ABSTRACT
Background
Vutrisiran and tafamidis are approved therapies for treating hereditary transthyretin-mediated (ATTRv/hATTR) amyloidosis with polyneuropathy, a rapidly progressive and fatal disease. To assist healthcare decision-makers, an indirect treatment comparison (ITC) was undertaken to explore the comparative efficacy of vutrisiran and tafamidis.
Research design and methods
Individual patient data (vutrisiran vs. placebo) and published results (tafamidis vs. placebo) from phase 3 randomized controlled trials were used in a Bucher analysis to assess differences in treatment effects between vutrisiran and tafamidis on: Neuropathy Impairment Score-Lower Limbs (NIS-LL), Norfolk Quality of Life-Diabetic Neuropathy (Norfolk QOL-DN) score, NIS-LL Response, and modified Body Mass Index (mBMI).
Results
Greater treatment effects were observed at 18 months with vutrisiran vs. tafamidis for all endpoints, with statistically significant improvements in polyneuropathy (relative mean change in NIS-LL: −5.3 [95% confidence interval (CI): −9.4, −1.2; p = 0.011]), health-related quality of life (HRQOL, relative mean change in Norfolk QOL-DN: −18.3 [95% CI: −28.6, −8.0; p < 0.001]), and nutritional status (relative mean change in mBMI: 63.9 [95% CI: 10.1, 117.7; p = 0.020]).
Conclusions
This analysis suggests vutrisiran has greater efficacy on multiple measures of polyneuropathy impairment and HRQOL compared to tafamidis in patients with ATTRv amyloidosis with polyneuropathy.
Plain Language Summary
Hereditary transthyretin-mediated (ATTRv/hATTR) amyloidosis is a rare genetic disease that runs in families, affecting about 50,000 people worldwide. This condition results in abnormal protein deposits building up, causing damage to multiple nerves (polyneuropathy) and other organs, which can shorten lifespan and have other harmful effects. Polyneuropathy symptoms include weakness, numbness, pain, dizziness, and diarrhea. Over time, everyday activities become more difficult as patients become increasingly disabled and dependent on others. Several treatments have been approved for the polyneuropathy of ATTRv amyloidosis. Many of these work in different ways to impact the disease process. An indirect treatment comparison is a well-established statistical method used by healthcare decision-makers to compare treatments when head-to-head trials are unavailable. Indirect treatment comparisons using a common approach, the Bucher method, yield similar conclusions to head-to-head studies over 90% of the time. This method was used to compare clinical trial data for tafamidis and vutrisiran, two approved treatments for ATTRv amyloidosis with polyneuropathy. The findings show that vutrisiran is more effective than tafamidis at addressing the polyneuropathy of ATTRv amyloidosis as measured by changes to sensory or motor nervous system functioning and nutritional status. Also, vutrisiran showed greater maintenance of health-related quality of life compared to tafamidis. The expected benefits of vutrisiran over tafamidis are large enough to be noticeable and clinically meaningful to a patient or clinician. This highlights the potential advantages of vutrisiran compared to tafamidis with regard to preservation of physical function and quality of life when treating ATTRv amyloidosis with polyneuropathy.
1. Introduction
Hereditary transthyretin-mediated (ATTRv) amyloidosis, also known as hATTR amyloidosis, is a rare, rapidly progressive, debilitating, and fatal disease caused by misfolded transthyretin (TTR) protein that accumulates as amyloid deposits in multiple organs and tissues, including the nerves, heart, gastrointestinal tract, and musculoskeletal tissues [Citation1–5]. An estimated 50,000 individuals are living with ATTRv amyloidosis worldwide [Citation1] and experience polyneuropathy as one of the common manifestations of the disease [Citation4,Citation5]. As the disease progresses, symptoms increase in severity, leading to significant disability, decreased health-related quality of life (HRQOL), loss of physical function, and death (median survival following diagnosis: 4.7 years) [Citation6–9]. Patients may lose their ability to walk unaided, have difficulty gripping and lifting objects, and become increasingly dependent on others for care [Citation1–4,Citation10–13]. Delays in treatment or the use of suboptimal therapy may result in polyneuropathy progression that cannot be fully reversed [Citation14]. Therefore, timely diagnosis and early and effective treatment are vital for improving patient outcomes, HRQOL, and overall wellbeing in ATTRv amyloidosis with polyneuropathy.
Currently, there are four available pharmacologic treatments approved by one or more regulatory authorities worldwide for ATTRv amyloidosis with polyneuropathy: vutrisiran, patisiran, inotersen, and tafamidis. Mechanistically, these drugs offer three unique pharmacologic treatment approaches. Vutrisiran and patisiran are ribonucleic acid interference (RNAi) therapies, inotersen is an antisense oligonucleotide, and tafamidis is a TTR tetramer stabilizer [Citation15–19]. The objective of this analysis was to conduct an indirect treatment comparison (ITC) of the efficacy of the RNAi therapeutic vutrisiran and the TTR tetramer stabilizer tafamidis for the treatment of ATTRv amyloidosis with polyneuropathy.
Tafamidis was evaluated for the treatment of TTR-FAP, currently known as ATTRv amyloidosis with polyneuropathy, in the pivotal phase 3 clinical trial, Fx-005 (NCT00409175). While, the primary analysis did not show a significant benefit of treatment with tafamidis versus placebo at 18 months, a statistically significant benefit for tafamidis versus placebo was observed in the post hoc analysis of patients who completed the trial per protocol (the smaller, efficacy-evaluable population) [Citation18]. Tafamidis was subsequently approved in some countries for use in patients with Stage 1 symptomatic polyneuropathy (Familial Amyloid Polyneuropathy [FAP] Stage 1). However, it is not approved in the United States (US) for the treatment of the polyneuropathy of ATTRv amyloidosis [Citation20].
