ABSTRACT
Introduction
The heterogeneity of Parkinson’s disease (PD) is evident from descriptions of non-motor (NMS) subtypes and Park Sleep, originally identified by Sauerbier et al. 2016, is one such clinical subtype associated with the predominant clinical presentation of sleep dysfunctions including excessive daytime sleepiness (EDS), along with insomnia.
Areas covered
A literature search was conducted using the PubMed, Medline, Embase, and Web of Science databases, accessed between 1 February 2023 and 28 March 2023. In this review, we describe the clinical subtype of Park Sleep and related ‘tests’ ranging from polysomnography to investigational neuromelanin MRI brain scans and some tissue-based biological markers.
Expert Opinion
Cholinergic, noradrenergic, and serotonergic systems are dominantly affected in PD. Park Sleep subtype is hypothesized to be associated primarily with serotonergic deficit, clinically manifesting as somnolence and narcoleptic events (sleep attacks), with or without rapid eye movement behavior disorder (RBD). In clinic, Park Sleep recognition may drive lifestyle changes (e.g. driving) along with therapy adjustments as Park Sleep patients may be sensitive to dopamine D3 active agonists, such as ropinirole and pramipexole. Specific dashboard scores based personalized management options need to be implemented and include pharmacological, non-pharmacological, and lifestyle linked advice.
1. Introduction
1.1. Prevalence of sleep dysfunction as related to the Park Sleep subtype in PD
Non-motor symptoms (NMS) in Parkinson’s disease (PD) have been shown to have a high prevalence and huge burden on the quality of life (QoL) and clinical expression of symptoms in PD [Citation1,Citation2]. A survey by Parkinson’s UK reported that NMS such as pain, anxiety, and sleep disorders were listed ahead of motor problems in clinical practice, and another study reported that seven out of 10 symptoms reported as most bothersome by patients with advanced PD were non-motor in nature, with sleep featuring as the fourth most bothersome symptom out of 10 in PD of 6 years or more duration [Citation1,Citation3]. While the motor subtypes of PD are well established, the non-motor subtyping concept is relatively new, and several recent papers have focused on the clinical differentiation of the non-motor subtypes, in addition to subtype-specific personalized treatment [Citation1,Citation4–7]. This approach is supported by animal model studies as well as clinical data-driven and cluster analysis-based studies [Citation8,Citation9].
Sleep dysfunction in Parkinson’s includes a wide range of symptoms and exploring each potential symptom is outside the scope of this review, however this has been widely published before [Citation10–14]. The focus of this review is on the sleep-related symptoms associated with the Park Sleep subtype, as well as the supportive investigations that can be used to clinically define this subtype. Sleep-related symptoms that occur in the Park Sleep subtype have their main anchors, which principally include excessive daytime somnolence (EDS) along with insomnia as a secondary phenomenon. Many such cases may also have co-morbid rapid eye movement (REM) behavior disorder (RBD) [Citation4].
1.2. The non-motor phenotypes and Park Sleep subtype
Cluster analysis studies have identified four specific clusters of Parkinson’s: mild, non-motor dominant, motor-dominant, and severe [Citation15]. Further analysis has outlined seven main NMS dominant subtypes: Park Sleep, Park Autonomic, Park Fatigue, Park Pain, Park Cognitive, Park Apathy, and Park Depression/Anxiety [Citation4,Citation16], based on varying neuropathology and neurotransmitter involvement. These non-motor subtypes are exhibited clinically, where specific NMS is predominantly expressed over other, NMS with a background of varying motor involvement, which may be minor [Citation4]. The Park-Sleep subtype is typically characterized by the expression of somnolence of varying degree, with or without associated insomnia. RBD, as mentioned before, may co-exist, and it is worth noting that, from a pathophysiological point of view, RBD has recently been proposed as a core feature of the noradrenergic subtype of PD [Citation17].
EDS is the inability to stay alert and awake during the day waking hours, resulting in periods of uncontrollable need for sleep or unintended lapses into drowsiness or sleep [Citation18]. EDS can be present in as high as 50% of People with Parkinson’s [Citation19], with the frequency rising in line with disease severity [Citation20,Citation21] as measured by Hoehn & Yahr (H&Y) staging, disease duration, and the Unified PD Rating Scale (UPDRS) Part III scores [Citation22,Citation23].
