also be of interest, although these will be difficult to fund.
Treatment to prevent progression (and potentially reverse) the structuralabnormalities of SSc-related digital vasculopathy. This is the ultimate goal: to prevent progression of the microvascular and digital arterial structural abnormality which leads to the poor digital blood supply, which in turn contributes to DU formation. Although it has been suggested that prostanoids, PDE5 inhibitors, ET-1 receptor antagonists and potentially some other vasoactive therapies might confer vascular protection [Citation97,Citation98] and through a variety of different mechanisms, including reduction of oxidative stress with sildenafil [Citation99] and stabilizing adherens junctions with iloprost [Citation55], prospective studies are required to address this issue. These studies will be complex as they will need to be large scale, identifying patients with early disease and (probably, although different study designs are possible) randomizing them to different treatment arms and including novel endpoints such as quantitative nailfold capillaroscopy [Citation100] which have the potential to detect subclinical change over time.
In summary, much has been achieved in the last 10 years to reduce disease burden in patients with SSc-related DUs, not least by recognizing that burden and the importance of ensuring that patients have access to a skilled multidisplinary team. However, much remains to be done: this should be achievable through advances in translational research and directing efforts into well-designed clinical trials.
Article highlights
SSc-related DUs are an unmet clinical need: DUs are painful, disabling and disfiguring with a major impact on quality of life.
Management is multidisciplinary, and pharmacological treatment plays a very important role.
Most recommendations for best practice pharmacological treatment suggest optimizing treatment for Raynaud’s phenomenon (calcium channel blockers are first line), followed by a PDE5 inhibitor, bosentan, and IV prostanoid therapy.
Advances in therapy are likely to be through new approaches to blocking the endothelin pathway and/or supplementing the NO and/or prostacyclin pathways, including the use of topical therapies.
Development of reliable and sensitive outcome measures for ‘DU burden’ is key to facilitating early and later phase clinical trials.
Declaration of interest
A L Herrick has received consultancy fees from Arena, Boehringer-Ingelheim, Camurus, CSL Behring, Galderma and Gesynta Pharma, speaker fees from Actelion and Janssen, and research funding from Actelion and Gesynta Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Hughes M, Allanore Y, Chung L, et al. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nature Reviews Rheumatol. 2020;16(4):208–221. DOI:10.1038/s41584-020-0386-4
- Nihtyanova SI, Brough GM, Black CM, et al. Clinical burden of digital vasculopathy in limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2008;67(1):120–123.
- Matucci-Cerinic M, Krieg T, Guillevin L, et al. Elucidating the burden of recurrent and chronic digital ulcers in systemic sclerosis: long-term results from the DUO Registry. Ann Rheum Dis. 2016;75(10):1770–1776. DOI:10.1136/annrheumdis-2015-208121
- Castellvi I, Eguiluz S, Escudero-Contreras A, et al. LAUDES Study: impact of digital ulcers on hand functional limitation, work productivity and daily activities, in systemic sclerosis patients. Rheumatol Int. 2019;39(11):1875–1882. DOI:10.1007/s00296-019-04436-z
- Hughes M, Pauling JD, Jones J, et al. Multicenter qualitative study exploring the patient experience of digital ulcers in systemic sclerosis. Arthritis Care Res. 2020;72(5):723–733. DOI:10.1002/acr.24127
- van Leeuwen NM, Ciaffi J, Liem SIE, et al. Health-related quality of life in patients with systemic sclerosis: evolution over time and main determinants. Rheumatology. 2021;60(8):3646–3655. DOI:10.1093/rheumatology/keaa827
- Morrisroe K, Stevens W, Sahhar J, et al. Digital ulcers in systemic sclerosis: their epidemiology, clinical characteristics, and associated clinical and economic burden. Arthritis Res Ther. 2019;21(1):299. DOI:10.1186/s13075-019-2080-y
- Nevskaya T, Calderon LM, Baron M, et al. on behalf of the canadian scleroderma research group. Health care utilization in systemic sclerosis patients with digital ulcers. Arthritis Care Research. 2022. doi:10.1002/acr.24902.