Vutrisiran was evaluated in the phase 3 clinical trial, HELIOS-A (NCT03759379) for the treatment of ATTRv amyloidosis with polyneuropathy. In this study, patients received vutrisiran or the standard dose of the RNAi therapeutic patisiran (i.e. the trial reference treatment). Significant treatment effects were observed for all primary and secondary efficacy endpoints at months 9 and 18 versus the pre-specified external placebo group from the APOLLO study of patisiran (NCT01960348) [Citation17,Citation21]. The treatment effect on polyneuropathy, HRQOL, and nutritional status (which may be adversely impacted by autonomic neuropathy) after 18 months of treatment with vutrisiran was similar to that observed with patisiran in HELIOS-A [Citation17]. Based on the results of HELIOS-A, vutrisiran was approved for the treatment of ATTRv amyloidosis with polyneuropathy by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other regulatory agencies.
To date, there has been no head-to-head comparison of vutrisiran and tafamidis. In the absence of head-to-head trials, ITCs are a well-accepted method for assessing the comparative efficacy of rare disease treatments. Evaluating the results of direct head-to-head comparisons of various interventions and ITCs of these same interventions using the Bucher methodology, Song et al. reported no evident statistically significant differences between treatment estimates yielded by head-to-head comparisons and by the corresponding ITCs in 93% (41 of 44) of all cases evaluated [Citation22]. In the rapidly evolving therapeutic area of ATTRv amyloidosis with polyneuropathy, ITCs are crucial in understanding the evidence base to better guide healthcare provision and treatment decision-making [Citation23–29].
2. Methods
The feasibility of conducting this ITC was assessed prior to designing the analyses described below. The selection of outcomes on which to compare vutrisiran and tafamidis in this ITC and the development of the statistical analysis plan predated the availability of the 18-month clinical study results of HELIOS-A, supporting the scientific validity and rigor of this ITC. A summary of the feasibility assessment and detailed analysis plan is included in Appendix Section 1.
2.1. Data sources
2.1.1. Tafamidis
A systematic literature review of bibliographic databases, conference repositories, and trial registries was performed in September 2021 to identify all relevant clinical and non-clinical evidence for the use of vutrisiran and tafamidis in the treatment of adult patients with ATTRv amyloidosis with polyneuropathy. Based on this review, there was only one relevant clinical study on tafamidis identified for the ITC, the Fx-005 trial. All related published data used in this ITC stemmed from this single clinical study (i.e [Citation18,Citation30–32]).
Fx-005, the pivotal phase 3 trial of tafamidis, was a multicenter, international, randomized, double-blinded, placebo-controlled study of patients with ATTRv amyloidosis with polyneuropathy and the valine to methionine at amino acid 30 (V30M) TTR variant [Citation18]. A total of 125 patients in the ITT population were randomized 1:1 to receive tafamidis 20 mg orally (n = 64) or placebo (n = 61) daily over a period of 18 months. In the trial, the co-primary endpoints were improvement or stabilization in Neuropathy Impairment Score-Lower Limbs (NIS-LL) at 18 months (a binary outcome referred to as ‘NIS-LL Responder’ and defined in study Fx-005 as patients with an increase from baseline in NIS-LL of < 2 points or any level of decrease from baseline in NIS-LL) and change from baseline in Norfolk Quality of Life – Diabetic Neuropathy questionnaire (Norfolk QOL-DN) total score at 18 months. The secondary endpoints were change from baseline in modified Body Mass Index (mBMI) at 18 months and change from baseline in NIS-LL at 18 months.
Further details of the Fx-005 study are included in Appendix, Supplementary Table S1.
2.1.2. Vutrisiran
In the ITC of vutrisiran vs. tafamidis, individual patient data were used from HELIOS-A (vutrisiran) and APOLLO (external placebo group for the comparison of vutrisiran vs. placebo) at 18 months. Details of the APOLLO study have been described previously by Adams et al. (2017; 2018) [Citation15,Citation33].
HELIOS-A is a phase 3, randomized, global, open-label study of vutrisiran in patients with ATTRv amyloidosis with polyneuropathy. HELIOS-A enrolled 164 patients who were randomized 3:1 to receive vutrisiran (n = 122; 25 mg subcutaneously [SC] every 3 months [Q3M]) or patisiran (n = 42; 0.3 mg/kg intravenously [IV] every 3 weeks [Q3W]), as the reference comparator for a period of 18 months. The primary endpoint of HELIOS-A was change from baseline in modified Neuropathy Impairment Score +7 (mNIS +7) at 9 months or 18 months, depending on the relevant jurisdiction. Select secondary and exploratory endpoints included change from baseline in Norfolk QOL-DN total score and change from baseline in mBMI at 9 and 18 months.
In HELIOS-A, with agreement by multiple regulators around the world, the placebo arm of the APOLLO study (phase 3 study of patisiran; NCT01960348) was used as the external placebo comparator arm for most endpoints. To date, the APOLLO placebo group is the largest available cohort of untreated patients with ATTRv amyloidosis with polyneuropathy in a double-blinded, placebo-controlled, phase 3 study setting (n = 77) [Citation15].
Further details of the HELIOS-A study are included in Appendix, Supplementary Table S1.