RBD is defined as parasomnia demonstrated by vivid dreams allied with simple or complex motor behaviors during REM sleep [Citation24]. Often the bed partner is the first to notice these behaviors during sleep, whilst the individual themselves may very well be unaware. The general population prevalence of RBD is less than 1%, but this rises to 50% in PD populations [Citation25]. Furthermore, RBD can be a prodromal NMS of PD manifesting for 10 years or more, prior to the motor features of PD itself [Citation26,Citation27]. More recently, RBD has been described as a component of the noradrenergic subtype for PD and is also considered a part of the cholinergic subtype [Citation28].
Secondary symptoms include insomnia, which may also co-exist with Park Sleep. Insomnia is defined as the difficulty in initiating, maintaining, and awakening from sleep on at least 3 days per week for 3 months [Citation29] and is reported in over 80% of the people with PD [Citation30]. Insomnia of Park Sleep could be multifactorial and in part could be secondary to EDS and manifest as ‘sleep onset insomnia’ the latter being associated with comorbidities such as mood disturbances [Citation31,Citation32].
Another important secondary cause of EDS to consider includes Obstructive Sleep Apnea (OSA), the repetitive pharyngeal collapse during sleep causing reduced airflow leading to periodic arrest in breathing [Citation33,Citation34]. Complication of OSA can lead to EDS, fatigue, cognitive changes, limb restlessness, among others. The prevalence and frequency of OSA in the PD population varies across different studies [Citation35–41]. Arguably, OSA-driven hypercapnic acidosis may additionally precipitate sleep fragmentation, and it can also lead to non-REM (NREM) parasomnia events via stimulation of serotonergic neurons, resulting in an increased excitability of motor neurons [Citation42–45].
Several lines of evidence suggest that circadian dysfunction may play an important role in somnolence and that it may present co-morbidity with other sleep disorders in patients with PD [Citation46]. For instance, dopaminergic therapy in PD is linked to a circadian phase advance and decrease in nighttime levels of melatonin [Citation47]. Melatonin is a pineal hormone thought to play a crucial role in enhancing the robustness of suprachiasmatic circadian function, which is also an indirect but practical indicator of suprachiasmatic function [Citation48]. Also, it has been shown that neurodegeneration affects the retina in PD, and frequently leads to impairment of retinal ganglion cells (RGCs), some of which are involved in circadian entrainment to light – dark cycles [Citation49].
2. Potential biomarkers
2.1. Polysomnography and electroencephalogram biomarkers
Polysomnography (PSG) is the gold standard for the diagnosis of RBD [Citation50] and is therefore an important existing diagnostic biomarker, although with limited availability in many countries, and at a high cost [Citation51]. A large study, with 2770 subjects without PD, was conducted using PSG, and those with longer total sleep time, lower REM sleep time, and higher minimum oxygen saturation during REM sleep had a higher risk of developing PD [Citation51,Citation52]* suggesting PSG could be a prodromal biomarker for PD. Whether this could apply as a prodromal biomarker for park-sleep phenotype needs investigation. Several studies suggest that RBD remains the gold standard for RBD diagnosis as well as an important prognostic biomarker and is currently the only instrument to objectively assess RBD severity [Citation53–55]. Alongside RBD, PSG is used for the diagnosis of NREM parasomnia, periodic limb movement disorder (PLMD), and sleep apnea, making it an important biomarker tool for Park Sleep subtypes of somnolence and RBD [Citation38–41].
Electroencephalogram (EEG), part of the PSG, has been shown to be an independent biomarker whereby studies report showing macro- and micro-structural sleep abnormalities prior to formal PD diagnosis, implying a neurodegeneration biomarker capability [Citation56–58]. Further supporting the notion PSG would provide good supporting evidence for confirmation of the Park Sleep subtype, specifically RBD.