- Suliman AS, Bruni C, Johnson SR, et al. Defining skin ulcers in systemic sclerosis: systematic literature review and proposed world scleroderma foundation (WSF) Definition. J Scleroderma Relat Disord. 2017;2(2):115–120. DOI:10.5301/jsrd.5000236
- Hughes M, Tracey A, Bhushan M, et al. Reliability of digital ulcer definitions as proposed by the UK scleroderma study group: a challenge for clinical trial design. J Scleroderma Relat Disord. 2018;3(2):170–174. DOI:10.1177/2397198318764796
- Hughes M, Murray A, Denton CP, et al. Should all digital ulcers be included in future clinical trials of systemic sclerosis-related digital vasculopathy? Med Hypotheses. 2018;116:101–104.
- Korn JH, Mayes M, Matucci-Cerinic M, et al. Digital ulcers in systemic sclerosis. Prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50(12):3985–3993. DOI:10.1002/art.20676
- Matucci-Cerinic M, Denton CP, Furst DE, et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2011;70(1):32–38. DOI:10.1136/ard.2010.130658
- Hachulla E, Hatron P-Y, Carpentier P, et al. Efficacy of sildenafil on ischaemic digital ulcer healing in systemic sclerosis: the placebo controlled SEDUCE study. Ann Rheum Dis. 2016;75(6):1009–1015. DOI:10.1136/annrheumdis-2014-207001
- Khanna D, Denton CP, Merkel PA, et al. Effect of macitentan on the development of new ischemic digital ulcers in patients with systemic sclerosis. DUAL-1 and DUAL-2 randomized clinical trials. JAMA. 2016;315:1975–1988.
- Seibold JR, Wigley FM, Schiopu E, et al. Digital ulcers in SSc treated with oral treprostinil: a randomized, double-blind, placebo-controlled study with open-label follow-up. J Scleroderma Relat Disord. 2017;2(1):42–49. DOI:10.5301/jsrd.5000232
- Barsotti S, Venturini V, Di Battista M, et al. The impact of skin calcinosis on digital ulcers in patients with SSc: clinical and prognostic stratification using the “wound bed score”. Int Wound J. 2020;17(6):1783–1790. DOI:10.1111/iwj.13467
- Giuggioli D, Magnani L, Spinella A, et al. Infections of scleroderma digital ulcers: a single center cohort retrospective study. Dermatol Reports. 2021;13:9075.
- Giuggioli D, Manfredi A, Colaci M, et al. Osteomyelitis complicating scleroderma digital ulcers. Clin Rheumatol. 2013;32(5):623–627. DOI:10.1007/s10067-012-2161-7
- Herrick AL. Raynaud’s phenomenon and digital ulcers: advances in evaluation and management. Curr Opin Rheumatol. 2021;33(6):453–462.
- Lebedoff N, Frech TM, Shanmugam VK, et al. Review of local wound management for scleroderma-associated digital ulcers. J Scleroderma Relat Disord. 2018;3(1):66–70. DOI:10.5301/jsrd.5000268
- Muir L, Herrick AL. Surgical approaches including sympathectomy. In: In: Matucci-Cerinic M Denton Ceditors Atlas of Ulcers in Systemic Sclerosis 2019. Springer;Cham:10.1007/978-3-319-98477-3_21
- Hughes M, Alcacer-Pitarch B, Gheorghiu AM, et al. Digital ulcer debridement in systemic sclerosis: a systematic literature review. Clin Rheumatol. 2020;39(3):805–811. DOI:10.1007/s10067-019-04924-4
- Braschi F, Bartoli F, Bruni C, et al. Lidocaine controls pain and allows safe wound bed preparation and debridement of digital ulcers in systemic sclerosis: a retrospective study. Clin Rheumatol. 2017;36(1):209–212. DOI:10.1007/s10067-016-3414-7
- Momeni A, Sorice SC, Valenzuela A, et al. Surgical treatment of systemic sclerosis - is it justified to offer peripheral sympathectomy earlier in the disease process? Microsurgery. 2015;35:441–446.