2.2. Outcomes
All similarly defined outcomes that could be obtained from a priori analyses or via post hoc derivations across the Fx-005, HELIOS-A, and APOLLO studies were analyzed in the current ITC. The full set of outcomes that could be compared across studies between baseline and month 18, and were thus analyzed in the ITC, were: NIS-LL, NIS-LL Responder, Norfolk QOL-DN, and mBMI. These outcomes have clinical relevance in assessing the impact of treatment on HRQOL and the polyneuropathy manifestations of ATTRv amyloidosis. Most of these outcomes are commonly used in clinical trials to assess polyneuropathy in ATTRv amyloidosis and evaluate the effectiveness of therapies, although NIS-LL Responder is an outcome measure unique to the Fx-005 study. Change from baseline in the NIS-LL score at month 18 and NIS-LL Responder were not prespecified endpoints for HELIOS-A and APOLLO. However, for this ITC, NIS-LL scores, which were defined consistently with those used in Fx-005, were derived from subcomponents (items) of the mNIS +7 and Neuropathy Impairment Score (NIS) assessments collected in the HELIOS-A and APOLLO studies. This enabled a like-for-like comparison of NIS-LL outcomes between vutrisiran and tafamidis. It was, however, not possible to calculate mNIS +7 from NIS-LL or from other data collected in Fx-005 [Citation34]. As such vutrisiran and tafamidis could not be compared with respect to the outcome of mNIS +7.
The NIS-LL is a composite score of standard measures of neuropathy-related weakness, reflex loss, and sensory loss across lower limbs on both sides of the body [Citation34,Citation35]. Norfolk QOL-DN is a standardized 35-item patient-reported outcomes measure that assesses neuropathy-related quality of life across 5 domains: physical function/large fiber neuropathy, activities of daily living, symptoms, small fiber neuropathy, and autonomic neuropathy [Citation18,Citation33,Citation36,Citation37]. It has been specifically validated as a measure of HRQOL in patients with ATTRv amyloidosis with polyneuropathy [Citation36–38]. Negative changes from baseline in the NIS-LL scale (possible score range: 0 to 88 points) and the Norfolk QOL-DN questionnaire (possible score range: −4 to 136 points) indicate an overall improvement [Citation34,Citation39].
Autonomic neuropathy may lead to impaired nutritional status, and cachexia (wasting) is a leading cause of death in ATTRv amyloidosis [Citation40,Citation41]. Therefore, measuring weight loss and wasting through nutritional status in patients with ATTRv amyloidosis with polyneuropathy is also important. In ATTRv amyloidosis, measuring nutritional status using traditional Body Mass Index (BMI) is complicated by fluid retention caused by disease-related hypoalbuminemia [Citation42,Citation43]. A modified measure of BMI (i.e. mBMI), (weight [kg]/height [m2]) × (albumin [g/L]), incorporating serum albumin levels to correct BMI for potential fluid retention, is therefore used [Citation18,Citation33]. A higher mBMI value indicates more favorable nutritional status.
2.3. Differences between HELIOS-A, APOLLO, and Fx-005 studies
There are differences in the HELIOS-A and Fx-005 study designs which are salient in the context of this ITC. First, Fx-005 patients were randomized to a concurrent, internal placebo group, whereas HELIOS-A used an external placebo group from the APOLLO study. To allow for robust comparison between vutrisiran and the APOLLO placebo group, HELIOS-A was designed to minimize cross-study differences with APOLLO. In particular, this included the use of similar inclusion and exclusion criteria, overlapping study sites, and the enrollment of similar proportions of patients with key baseline characteristics. As a result, the HELIOS-A and APOLLO study populations were largely overlapping and are clinically comparable. Any remaining potential impact on the calculated treatment effects between vutrisiran and the APOLLO placebo group caused by differences in patient baseline characteristics were minimized in the context of this ITC by calculating treatment effects with adjustment for such differences based on propensity scores. Calculation of these propensity-score adjusted treatment effects followed the method used in pre-specified sensitivity analyses of the HELIOS-A study, using regression adjustment for continuous outcomes and stabilized inverse probability weighting for binary outcomes [Citation17]. Adjustment of treatment effect estimates based on these propensity scores served as an additional measure (involving a widely used and well-validated approach, see [Citation44]) to control for the possible effect of baseline differences between the HELIOS-A vutrisiran group and the APOLLO placebo group.
Differences in the study inclusion criteria between Fx-005 and HELIOS-A are also relevant. While FAP Stage 1 is not explicitly an inclusion criterion for Fx-005, it is assumed that the entire Fx-005 population was FAP Stage 1 based on determinations by regulatory and health technology assessment agencies [Citation31,Citation45,Citation46]. Patients with FAP Stage 1 experience mild neuropathy in their legs and feet but can walk unassisted [Citation34,Citation40,Citation47]. HELIOS-A and APOLLO enrolled patients with FAP Stage 1 or 2 at baseline. At FAP Stage 2, patients experience neuropathy throughout the body and need assistance from crutches or sticks to walk. FAP Stage 3 patients, typically those who experience severe neuropathy and are bedridden or wheelchair bound, were not included in these studies [Citation34,Citation40,Citation47]. In the interest of comparability with the Fx-005 patient population, the primary ITC analysis utilized HELIOS-A and APOLLO data from the subset of patients with FAP Stage 1 at baseline. This was a conservative approach in that it limited statistical power, although there is no evidence to suggest that FAP stage is an effect modifier.
When estimating the treatment effect of tafamidis on change in NIS-LL from baseline in Fx-005, mixed-effects model for repeated measures (MMRM) analyses were used with three approaches for handling missing data. Specifically, analyses were conducted using: (1) observed data only, (2) imputed data where missing outcomes were estimated based on non-missing data within the same treatment group, and (3) imputed data where missing outcomes were estimated based on non-missing data in the placebo group. Estimated mean differences in change from baseline in NIS-LL between tafamidis and placebo were similar regardless of the method used to handle missing data. For NIS-LL Responder and change in Norfolk QOL-DN from baseline in Fx-005, the last-observation-carried-forward (LOCF) method was used to impute missing data. Change in mBMI from baseline was estimated using MMRM based on observed data only.