2.2. Neuroimaging biomarkers
Several neuroimaging markers could be considered as potential tests to support the Park Sleep subtype; however, at this time, none have a proven evidence base. Dopamine transporter (DaT) single-photon emission computed tomography (DaTScan) using tracers such as 99mTc-TRODAT-1 and 123I–2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)-nortropane (123I-FP-CIT) in iRBD patients shows that those with decreased update have a higher relative risk of developing synucleinopathies, primarily PD [Citation59–61]. Wasserman and colleagues 2021 have shown iRBD patients having a strong positive association between the reduced dopaminergic availability and poor quality of sleep, as shown with reduced uptake in the left caudate (r = −0.630, p = 0.028) on DaTScan [Citation10], thus postulating those with reduced dopaminergic availability and poorer sleep quality, tend to have higher risk of phenoconversion to synucleinopathies. This has led many studies to be consistent with their findings in reporting DaTScans ability to be 75% sensitive, 51% specific, 44% positive predictive value, and 80% negative predictive value, with a likelihood ratio of 1.54 to predict the phenoconversion of iRBD [Citation60]. DaTScans in those with somnolence and PD shows there to be an inverse correlation between Epworth Sleepiness Scale (ESS) score and the mean DaT binding in the striatum (r = −0.627, p = 0.03); however, this is only seen with subjective somnolence reporting [Citation62]. Mirroring these findings, others have found abnormal caudate dopaminergic uptake (p = 0.030) and disease duration (p = 0.018), were both predictors for the development of EDS in PD [Citation63]. DaTScan has been proposed as a biomarker for other sleep dysfunction, such as narcolepsy with limited supportive results [Citation64].
Cerebral glucose metabolism in iRBD patients, as analyzed with 18F-fluorodeoxyglucose (18F-FDG)-PET, whereby studies have shown involvement in the amygdala, cerebellum, frontal cortical areas, and basal ganglia, corresponding to phenoconversion to PD [Citation65,Citation66]. A recent study by Diaz-Glavan and colleagues 2023 has shown that iRBD patients at higher risk of phenoconversion to PD have lower FDG uptake in the substantia nigra, thalamus, and angular gyrus [Citation67].
Magnetic resonance imaging (MRI) can be used to assess regional volume changes by applying voxel-based morphometry. Studies show significant reduction of gray matter volume, especially in the right superior temporal gyrus, right thalamus, and the posterior regions of PD-RBD patients compared to PD populations without RBD [Citation68–71]. Likewise, neuromelanin (NM) MRI has been reported to reveal changes in the locus coeruleus, sub-coeruleus complex, and substantia nigra of patients with iRBD, indicating their value as potential biomarkers [Citation72,Citation73].
Serotonergic (SERT) transporter activity (DASB) position emission tomography (PET) scans have shown lower activity in Park Sleep subtype in a small study and relate to excessive somnolence, which needs to be further investigated to establish whether central SERT deficit in the graph could underpin the Park Sleep subtype, at least in part [Citation74,Citation75]. Abnormal sensitivity to dopamine D3 receptors in the ventral striatum has been implicated and may explain the susceptibility of Park Sleep patients to highly dopamine D3 receptor selective dopamine agonists such as pramipexole and ropinirole [Citation76,Citation77].
123I-labeled meta-iodobenzylguanidine (MIBG) cardiac uptake has been shown to be reduced in clinical iRBD at similar levels to those developing PD and dementia with Lewy bodies (DLB), and therefore needs to be investigated as a potential biomarker [Citation78].
Sommerauer and colleagues 2018, conduct a study with 30 non-demented PD patients (16 of whom had PSG-confirmed RBD) undergoing cognitive function assessments using a neuropsychological battery of tests and assessment of blood pressure changes on tilting. They report PD-RBD patients had decreased locus coeruleus NM-MRI signaling (p < 0.001), and extensive reduction of 11C-MeNER update that correlated with the amount of REM sleep without atonia they had [Citation73].
Combining several imaging modalities together, as discussed above, a study by Knudsen and colleagues 2018 find that compared to controls, iRBD patients had decreased colonic 11C-donepezil uptake (p = 0.0020), 123I-MIBG heart:mediastinum ratio (p < 0.0001), NM-MRI locus coeruleus:pons ratio (p = 0.0028), and putaminal 18F-DOPA uptake (p = 0.0013). The iRBD patients had pathology of that observed in PD, yet mostly had normal putaminal dopaminergic storage but had noradrenergic thalamic denervation pathology [Citation79], thus suggesting, in part, a noradrenergic basis of iRBD.