- Daumas A, Magalon J, Jouve E, et al. Long-term follow-up after autologous adipose-derived stromal vascular fraction injection into fingers in systemic sclerosis patients. Current Res Translational Med. 2017;65(1):40–43. DOI:10.1016/j.retram.2016.10.006
- Ahijon-Lana M, Baragano-Ordonez E, Veiga-Cabello R, et al. Treatment of raynaud phenomenon and ischemic ulcers associated to systemic sclerosis with hyperbaric oxygen. Reumatol Clin. 2022;18(4):246–248. DOI:10.1016/j.reumae.2021.05.004
- van Rhijn-Brouwer FCC, Gremmels H, Fledderus JO, et al. A randomised placebo-controlled double-blind trial to assess the safety of intramuscular administration of allogeneic mesenchymal stromal cells for digital ulcers in systemic sclerosis: the MANUS Trial protocol. BMJ Open. 2018;8(8):e020479. DOI:10.1136/bmjopen-2017-020479
- Costedoat I, Masson M, Barnetche T, et al. Locoregional treatments for digital ulcers in systemic sclerosis: a systematic review. Acta Derm Venereol. 2021;101(6):adv00478. DOI:10.2340/00015555-3839
- Rirash F, Tingey PC, Harding SE, et al. Calcium channel blockers for primary and secondary Raynaud’s phenomenon. Cochrane Database Syst Rev. 2017;12(12):CD000467. DOI:10.1002/14651858.CD000467.pub2
- Roustit M, Blaise S, Allanore Y, et al. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud’s phenomenon: systematic review and meta-analysis of randomised trials. Ann Rheum Dis. 2013;72:1696–1699.
- Khouri C, Lepelley M, Bailly S, et al. Comparative efficacy and safety of treatments for secondary Raynaud’s phenomenon: a systematic review and network meta-analysis of randomised trials. Lancet Rheumatol. 2019;1:e237–46.
- Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76:1327–1339.
- de Vries-Bouwstra JK, Allanore Y, Matucci-Cerinic M, et al. Worldwide expert agreement on updated recommendations for the treatment of systemic sclerosis. J Rheumatol. 2020;47:249–254.
- Walker KM, Pope J, participating members of the Scleroderma Clinical Trials Consortium (SCTC); Canadian Scleroderma Research Group (CSRG). Treatment of systemic sclerosis complications: what to use when first-line treatment fails–a consensus of systemic sclerosis experts. Sem Arthritis Rheum. 2012;42:42–45.
- Fernandez-Codina A, Walker KM, Pope JE, et al. Treatment algorithms for systemic sclerosis according to experts. Arthritis Rheum. 2018;70:1820–1828.
- Tingey T, Shu J, Smuczek J, et al. Meta-analysis of healing and prevention of digital ulcers in systemic sclerosis. Arthritis Care Res. 2013;65:1460–1471.
- Denton CP, Hughes M, Gak N, et al. BSR and BHPR guideline for the treatment of systemic sclerosis. Rheumatology. 2016;55:1906–1910.
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731.
- Phat TN, Luong VH, Minh VN, et al. Bosentan versus nifedipine in the treatment of vasculopathy in systemic sclerosis patients: a randomized control trial. Asian Pac J Allergy Immunol. 2022. DOI:10.12932/AP-070722-1406)
- Clinical commissioning policy: sildenafil and bosentan for the treatment of digital ulceration in systemic sclerosis in adults reference: nHS England 210302P [1911] first published: may 2021 version number: 1.0. https://www.england.nhs.uk/wp-content/uploads/2022/01/clinical-commissioning-policy-sildenafil-bosentan-treatment-of-digitalulceration-in-systemic-sclerosis.pdf
- Chang SH, Jun JB, Lee YJ, et al. A clinical comparison of an endothelin receptor antagonist and phosphodiesterase type 5 inhibitors for treating digital ulcers of systemic sclerosis. Rheumatology. 2021;60:5814–5819.
- Curtiss P, Schwager Z, Lo Sicco K, et al. The clinical effects of l-arginine and asymmetric dimethylarginine: implications for treatment in secondary Raynaud’s phenomenon. J Eur Acad Dermatol Venereol. 2019;33:497–503.
- Teh L-S, Manning J, Moore T, et al. Sustained-release transdermal glyceryl trinitrate patches as a treatment for primary and secondary Raynaud’s phenomenon. Br J Rheumatol. 1995;34:636–641.