In the main analyses specified in the statistical analysis plan for the HELIOS-A study (vutrisiran), different methods were used for handling missing data compared to those used in Fx-005. In this ITC, these differences were addressed by conducting new analyses of the vutrisiran arm in HELIOS-A vs. the APOLLO placebo arm using approaches for handling missing data that were similar to those used in the corresponding analyses comparing tafamidis vs. placebo in Fx-005. Specifically, MMRM models were fit to observed data to estimate mean differences in change from baseline in NIS-LL between vutrisiran and APOLLO placebo with adjustment for baseline NIS-LL, in a manner consistent with that reported by Keohane et al. (2017) for the comparison of tafamidis and placebo in Fx-005 [Citation30]. A similar MMRM approach was used for the analysis of observed changes in mBMI, with adjustment for baseline mBMI. In addition, as in the Fx-005 study, the LOCF approach was implemented to impute missing data for the analyses of NIS-LL Responder (derived from continuous change in NIS-LL) and change from baseline in Norfolk QOL-DN with vutrisiran vs. APOLLO placebo at month 18 among patients with at least one follow-up observation. In the case of Norfolk QOL-DN, an MMRM model was fit to the resulting data set (with LOCF imputation) to estimate mean differences in change from baseline between vutrisiran and APOLLO placebo.
Methods of handling missing data by outcome in the ITC are summarized in .
Table 1. Methods of handling missing data by outcome.
Finally, unlike APOLLO and Fx-005, HELIOS-A was conducted over the course of the ongoing coronavirus disease 2019 (COVID-19) pandemic (with the first dose administered 14 February 2019, and the 18-month data cut off occurring on 26 August 2021). To account for possible confounding effects, outcome assessments of vutrisiran arm patients were censored (i.e. considered missing) on or after the onset of a serious COVID-19 adverse event (AE). Further details are provided in Appendix Section 2.
2.4. Statistical analysis
The Bucher method is an established approach for conducting pairwise ITCs across randomized, controlled trials (RCTs) [Citation22,Citation48] and is recommended by multiple health technology assessment organizations as a preferred approach for conducting cross-trial ITCs [Citation48–50]. The use of an external placebo group in clinical trials and the inclusion of trials with external placebo groups in ITCs are established practices in rare diseases where the natural history of the disease course is well understood [Citation51–54], assuming there are overall similarities between patients in the external placebo group and in the treatment arm of the trial of interest (see, for example, Cheng et al. (2020) and Parikh et al. (2021) [Citation55,Citation56]). To bolster the clinical comparability between the vutrisiran arm of HELIOS-A and the APOLLO placebo arm, the methods described in Section 2.3 were used to minimize the impact of any differences between these treatment arms.
Adopting the Bucher method, the effect of vutrisiran relative to tafamidis, as captured by difference in mean change from baseline for NIS-LL, Norfolk QOL-DN, and mBMI at 18 months and by risk difference (RD), odds ratio (OR), and risk ratio (RR) for the binary outcome of NIS-LL Responder, was determined using the estimates of treatment effect difference for each treatment vs. placebo (APOLLO placebo arm for vutrisiran and Fx-005 placebo arm for tafamidis) [Citation48,Citation57]. For the changes in NIS-LL, Norfolk QOL-DN, and mBMI at 18 months, the propensity-score adjusted estimate of the least squares mean difference (LSMD) between the vutrisiran arm in HELIOS-A and the placebo arm in APOLLO was compared to the published difference in mean change between tafamidis and placebo from the Fx-005 study to obtain relative effect estimates for vutrisiran vs. tafamidis. For the NIS-LL Responder outcome, the proportion of vutrisiran arm patients in HELIOS-A and placebo arm patients in APOLLO deemed to be NIS-LL Responders at 18 months was calculated. Analysis of this outcome used inverse probability of treatment weighting to balance baseline differences between the vutrisiran arm in HELIOS-A and the placebo arm in APOLLO in the calculation of RD, OR, and RR for vutrisiran vs. placebo. These relative efficacy measures were then compared to the corresponding published figures for tafamidis vs. placebo to obtain relative effect estimates for vutrisiran vs. tafamidis. Further details of propensity score matching can be found in Appendix Section 3.
Primary ITC analyses were conducted using the ITT population of Fx-005 and the subgroup of patients with FAP Stage 1 in HELIOS-A and APOLLO. Secondary analyses were conducted using the ITT population of Fx-005 and the full, modified ITT (mITT) population of vutrisiran-treated patients from the HELIOS-A study and placebo-treated patients from the APOLLO study.
3. Results
The results of the primary and secondary ITCs of vutrisiran and tafamidis are presented in . Overall, vutrisiran demonstrated greater treatment effects on neuropathy, nutritional status, and HRQOL than tafamidis in patients with ATTRv amyloidosis with polyneuropathy. Results were consistent and robust across primary and secondary analyses for the continuous outcomes and numerically favored vutrisiran in all binary outcomes.
Table 2. Primary and secondary Bucher ITC analyses of vutrisiran versus tafamidis.
3.1. Primary analysis
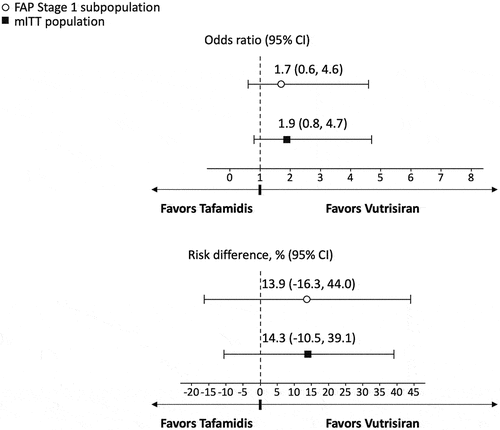
In the primary analysis, the difference in mean change from baseline at 18 months favored vutrisiran compared to tafamidis for NIS-LL (−5.3 [95% Confidence Interval (CI) −9.4 to −1.2, p = 0.011]; ), Norfolk QOL-DN (−18.3 [95% CI −28.6 to −8.0, p < 0.001]; ), and mBMI (63.9 [95% CI 10.1 to 117.7, p = 0.020]; ). Vutrisiran also had numerically better efficacy in terms of the NIS-LL Responder outcome at 18 months compared to tafamidis, with an OR for NIS-LL response of 1.7 (95% CI 0.6 to 4.6, p = 0.282; ) and an RD of 13.9% (95% CI −16.3% to 44.0%, p = 0.368; ).