2.3. Biofluid and tissue biomarkers
To date, no single tissue or blood-based biomarker has an evidence base to support a clinical subtype, and specifically for Park Sleep. Despite this, circulating microRNA alterations, specifically downregulated in iRBD, have been demonstrated in several neurodegenerative studies [Citation80], suggesting this downregulation in iRBD could be a biomarker for PD. Furthermore, systemic inflammatory markers, specifically C-reactive protein (CRP) and interleukin-6 (IL6), have been found to be consistently associated with sleep disturbances including insomnia although no specific link with somnolence of Park Sleep has been described [Citation81].
Likewise, plasma melatonin levels have been shown to be significantly higher in PD populations than in controls (mean 19.40 ± SEM 4.23 vs. 12.82 ± 4.85, p < 0.001) with a significantly negative correlation between plasma melatonin levels and daytime somnolence (Spearman rank = −0.308, p < 0.05) and general sleep quality as assessed by PD sleep scale (spearman = 0.336, p < 0.05) [Citation82]. These findings have been echoed by others finding somnolence in PD as having a more significant negative correlation with plasma melatonin than other NMS [Citation83].
Cerebrospinal fluid (CSF)-based biomarkers have enabled the identification of real-time quaking-induced conversion, which detects pathogenic alpha-synuclein in CSF of iRBD patients with a high specificity and sensitivity implying an increased risk of phenoconversion of iRBD to PD [Citation84,Citation85]. Furthermore, low alpha-synuclein CSF levels have been associated with EDS, insomnia, and narcolepsy, however, levels were also found to be influenced by other external factors, limiting its potential as a stand-alone biomarker [Citation86,Citation87].
PD-related narcolepsy, EDS, sleep attacks, and cataplexy have loss of hypocretin (orexin) neurons with lower orexin levels in CSF observed in some studies, however these findings remain controversial [Citation88–92]. Glial fibrillary acidic protein (GFAP) from CSF has been shown to be elevated in PD populations with narcolepsy, potentiating it as a biomarker, but studies on GFAP remain limited at present [Citation93].
Furthermore, gut microbiome in PD has gained significant momentum in recent decades given our understanding on the guts impact [Citation94–100]* on PD progression and prognosis, but further evidence is now coming to light for the similarity of iRBD gut microbiome to that of people with PD, suggesting evaluating gut microbiome of iRBD as a biomarker for phenoconversion risk to PD [Citation101,Citation102].
2.4. Biomarkers in Park Sleep summary
Recognition of Park Sleep is important not just for research into the natural history of this subtype but also for delivery of bespoke personalized treatment (). Much work still needs to be done regarding tests and potential biomarkers, but at this time PSG, if available, remains a useful surrogate to ascertain the diagnosis of RBD, while Multiple Sleep Latency Test (MSLT) may be a useful test for abnormal sleepiness along with simple clinical screening tests such as sleep items in NMS questionnaire (NMSQuest) (items 22–26) as well as the ESS. RBD in particular also appears to predict parkinsonian phenotypes which have faster progression, alongside cognitive and autonomic deficits [Citation17,Citation103]. In the future, serotonin PET studies using DASB as a ligand may be useful based on preliminary proof of concept data.
Figure 1. A proposed algorithm for identifying causes and establishing pathway for personalized management of somnolence in people with Park Sleep.
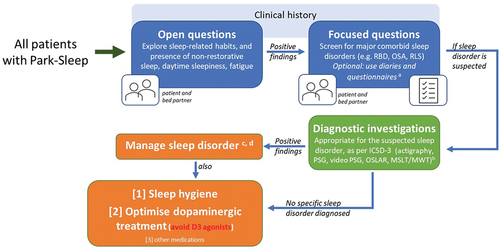
3. Importance of clinical recognition
As a result of the wide range and complex combinations of sleep disorders in PD, the recognition and diagnosis of sleep dysfunction, and as such the Park Sleep subtype, remains a challenge [Citation104]. However, there are several clinical implications of the diagnosis of the Park Sleep subtype in our routine clinical practice. The narcoleptic subtype, or indeed a propensity to sudden onset of sleep, in PD can be triggered by Dopamine D3 receptor sensitivity in the ventral striatum [Citation105]. There are also studies suggesting that specific dopamine D3 receptor agonists, such as pramipexole and ropinirole, which have a high D3 receptor affinity, may precipitate ‘sleep attacks’ in PD. In fact, this has been described originally by Frucht and colleagues (1999) who first described a phenomenon of falling asleep at the wheel in patients taking pramipexole or ropinirole [Citation106]. Road traffic accidents in such patients have serious functional consequences. Therefore, clinical recognition of this subtype would mean that subtype-specific treatment [Citation107] needs to be considered including specifically avoiding dopamine D3 receptor agonists, as well as lifestyle advice such as avoiding driving, swimming alone, and operating heavy machinery. shows an algorithm to consider a pathway to screen patients in the clinic for early clinical recognition and subsequent bespoke personalized management of Park sleep.