- Anderson ME, Moore TL, Hollis S, et al. Digital vascular response to topical glyceryl trinitrate, as measured by laser Doppler imaging, in primary Raynaud’s phenomenon and systemic sclerosis. Rheumatology. 2002;41:324–328.
- Hughes M, Moore T, Manning J, et al. Reduced perfusion in systemic sclerosis digital ulcers (both fingertip and extensor) can be increased by topical application of glyceryl trinitrate. Microvasc Research. 2017;111:32–36.
- Moinzadeh P, Riemekasten G, Siegert E, et al. Vasoactive therapy in systemic sclerosis: real-life therapeutic practice in more than 3000 patients. J Rheumatol. 2016;43:66–74.
- Panopoulos S, Chatzidionysiou K, Tektonidou MG, et al. Treatment modalities and drug survival in a systemic sclerosis real-life patient cohort. Arthritis Res Ther. 2020;22:56.
- Blagojevic J, Abignano G, Avouac J, et al. Use of vasoactive/vasodilating drugs for systemic sclerosis (SSc)-related digital ulcers (DUs) in expert tertiary centres: results from the analysis of the observational real-life DeSScipher study. Clin Rheumatol. 2020;39:27–36.
- Bellando-Randone S, Lepri G, Bruni C, et al. Combination therapy with bosentan and sildenafil improves Raynaud’s phenomenon and fosters the recovery of microvascular involvement in systemic sclerosis. Clin Rheumatol. 2016;35:127–132.
- Rademacher JG, Wincup C, Tampe B, et al. Combination therapy with bosentan and sildenafil for refractory digital ulcers and Raynaud’s phenomenon in a 30-year-old woman with systemic sclerosis: case report and literature review. J Scleroderma Related Disorders. 2020;5:159–164.
- Nagaraja V, Spino C, Bush E, et al. A multicenter randomized, double-blind, placebo-controlled pilot study to assess the efficacy and safety of riociguat in systemic sclerosis-associated digital ulcers. Arthritis Res Ther. 2019;21:202.
- Wigley FM, Wise RA, Seibold JR, et al. Intravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis. A multicenter, placebo-controlled, double-blind study. Ann Intern Med. 1994;120:199–206.
- Pope J, Fenlon D, Thompson A, et al. Iloprost and cisaprost for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database Syst Rev. 2000;1998(2):CD000953.
- Tsou PS, Palisoc PJ, Flavahan NA, et al. Dissecting the cellular mechanism of prostacyclin analog iloprost in reversing vascular dysfunction in scleroderma. Arthritis Rheumatol. 2021;73:520–529.
- Cruz JE, Ward A, Anthony S, et al. Evidence for the use of epoprostenol to treat Raynaud’s phenomenon with or without digital ulcers. Ann Pharmacother. 2016;50:1060–1067.
- Law ST, Farber HW, Simms RW. Use of intravenous epoprostenol as a treatment for the digital vasculopathy associated with the scleroderma spectrum of diseases. J Scleroderma Relat Disord. 2017;2:208–212.
- Marasini B, Massarotti M, Bottasso B, et al. Comparison between iloprost and alprostadil in the treatment of Raynaud’s phenomenon. Scand J Rheumatol. 2004;33:253–256.
- Bellando-Randone S, Bruni C, Lepri G, et al. The safety of iloprost in systemic sclerosis in a real-life experience. Clin Rheumatol. 2018;37:1249–1255.
- Negrini S, Magnani O, Matucci-Cerinic M, et al. Iloprost use and medical management of systemic sclerosis-related vasculopathy in Italian tertiary referral centers: results from the PROSIT study. Clin Exp Med. 2019;19:357–366.
- Barsotti S, Lorenzoni V, Di Battista M, et al. Prostanoids in scleroderma microangiopathy: clinical and pharmacoeconomic comparison between two intravenous regimens. Scand J Rheumatol. 2021;50:307–313.
- Bixio R, Adami G, Bertoldo E, et al. Higher body mass index is associated with a lower iloprost infusion rate tolerance and higher iloprost-related adverse events in patients with systemic sclerosis. Ther Adv Musculoskelet Dis. 2022;14:1759720X221137125.