Figure 1. Mean difference between vutrisiran and tafamidis on 18-month changes from baseline in NIS-LL.
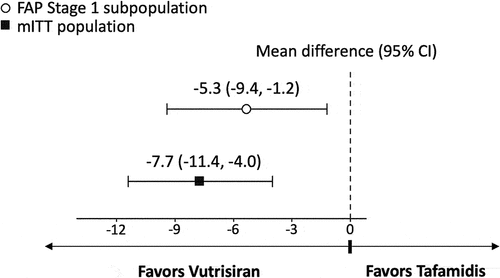
Figure 2. Mean difference between vutrisiran and tafamidis on 18-month changes from baseline in Norfolk QOL-DN.
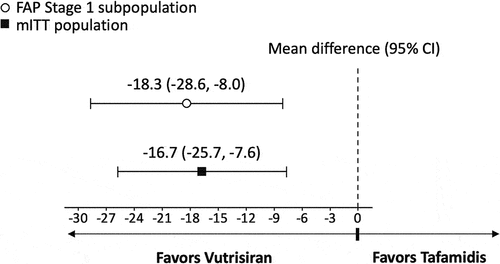
Figure 3. Mean difference between vutrisiran and tafamidis on 18-month changes from baseline in mBMI.
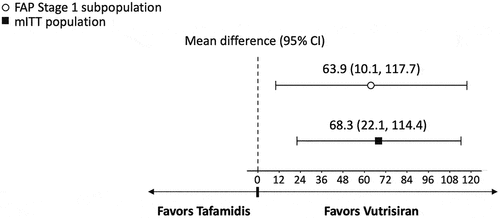
3.2. Secondary analysis
Significantly greater treatment effects with vutrisiran versus tafamidis were observed across the secondary analyses that considered data from the full mITT population of vutrisiran-treated patients from HELIOS-A and placebo-treated patients from APOLLO. The difference in mean change from baseline at 18-months for vutrisiran vs. tafamidis was significant for NIS-LL (−7.7 [95% CI −11.4 to −4.0, p < 0.001]; ), Norfolk QOL-DN (−16.7 [95% CI −25.7 to −7.6, p < 0.001]; ), and mBMI (68.3 [95% CI 22.1 to 114.4, p = 0.004]; ). Similar to the results of the primary analysis, the binary NIS-LL Responder outcome at 18 months numerically favored vutrisiran over tafamidis (OR 1.9 [95% CI 0.8 to 4.7, p = 0.172]; RD 14.3% [95% CI −10.5% to 39.1%, p = 0.258]; ).
4. Discussion
ATTRv amyloidosis with polyneuropathy causes potentially irreversible and progressive disability and shortened lifespan. With the growing number of therapeutic options indicated to treat the polyneuropathy of ATTRv amyloidosis, healthcare decision-makers must weigh the effectiveness and safety of each option in order to optimize patients’ clinical outcomes, often without comparative evidence from head-to-head clinical trials. This a priori designed ITC presents compelling evidence that vutrisiran offers greater treatment effects compared to tafamidis on multiple disease-relevant outcomes of ATTRv amyloidosis with polyneuropathy, including sensorimotor neuropathy, HRQOL, and nutritional status. As such, this analysis provides important evidence for future decision-making of health technology assessment agencies, policymakers, clinicians, and other stakeholders.
Results were consistent across all analytic methods and outcome measures, which all statistically or numerically favored vutrisiran over tafamidis. Compared with placebo, tafamidis demonstrated an approximate 3-point decrease in mean change in NIS-LL scores, equivalent to 52% less neurologic deterioration than was observed in the placebo arm, while vutrisiran demonstrated a greater treatment effect in preserving neurologic function with an approximate 8.3-point decrease in mean change in NIS-LL scores, equivalent to 93% less neurologic deterioration compared to the corresponding placebo arm. When indirectly compared to tafamidis, vutrisiran was associated with a 5.3-points decrease in 18-month change in NIS-LL. This 5.3-point advantage in NIS-LL score change favoring vutrisiran over tafamidis in the primary analysis and 7.7-point advantage in the secondary analysis is 2–3 times the margin for the minimum clinically recognizable degree of change, which has been described as 2 points in NIS-LL [Citation34]. The favorable outcomes for vutrisiran compared to tafamidis on this outcome measure were robust, as observed findings were consistent between the primary analysis of patients with early-stage neuropathy (FAP Stage 1) and the secondary analysis including mITT patients with later stage polyneuropathy (FAP Stages 1 and 2) in HELIOS-A and APOLLO.
Similarly, treatment with vutrisiran improved Norfolk QOL-DN by 18.3 points compared with tafamidis, which is more than double the 8.8 point threshold proposed to be indicative of a clinically relevant benefit [Citation58]. For the proportion of patients with an NIS-LL response (NIS-LL Responders), vutrisiran also numerically outperformed tafamidis. The difference between vutrisiran and tafamidis on this specific response measure was not statistically significant, largely due to the wide CI of the NIS-LL Responder rate in the Fx-005 study. The point estimate of the treatment effect of vutrisiran relative to tafamidis observed on the NIS-LL Responder outcome in this ITC was of a similar magnitude to the point estimate of the effect of patisiran relative to tafamidis observed in a previously conducted ITC study [Citation23].
