1. Introduction
Despite improvements in indolent non-Hodgkin lymphoma (iNHL) therapy, they remain generally incurable and new treatments are needed. Will phosphatidylinositol 3-kinase (PI3K) inhibitors be among these?
PI3K exists in four isoforms (α, β, γ, and δ). The δ isoform, preferentially expressed in hematopoietic cells, plays a key role in B-cell development and function [Citation1]. PI3K, activated in B-cells by various upstream signals including the B-cell receptor, signals downstream to regulate B-cell survival, proliferation, and mobility [Citation2] (). As dysregulated PI3K signaling contributes to iNHL B cell survival and proliferation, PI3K became a rational therapeutic target for drug development, particularly PI3Kδ selective agents to promote lymphocyte specificity, reducing off-target toxicity [Citation2]. For a comprehensive understanding of the role of PI3K in B-cell lymphoma and its therapeutic implications, we refer readers to a prior review on this topic, Gottfried von Keudell & Alison J. Moskowitz, 2019 [Citation3]. Several PI3K inhibitors (PI3Ki) with varying isoform specificity but including δ demonstrated durable responses and improved progression-free survival (PFS) in iNHL and chronic lymphocytic leukemia (CLL), leading to various therapeutic approvals. While initially considered small-molecule inhibitors of B cells, accumulating data reveal additional immune and other off-target toxicity, highlighting several critical issues including suboptimal dosing regimens, limitations of single-arm trial interpretation, and ultimately concerns about adverse effects on overall survival. Moreover, the optimal approach to developing future PI3Kis for lymphoma remains a matter of debate. The Food and Drug Administration (FDA) has issued specific directives regarding the use of PI3Kis in hematologic malignancies and requires randomized studies for future PI3Kis approval decisions using overall survival (OS) as a clinical outcome endpoint [Citation4].
Figure 1. PI3K pathway.
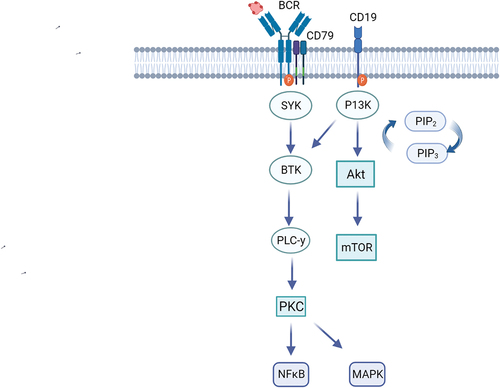
This editorial review addresses existing data on PI3Ki in B-cell iNHL (not CLL or T cell lymphoma), and potential pathways to continue PI3Ki drug development in light of the US FDA discussion of the use of PI3Ki in hematological malignancies [Citation5]. The role of PI3Ki in T-cell lymphoma was beyond the scope of the article, however this is an area of active investigation with evidence that PI3Ki may play a valuable role, especially as the risk:benefit calculation differs.
2. Efficacy vs toxicity of PI3Ki
Four PI3Ki received accelerated FDA approval for the treatment of relapsed/refractory (R/R) iNHL based on durable overall response rates (ORR) observed in single-arm trials including idelalisib (targeting δ), copanlisib (a pan-PI3K inhibitor with predominance for α and δ), duvelisib (targeting δ and ɣ), and umbralisib (targeting δ with additional inhibition of casein kinase−1 epsilon).
In their pivotal single-agent phase 2 studies for R/R iNHL, idelalisib, copanlisib, duvelisib, and umbralisib demonstrated activity with ORR 40%−70%, median response duration 10–22 months, and PFS of 9.5–12.5 months [] [Citation6–9]. PI3Ki in combination with rituximab and/or bendamustine in R/R iNHL demonstrated promising efficacy but were associated with a relatively increased risk of death from non-lymphomatous causes [Citation10–14]. Thus, while PI3Kis have shown clinical efficacy, toxicity remains a significant issue.
Table 1. Studies of monotherapy PI3K δ inhibitors.
In the phase 2 studies of idelalisib, copanlisib, duvelisib, and umbralisib adverse events (AEs) led to frequent drug discontinuation (15%−31%) and dose reduction (11%−34%), with serious AEs up to 65% and severe (Grade ≥3) adverse reactions up to 87%. The significant AEs included a wide range of immune related adverse events (IR-AEs), such as hepatitis, colitis, and pneumonitis, leading to fatal hepatotoxicity, intestinal perforation, and respiratory failure. Increased incidence of severe infections has also been seen with all the agents. IR-AEs have impeded the clinical utility and development of the PI3Kδ inhibitors.
PI3Kδ inhibition related autoimmune toxicity (IR-AEs) is now understood to be primarily mediated by dysregulation of regulatory T cells (Treg) and activation of Th17. However, the impact of PI3Kδi on T cell function was not fully appreciated during the early trials.