- Schioppo T, Orenti A, Boracchi P, et al. Acute and chronic effects of two different intravenous iloprost regimens in systemic sclerosis: a pragmatic non-randomized trial. Rheumatology. 2018;57:1408–1416.
- Ingegnoli F, Schioppo T, Allanore Y, et al. Practical suggestions on intravenous iloprost in Raynaud’s phenomenon and digital ulcer secondary to systemic sclerosis: systematic literature review and expert consensus. Sem Arthritis Rheum. 2019;48:686–693.
- Jamart C, Levesque H, Thietart S, et al. Iloprost duration for digital ulcers in systemic sclerosis: french retrospective study at two centers and literature review. Frontiers Med. 2022;9:878970.
- Fraticelli P, Martino GP, Murri M, et al. A novel iloprost administration method with portable syringe pump for the treatment of acral ulcers and Raynaud’s phenomenon in systemic sclerosis patients. A pilot study (ILOPORTA). Clin Exp Rheumatol. 2017;35(Suppl 106):173–178.
- Braga Temido MH F, Gomes M, Parente F, et al. Iloprost infusion through elastomeric pump in the treatment of Raynaud’s phenomenon and digital ulcers. J Scleroderma Rel Disord. 2019;4:NP1–4.
- Shah AA, Schiopu E, Chatterjee S, et al. The recurrence of digital ulcers in patients with systemic sclerosis after discontinuation of oral treprostinil. J Rheumatol. 2016;43:1665–1671.
- Ughi N, Crotti C, Ingegnoli F. Effectiveness and safety of oxycodone/naloxone in the management of chronic pain in patients with systemic sclerosis with recurrent digital ulcers: two case reports. Clinical Intervent Aging. 2016;11:307–311.
- Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol. 2008;35:1801–1808.
- Garaiman A, Steigmiller K, Gebhard C, et al. Use of platelet inhibitors for digital ulcers related to systemic sclerosis: eUSTAR study on derivation and validation of the DU-VASC model. Rheumatology. 2023;62(SI):SI91–100.
- Lautenbach G, Dobrota R, Mihai C, et al. Evaluation of botulinum toxin a injections for the treatment of refractory chronic digital ulcers in patients with systemic sclerosis. Clin Exp Rheumatol. 2020;38(Suppl 125):S154–60.
- Martina E, Diotallevi F, Radi G, et al. Therapeutic use of botulinum neurotoxins in dermatology: systematic review. Toxins (Basel). 2021;13:120.
- Ennis D, Ahmad Z, Anderson MA, et al. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Practice Res Clin Rheum. 2021;35:101684.
- Motegi SI, Sekiguchi A, Saito S, et al. Successful treatment of Raynaud’s phenomenon and digital ulcers in systemic sclerosis patients with botulinum toxin B injection: assessment of peripheral vascular disorder by angiography and dermoscopic image of nail fold capillary. J Dermatol. 2018;45:349–352.
- Zhou Y, Liu Y, Hao Y, et al. The mechanism of botulinum a on Raynaud syndrome. Drug Des Dev Ther. 2018;12:1905–1915.
- Shenavandeh S, Sepaskhah M, Dehghani S, et al. A 4-week comparison of capillaroscopy changes, healing effect, and cost-effectiveness of botulinum toxin-A vs prostaglandin analog infusion in refractory digital ulcers in systemic sclerosis. Clin Rheumatol. 2022;41:95–104.
- Bello RJ, Cooney CM, Melamed E, et al. The therapeutic efficacy of botulinum toxin in treating scleroderma-associated Raynaud’s phenomenon: a randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheum. 2017;69:1661–1669.
- Senet P, Maillard H, Diot E, et al. Efficacy and safety of botulinum toxin in adults with Raynaud’s phenomenon secondary to systemic sclerosis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2023;75(3):459–467 .
- Goldberg SH, Akoon A, Kirchner HL, et al. The effects of botulinum toxin a on pain in ischemic vasospasm. J Hand Surg Am. 2021;46:513.e1.
- Bruni C, Ngcozana T, Braschi F, et al. Preliminary validation of the digital ulcer clinical assessment score in systemic sclerosis. J Rheumatol. 2019;46:603–608.