Safety outcomes were not compared as part of this ITC as different definitions of AEs and serious AEs were used across the studies. However, the HELIOS-A and Fx-005 studies demonstrate that both vutrisiran and tafamidis are well tolerated [Citation18,Citation59,Citation60]. An open-label study also showed that tafamidis, evaluated at up to 6 years of use, was generally well tolerated [Citation60]. HELIOS-A patients are currently able to take part in an extension study for longer-term follow-up and these data will be forthcoming.
There are some limitations of this ITC. As is expected in rare conditions such as ATTRv amyloidosis, the number of patients enrolled in the studies included in the ITC is relatively low. Sensitivity analysis via matching-adjusted indirect comparisons (MAICs) adjusting for multiple other baseline characteristics (aside from FAP stage) that differ between studies was not conducted due to sample size considerations. While there are differences in patient characteristics between HELIOS-A and Fx-005, the characteristics that differ between these studies are not currently known to be relative treatment effect modifiers. Therefore, based on extant knowledge, the differences in baseline characteristics between HELIOS-A and Fx-005 do not compromise the validity of the primary and secondary analyses in this ITC. As is common to all ITCs, it was not possible to adjust for any unobserved cross-trial differences.
This ITC drew on data from an open-label study with an external placebo group. While randomized controlled parallel-arm trial data are traditionally used in ITC analyses, the use of trials involving an external comparison group in an ITC is acceptable practice. Methods were used to reinforce the clinical comparability of the HELIOS-A vutrisiran arm and the external placebo group (APOLLO) included in this ITC, as described in Sections 2.3 and 2.4. The lack of randomization between these two arms is an important limitation that must be acknowledged. However, a Bucher approach is suitable here and remains the best approach based on available evidence. Other ITC approaches were considered (i.e. unanchored comparison, network meta-analysis, MAIC, and simulated treatment comparison), but these methods were deemed less appropriate or infeasible. The HELIOS-A study design does mean, however, that there is no randomization on potential, unknown treatment effect modifiers for the vutrisiran arm versus the external placebo arm of the APOLLO study. Further research in the form of a head-to-head RCT would provide the most robust evidence of the comparative efficacy of vutrisiran and tafamidis.
5. Conclusions
In the absence of a head-to-head trial, this ITC provides important information on the comparative efficacy of vutrisiran and tafamidis. In particular, the results highlight the statistically greater treatment effect of vutrisiran over tafamidis for patients with ATTRv amyloidosis for polyneuropathy manifestations, HRQOL, and nutritional status, as measured by NIS-LL, Norfolk QOL-DN, and mBMI. The difference in effect between vutrisiran and tafamidis is clinically relevant for patients, as evidenced by the large differences in treatment effects between vutrisiran and tafamidis which surpassed published minimum clinically important difference thresholds. Furthermore, the robustness of these results is confirmed by the consistency of findings across primary and secondary analyses of all endpoints. The statistical and clinical significance of these findings underscore the potential advantages of vutrisiran compared to tafamidis with regard to preservation of physical function and quality of life when treating ATTRv amyloidosis with polyneuropathy.
Declaration of interests
M Merkel, D Danese, C Chen, and H Lin are employees and stockholders of Alnylam Pharmaceuticals. A Wu and H Yang are employees of Analysis Group, Inc., which received consultancy fees from Alnylam Pharmaceuticals for conducting the research. J Wang was an employee of Analysis Group, Inc when the study was conducted.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
M Merkel, H Lin, J Wang, and H Yang were involved in the conception of this study and, together with C Chen, designed the study. C Chen acquired the data. J Wang, A Wu, and H Yang completed the data analysis. All authors were involved in the interpretation of the data, and drafting and revising the manuscript. All authors reviewed and approved this manuscript for publication and agree to be accountable for all aspects of the work.
Vutrisiran_vs_Tafamidis_ITC_Table_of_abbreviation_v1.0.docx
Download MS Word (31.9 KB)Vutrisiran_Vs_Tafamidis_ITC_Supplementary_Materials_v15_Clean.docx
Download MS Word (47.8 KB)Acknowledgments
The authors would like to thank Denise Yi, PhD, Chunyi Xu, ScM, and Chujun He, BA, from Analysis Group, for their contribution to data analysis. Medical writing and editorial support were provided by David Wyatt, PhD, and Susan Bartko-Winters, PhD, from Stratenym Inc., Toronto, Ontario, and funded by Alnylam Pharmaceuticals.
Data availability statement
Due to the sensitive nature of the data used in this study, the dataset will not be made available to other researchers. However, requests from qualified researchers for additional analyses may be sent to Alnylam Pharmaceuticals ([email protected]).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14656566.2023.2215925.
Additional information
Funding
References
- Gertz M. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care. 2017;23(7 Suppl):S107–112.
- Lovley A, Raymond K, Guthrie S, et al. Patient-reported burden of hereditary transthyretin amyloidosis on functioning and well-being. J Patient Rep Outcomes. 2021;5(1):3. doi:10.1186/s41687-020-00273-y.
- Ando Y, Coelho T, Berk J, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8(1):31. doi:10.1186/1750-1172-8-31.