In some prospective randomized trials of PI3Ki, the investigational arm demonstrated superior PFS, but no difference or even negative impact on OS. This justifiably raised concerns about late toxicity being the main driver of these outcomes [] [Citation4,Citation10–14]. Caution is required in interpreting OS analysis of these trials as they were not powered for this endpoint and the OS differences were not significant. Further, OS may not be a reliable estimate of therapy impact given the long natural history of indolent lymphoma, and analysis does not account for subsequent treatment, including crossover to investigational drugs.
Table 2. Randomized control trials with PI3KI in relapsed/refractory indolent non-Hodgkin lymphoma.
3. FDA deliberation on the use of PI3K inhibitors in hematologic malignancies
The Oncologic Drugs Advisory Committee (ODAC) in April 2022 voted 16–0 (1 abstention) to require randomized studies of future PI3K inhibitors in hematologic malignancies rather than basing approval decisions on single-arm trial data.
In subsequent commentary, members of the Center for Drug Evaluation and Research of the FDA addressed further development of this class of drugs. They advocated appropriately for careful evaluation to identify the optimal biologic dose, recommending this optimal dose be determined by a randomized trial exploring different dose levels rather than the more traditional sequential-dose cohort. The randomized trial requirement represents an enormous hurdle for early drug development and may expose more patients to potentially toxic doses. The basophil activation test (BAT), a simple flow cytometric assay, can assess PI3Kδ inhibition in vivo [Citation15]. Thus, optimal biologic dose and schedule could be established by examining inhibition by BAT assays and development of toxicity. Importantly, the exploration of both dose and schedule is critical in optimizing PI3Kδi as there will likely be a differential impact on the tumor versus Treg. Our view is that PI3K inhibitors should be evaluated based on their individual properties, rather than assessing them as a class.
For approval, randomized trials are also appropriately favored over single-arm trials. Clinical efficacy endpoint selection, however, is not clear. While OS is the ultimate endpoint of interest, given iNHL long natural histories, a primary OS endpoint would prolong clinical development, discouraging, if not halting, development of this class of drugs. A key goal of early drug development should be identifying a dose and schedule that minimizes IR-AEs. Randomized studies should then be conducted for efficacy, along with close attention to IR-AEs. Non-lymphoma related deaths should be carefully monitored; however, this class of drug should not be singled out to disallow PFS as an endpoint for approval.
4. “Next Generation” PI3Kδ inhibitor – influence of dose and schedule in efficacy and immune toxicity
Novel schedules for PI3Kδ administration have been explored for both parsaclisib and zandelisib, which have preserved efficacy with reduced toxicity. Here, we focus on zandelisib as a model for future development of PI3Ki.
Zandelisib is a novel, orally bioavailable, potent, and selective PI3Kδ inhibitor with a distinct molecular structure from other PI3Kδi. Its plasma half-life of 28 h allows for daily dosing. In vitro, PI3Kδ target binding takes at least 5 h. High volume of distribution predicts higher tissue exposure relative to plasma. An optimal biologic dose was established based on the BAT assay and demonstrated excellent efficacy in both follicular lymphoma (FL) and CLL, diseases with established response benchmarks set by other PI3Kis [Citation16]. Efficacy did not improve at higher doses.
Furthermore, Pagel and colleagues hypothesized, based on these pharmacological findings, that intermittent dosing of zandelisib on days 1–7 of 28-day cycles could reduce immune-mediated adverse events associated with continuous PI3Kδ inhibition by facilitating Treg repopulation, while maintaining efficacy. To evaluate this hypothesis, they conducted a phase 1b clinical trial to assess the safety and efficacy of zandelisib as monotherapy or in combination with rituximab, using either continuous or intermittent dosing regimens in R/R B cell lymphoma [Citation17].
The intermittent dosing strategy resulted in a lower cumulative risk of grade ≥3 AEs of special interest beyond cycle 3, with grade ≥3 diarrhea or colitis occurring in 8% of the patients, grade ≥3 rash in 5%, and no grade 4 AEs of special interest. The intermittent dosing did not result in reduced efficacy, as patients with iNHL had high overall response rates, both overall (82% in FL and 100% in both MZL and CLL) and across different subgroups by treatment and by dosing schedule.
A confirmatory phase 2 trial in third-line or later FL confirmed the activity with an ORR of 72%, CRR 36%, and a median PFS of 9.7 months [Citation18]. An international phase 3 study of zandelisib plus rituximab versus chemoimmunotherapy after the failure of a previous immunochemotherapy (COSTAL trial, NCT04745832) was launched. Unfortunately, the trial has been terminated because the additional OS information requested by the FDA made it impossible for the study to be completed in a timely manner. A correlation analysis of T cell subsets is planned to assess the effect of intermittent dosing on regulatory T-cell function.
5. Summary and future directions
In summary, PI3Ki is an important class of drugs with activity across a range of B-cell lymphoma/leukemias including FL, MZL, and CLL, but whose utility has been limited by IR-AEs. Improved understanding of IR-AEs and availability of novel PI3Kδ inhibitors with different pharmacokinetic properties permit exploration of novel dosing schedules, informed by target inhibition assays. Data from zandelisib phase Ib and II studies demonstrated that efficacy was maintained with an intermittent dosing schedule that substantially reduced IR-AEs. Future studies of zandelisib and other PI3Kis to further optimize pharmacological parameters, and correlative studies to address target inhibition and immune effects may improve risk:benefit. The challenges for drug approval raised by the recent FDA decision regarding this class of drugs may keep such studies from proceeding in B cell lymphoma/leukemia.