- Mouthon L, Poiraudeau S, Vernon M, et al. Psychometric validation of the hand disability in systemic sclerosis-digital ulcers (HDISS-DU) patient-reported outcome instrument. Arthritis Res Ther. 2020;22:3.
- Williams HJ, Furst DE, Dahl SL, et al. Double-blind, multicenter controlled trial comparing topical dimethyl sulfoxide and normal saline for treatment of hand ulcers in patients with systemic sclerosis. Arthritis & Rheumatism. 1985;28:308–314.
- Simpson V, Hughes M, Wilkinson J, et al. Quantifying digital ulcers in systemic sclerosis: reliability of computer-assisted planimetry in measuring lesion size. Arthritis Care Res. 2018;70:486–490.
- Davison AK, Dinsdale G, New P, et al. Feasibility study of mobile phone photography as a possible outcome measure of systemic sclerosis-related digital lesions. Rheumatology Adv Practice. 2022;6(3):rkac105.
- Hughes M, Moore T, Manning J, et al. A pilot study using high-frequency ultrasound to measure digital ulcers: a possible outcome measure in systemic sclerosis clinical trials? Clin Exp Rheumatol. 2017;35(Suppl 106):S218–219.
- Suliman YA, Kafaja S, Fitzgerald J, et al. Ultrasound characterization of cutaneous ulcers in systemic sclerosis. Clin Rheumatol. 2018;37:1555–1561.
- Barsotti S, d’Ascanio A, Valentina V, et al. Is there a role for laser speckle contrast analysis (LASCA) in predicting the outcome of digital ulcers in patients with systemic sclerosis? Clin Rheumatol. 2020;39:69–75.
- Marjanovic E, Moore TL, Manning JB, et al. Systemic sclerosis-related digital ulcers: a pilot study of cutaneous oxygenation and perfusion. Rheumatology. 2020;59:3573–3575.
- Fernandez-Codina A, Kazem M, Pope JE. Possible benefit of tadalafil cream for the treatment of Raynaud’s phenomenon and digital ulcers in systemic sclerosis. Clin Rheumatol. 2020;39:963–965.
- Guigui A, Mazet R, Blaise S, et al. Treprotinil hydrogel iontophoresis in systemic sclerosis-related digital skin ulcers: a safety study. J Clin Pharmacol. 2020;60:758–767.
- Spinella A, de Pinto M, Baraldi C, et al. Topical cannabidiol in the treatment of digital ulcers in patients with scleroderma: comparative analysis and literature review. Adv Skin Wound Care. 2023;36:18–23.
- Naef R, Tenor H, Koch G. TOP-N53: a clinical drug candidate for the treatment of non-healing wounds. Chimia (Aarau). 2020;74:814–817.
- Karalilova RV, Batalov ZA, Sapundzhieva TL, et al. Tofacitinib in the treatment of skin and musculoskeletal involvement in patients with systemic sclerosis, evaluated by ultrasound. Rheum Int. 2021;41:1743–1753.
- Hou Z, Su X, Han G, et al. JAK1/2 inhibitor baricitinib improves skin fibrosis and digital ulcers in systemic sclerosis. Front Med (Lausanne). 2022;9:859330.
- Seibold JR, Jageneau AH. Treatment of Raynaud’s phenomenon with ketanserin, a selective antagonist of the serotonin2 (5-HT2) receptor. Arthritis Rheum. 1984;27:139–146.
- Bruni C, Cometi L, Gigante A, et al. Prediction and primary prevention of major vascular complications in systemic sclerosis. Eur J Int Med. 2021;87:51–58.
- Zanin-Silva DC, Santana-Goncalves M, Kawashima-Vasconcelos MY, et al. Management of endothelial dysfunction in systemic sclerosis: current and developing strategies. Frontiers Med. 2021;8:788250.
- Di Luigi L, Sgro P, Duranti G, et al. Sildenafil reduces expression and release of IL-6 and IL-8 induced by reactive oxygen species in systemic sclerosis fibroblasts. Int J Molecular Sci. 2020;21:3161.
- Herrick AL, Berks M, Taylor C. Quantitative nailfold capillaroscopy - update and possible next steps. Rheumatology. 2021;60:2054–2065.