- Hawkins P, Ando Y, Dispenzeri A, et al. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–638. DOI:10.3109/07853890.2015.1068949
- Schmidt H, Waddington‐cruz M, Botteman M, et al. Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2018;57(5):829–837. DOI:10.1002/mus.26034
- Koike H, Tanaka F, Hashimoto R, et al. Natural history of transthyretin Val30Met familial amyloid polyneuropathy: analysis of late-onset cases from non-endemic areas. J Neurol Neurosurg Psychiatry. 2012;83(2):152–158. DOI:10.1136/jnnp-2011-301299
- Adams D, Coelho T, Obici L, et al. Rapid progression of familial amyloidotic polyneuropathy: a multinational natural history study. Neurology. 2015;85(8):675–682. DOI:10.1212/WNL.0000000000001870
- Adams D, Suhr O, Hund E, et al. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol. 2016;29(Supplement 1):S14. DOI:10.1097/WCO.0000000000000289
- Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. DOI:10.1161/CIRCULATIONAHA.118.038169
- Coelho T, Maurer M, Suhr O. THAOS – the transthyretin amyloidosis outcomes survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63–76.
- Stewart M, Shaffer S, Murphy B, et al. Characterizing the high disease burden of transthyretin amyloidosis for patients and caregivers. Neurol Ther. 2018;7(2):349–364. DOI:10.1007/s40120-018-0106-z
- Yarlas A, Gertz M, Dasgupta N, et al. Burden of hereditary transthyretin amyloidosis on quality of life. Muscle Nerve. 2019;60(2):169–175. DOI:10.1002/mus.26515
- Amyloidosis Research Consortium. The voice of the patient report: amyloidosis research consortium; 2016 [cited 2022 Jan 20]. Available from: https://arciorg.wpengine.com/wp-content/uploads/2018/05/Voice-of-the-Patient.pdf
- Planté-Bordeneuve V, Gorram F, Salhi H, et al. Long-term treatment of transthyretin familial amyloid polyneuropathy with tafamidis: a clinical and neurophysiological study. J Neurol. 2017;264(2):268–276. DOI:10.1007/s00415-016-8337-3
- Adams D, Gonzalez-Duarte A, O’Riordan W, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. New Engl J Med. 2018;379(1):11–21. DOI:10.1056/NEJMoa1716153
- Benson M, Waddington-Cruz M, Berk J, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22–31. DOI:10.1056/NEJMoa1716793
- Adams D, Tournev IL, Taylor MS, et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid. 2022;30(1):1–9. doi:10.1080/13506129.2022.2091985.
- Coelho T, Maia L, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–792. doi:10.1212/WNL.0b013e3182661eb1.
- Ando Y, Adams D, Benson MD, et al. Guidelines and new directions in the therapy and monitoring of ATTRv amyloidosis. Amyloid. 2022;29(3):143–155. DOI:10.1080/13506129.2022.2052838.
- Jeffrey S FDA declines approval for tafamidis in TTR-FAP 2012 [cited 2021 Nov 14]. Available from: https://www.medscape.com/viewarticle/765981
- Adams D, Tournev I, Taylor M, et al. HELIOS-A: 9-month results from the phase 3 study of vutrisiran in patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy. Neurology. 2021;96:1234.
- Song F, Altman D, Glenny A-M, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326(7387):472. DOI:10.1136/bmj.326.7387.472
- Planté-Bordeneuve V, Lin H, Gollob J, et al. An indirect treatment comparison of the efficacy of patisiran and tafamidis for the treatment of hereditary transthyretin-mediated amyloidosis with polyneuropathy. Expert Opin Pharmaco. 2019;20(4):473–481. doi:10.1080/14656566.2018.1554648.
- Gorevic P, Franklin J, Chen J, et al. Indirect treatment comparison of the efficacy of patisiran and inotersen for hereditary transthyretin-mediated amyloidosis with polyneuropathy. Expert Opin Pharmaco. 2021;22(1):121–129. DOI:10.1080/14656566.2020.1811850
- Jansen J, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ispor task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428. DOI:10.1016/j.jval.2011.04.002
- Jansen J, Trikalinos T, Cappelleri J, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC good practice task force report. Value Health. 2014;17(2):157–173. DOI:10.1016/j.jval.2014.01.004
- Es-Skali I, Spoors J. Analysis of indirect treatment comparisons in national health technology assessments and requirements for industry submissions. J Comp Eff Res. 2018;7(4):397–409.
- Torbicki A, Bacchi M, Delcroix M, et al. Integrating data from randomized controlled trials and observational studies to assess survival in rare diseases. Circ-Cardiovasc Qual. 2019;5(5):e005095. DOI:10.1161/CIRCOUTCOMES.118.005095
- Wells G, Sultan S, Chen L, et al. Indirect evidence: indirect treatment comparisons in meta-analysis ottawa: Canadian Agency for Drugs and Technologies in Health; 2009 [cited 2022 Jan 22]. Available from: https://www.cadth.ca/sites/default/files/pdf/H0462_itc_tr_e.pdf
- Keohane D, Schwartz J, Gundapaneni B, et al. Tafamidis delays disease progression in patients with early stage transthyretin familial amyloid polyneuropathy: additional supportive analyses from the pivotal trial. Amyloid. 2017;24(1):30–36. DOI:10.1080/13506129.2017.1301419
- European Medicines Agency (EMA). Vyndaqel (tafamidis) 20 mg soft capsules: annex 1 Summary of product characteristics 2013 [cited 2021 Nov 1]. Available from: https://www.ema.europa.eu/en/documents/product-information/vyndaqel-epar-product-information_en.pdf
- European Medicines Agency (EMA). Public assessment report for Vyndaqel (tafamidis) 2011 [cited 2021 Nov 1]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/vyndaqel-epar-public-assessment-report_en.pdf
- Adams D, Suhr O, Dyck P, et al. Trial design and rationale for APOLLO, a Phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC neurol. 2017;17(1):181. DOI:10.1186/s12883-017-0948-5
- Dyck P, González-Duarte A, Obici L, et al. Development of measures of polyneuropathy impairment in hATTR amyloidosis: from NIS to mNIS + 7. J Neurol Sci. 2019;405:116424.