6. Expert opinion
The FDA’s requirement for overall survival as an outcome in randomized control trials for PI3Ki drugs has created significant obstacles in their development. While the risks and benefits of these drugs should be carefully evaluated, we believe that PI3Ki drugs have a critical role in the treatment of R/R iNHL. However, they should be evaluated individually rather than as a class effect. To optimize their use, we propose exploring novel dosing schedules that consider target inhibition assays and the distinct pharmacokinetic properties of next-generation PI3Kδ inhibitors. For example, zandelisib has demonstrated that efficacy can be maintained with an intermittent dosing schedule that substantially reduces IR-AEs. Further studies should focus on optimizing pharmacological parameters and conducting correlative studies to improve the risk:benefit ratio and address target inhibition and immune effects.
While published overall survival data should be treated with caution, continued investigation into PI3Kδ inhibitors could offer significant benefits in the treatment of B-cell lymphoma/leukemia. However, the recent FDA decision may impede further studies in this area. Therefore, close collaboration between regulatory bodies, drug developers, and academic institutions is essential to ensure the continued progress of PI3Ki drug development while minimizing the risks of immune-related adverse effects.
Declaration of interest
A D Zelenetz has received research support from Genentech/Roche, MEI, AbbVie, and BeiGene. They have also been Consultants for BMS, Genentech/Roche, Gilead, BeiGene, Janssen, Amgen, AstraZeneca, Pharmacyclics, Novartis, and MEI pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Vanhaesebroeck B, Ali K, Bilancio A, et al. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30(4):194–204. doi:10.1016/j.tibs.2005.02.008
- Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–330. doi:10.1038/nri1056.
- von Keudell G, Moskowitz AJ. The role of PI3K inhibition in lymphoid malignancies. Curr Hematol Malignancy Rep. 2019;14(5):405–413. doi:10.1007/s11899-019-00540-w.
- [FDA Briefing Document]; Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fda.gov/media/157762/download.
- Richardson NC, Kasamon Y, Pazdur R, et al. The saga of PI3K inhibitors in haematological malignancies: survival is the ultimate safety endpoint. Lancet Oncol. 2022;23(5):563–566. doi:10.1016/S1470-2045(22)00200-5
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. doi:10.1056/NEJMoa1314583
- Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905. doi:10.1200/JCO.2017.75.4648
- Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37(11):912–922. doi:10.1200/JCO.18.00915
- Fowler NH, Samaniego F, Jurczak W, et al. Umbralisib, a dual PI3Kδ/CK1ε Inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol. 2021;39(15):1609–1618. doi:10.1200/JCO.20.03433
- Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18(3):297–311. doi:10.1016/S1470-2045(16)30671-4
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi:10.1056/NEJMoa1315226
- Jones JA, Robak T, Brown JR, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 2017;4(3):e114–e126. doi:10.1016/S2352-3026(17)30019-4
- Matasar MJ, Capra M, Özcan M, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(5):678–689. doi:10.1016/S1470-2045(21)00145-5
- Gribben JG, Jurczak W, Jacobs RW, et al. Umbralisib plus ublituximab (U2) is superior to obinutuzumab plus chlorambucil (O+chl) in patients with treatment naïve (TN) and Relapsed/Refractory (R/R) chronic lymphocytic leukemia (CLL): results from the phase 3 unity-CLL study. Blood. 2020;136(Supplement 1):37–39. doi:10.1182/blood-2020-134783
- Winkler DG, Faia K, DiNitto J, et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20(11):1364–1374. doi:10.1016/j.chembiol.2013.09.017
- Moreno O, Butler T, Zann V, et al. Safety, pharmacokinetics, and pharmacodynamics of ME-401, an oral, potent, and selective inhibitor of phosphatidylinositol 3-kinase P110δ, following single ascending dose administration to healthy volunteers. Clin Ther. 2018;40(11):1855–1867. doi:10.1016/j.clinthera.2018.09.006
- Pagel JM, Soumerai JD, Reddy N, et al. Zandelisib with continuous or intermittent dosing as monotherapy or in combination with rituximab in patients with relapsed or refractory B-cell malignancy: a multicentre, first-in-patient, dose-escalation and dose-expansion, phase 1b trial. Lancet Oncol. 2022;23(8):1021–1030. doi:10.1016/S1470-2045(22)00333-3.
- Zelenetz AD, Jurczak W, Ribrag V, et al. Efficacy and safety of single-agent zandelisib administered by intermittent dosing in patients with relapsed or refractory (R/R) Follicular Lymphoma (FL): final results of the tidal phase 2 study. Blood. 2022;140(Supplement 1):3595–3597. doi:10.1182/blood-2022-165409.