- Dyck P, Davies J, Litchy W, et al. Longitudinal assessment of diabetic polyneuropathy using a composite score in the rochester diabetic neuropathy study cohort. Neurology. 1997;49(1):229–239. DOI:10.1212/WNL.49.1.229
- Vinik E, Hayes R, Oglesby A, et al. The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol The. 2005;7(3):497–508. DOI:10.1089/dia.2005.7.497
- Vinik E, Vinik A, Paulson J, et al. Norfolk QOL-DN: validation of a patient reported outcome measure in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2014;19(2):104–114. DOI:10.1111/jns5.12059
- Coelho T, Vinik A, Vinik E, et al. Clinical measures in transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2017;55(3):323–332. DOI:10.1002/mus.25257
- Obici L, Berk J, González-Duarte A, et al. Quality of life outcomes in APOLLO, the phase 3 trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. Amyloid. 2020;27(3):153–162. DOI:10.1080/13506129.2020.1730790
- Coutinho P, Martins da Silva A, Lopes Lima J, et al. Forty years of experience with type I amyloid neuropathy. Review of 483 cases. In: Glenner G, Pinho E, Costa P Falcao de Freitas A, editors. Amyloid and amyloidosis. Amsterdam: Excerpta Medica; 1980. p. 88–98.
- Gertz M, Kyle R, Thibodeau S. Familial amyloidosis: a study of 52 North American-born patients examined during a 30-year period. Mayo Clin Proc. 1992;67(5):428–440.
- Oh T, Lee J, Hwang J-W, et al. Value of preoperative modified body mass index in predicting postoperative 1-year mortality. Sci Rep. 2018;8(1):4614. DOI:10.1038/s41598-018-22886-6
- Suhr O, Danielsson Å, Holmgren G, et al. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med. 1994;235(5):479–485. DOI:10.1111/j.1365-2796.1994.tb01106.x
- Borah B, Moriarty J, Crown H, et al. Applications of propensity score methods in observational comparative effectiveness and safety research: where have we come and where should we go? J Comp Eff Res. 2014;3(1):63–78. DOI:10.2217/cer.13.89
- European Medicines Agency (EMA). Vyndaqel (tafamidis) EPAR Summary for the Public 2016 [cited 2021 Nov 1]. Available from: https://www.ema.europa.eu/en/documents/overview/vyndaqel-epar-summary-public_en.pdf
- Faria R, Walker S, Palmer S, et al. Tafamidis for transthyretin familial polyneuropathy (TTR-FAP) evidence review group assessment of manufacturer submission: cRD/CHE Technology assessment group (centre for reviews and dissemination/centre for health economics) University of York; 2012 [cited 2021 Dec 7]. Available from: https://www.york.ac.uk/media/crd/Tafamidis%20ERG%20Report_CRDCHE%20September%204%202013.pdf
- Adams D. Recent advances in the treatment of familial amyloid polyneuropathy. Ther Adv Neurol Disord. 2013;6(2):129–139.
- Bucher H, Guyatt G, Griffith L, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. DOI:10.1016/S0895-4356(97)00049-8
- Phillippo D, Ades A, Dias S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE 2016 [cited 2021 Nov 6]. Available from: https://www.sheffield.ac.uk/media/34216/download?attachment
- Kiefer C, Sturtz S, Bender R. Indirect comparisons and network meta-analyses. Dtsch Arztebl Int. 2015;112:803–808.
- International Conference on Harmonization (ICH). Choice of control group and related issues in clinical trials-E10 2000 [cited 2021 Nov 8]. Available from: https://database.ich.org/sites/default/files/E10_Guideline.pdf
- European Medicines Agency (EMA). Committee for medicinal products for human use: guideline on clinical trials in small populations 2006 [cited 2021 Nov 8]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-trials-small-populations_en.pdf
- United States Food and Drug Administration (FDA). Proposed FDA work plan for 21st century cures act innovation account activities 2016 [cited 2021 Nov 9]. Available from: https://www.fda.gov/files/Proposed-FDA-Work-Plan-for-21st-Century-Cures-Act-Innovation-Account-Activities-as-Submitted-to-Science-Board.pdf
- Amvuttra (Vutrisiran). Prescribing information. Cambridge: MA: Alnylam Pharmaceuticals, Inc.; 2022.
- Cheng D, Ayyagari R, Signorovitch J. The statistical performance of matching-adjusted indirect comparisons: estimating treatment effects with aggregate external control data. Ann Appl Stat. 2020;14(4):1806–1833.
- Parikh N, Marshall A, Betts K, et al. Network meta-analysis of nivolumab plus ipilimumab in the second-line setting for advanced hepatocellular carcinoma. J Comp Effect Res. 2021;10(5):343–352. DOI:10.2217/cer-2020-0236
- Song F, Clark A, Bachmann M, et al. Simulation evaluation of statistical properties of methods for indirect and mixed treatment comparisons. BMC Med Res Methodol. 2012;12(1):138. DOI:10.1186/1471-2288-12-138
- Yarlas A, Lovley A, Brown D, et al. Responder analysis for neuropathic impairment and quality-of-life assessment in patients with hereditary transthyretin amyloidosis with polyneuropathy in the NEURO-TTR study. J Neurol. 2022;269(1):323–335.
- Adams D, Tournev I, Taylor M, et al. HELIOS-A: study of vutrisiran in patients with hATTR amyloidosis. 26ème édition des Journées de la Société Francophone du Nerf Périphérique (SFNP); 2022/Jan/21.
- Barroso F, Judge D, Ebede B, et al. Long-term safety and efficacy of tafamidis for the treatment of hereditary transthyretin amyloid polyneuropathy: results up to 6 years. Amyloid. 2017;24(3):194–204. DOI:10.1080/13506129.2017.1357545