In the future, objective quantification tools, as shown in , may provide a better evaluation of the causes of daytime somnolence in PD. The standard objective tests of daytime sleepiness are the MSLT and the maintenance of wakefulness test (MWT) [Citation108]. MSLT and MWT provide quantitative measures of daytime sleepiness and ability to maintain vigilance. Both tests are based on latency to electroencephalographic sleep onset. However, both investigations are labor intensive, and they require technician attendance for daytime EEG monitoring. Some centers and studies have similarly argued for the utility of a behavioral test, called the Oxford sleep resistance (OSLER) test, which utilizes a computerized, non-assisted method for monitoring wakefulness and detecting sleep onset [Citation109].
4. Management
Detailed management strategies for all the components of the Park Sleep subtype are beyond the scope of this review. Clinical management of Park sleep should focus on two fundamental principles, one being pharmacotherapy and the second being non-pharmacological interventions, such as regular exercise during the day. provide a recommended algorithm to consider when approaching management strategies in Park Sleep subtype patients with somnolence, insomnia, and RBD.
Figure 2. Algorithm of managing poor nighttime sleep in Park Sleep subtype.
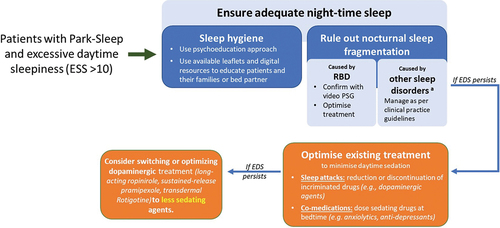
Pharmacotherapy needs to be addressed based on dominant clinical features, which could range from RBD, EDS, and insomnia. outlines the interventions, alongside pharmacological and non-pharmacological management strategies for the treatment of several sleep disorders in PD. Specific management strategies for these symptoms are already available as part of the Movement Disorder Society's evidence-based review of management of NMS [Citation110].
Table 1. Interventions, alongside pharmacological and non-pharmacological management strategies for the treatment of several sleep disorders in PD.
Modafinil, a wake-promoting agent, is approved for treatment of EDS in narcolepsy having been shown to provide modest improvement of EDS in PD [Citation111,Citation112], however much of the data shows modafinil’s improvement is rather subjective than objective [Citation111–115]. Modafinil has been proposed to have additional anti-parkinsonian actions and, intriguingly, possible neuroprotective action [Citation116–120], giving it more potential for usefulness in Park Sleep subtype, theoretically when combined with non-pharmacological therapy too.
Pitolisant, a novel stimulant drug, with potential for use in Park Sleep subtype. It is a histamine-3 receptor competitive antagonist and inverse agonist promoting histamine release in the brain, which given that in PD histamine neurons are spared suggests the potential use of this drug in treating EDS in PD patients, especially in Park Sleep subtype or in those with comorbid narcolepsy [Citation121]. One of the possible caveats could be related to the caution in potentiating the histaminergic system, since the local release of histamine in the substantia nigra may accelerate neurodegeneration. However, so far, there are no indications that the pitolisant mechanism of action compromises the effect of the anti-parkinsonian drugs.
Solriamfetol, an emerging drug that has dual action of both dopaminergic and noradrenergic reuptake inhibitor, used for EDS in narcolepsy [Citation122]. Solriamfetol has been shown to be safe and effective in maintaining wakefulness, but as a phase II Proof of Concept trial found, it does not show any significant improvement for ESS in PD [Citation123]. Unlike Pitolisant, Solriamfetol remains controversial in its effectiveness for cataplexy and similar sleep disturbances [Citation124]. Nonetheless, both Solriamfetol and Pitolisant are new drugs with the potential to be effective in Park Sleep subtype, given there is evidence for their role in EDS management.
Dual orexin receptor antagonists, including suvorexant and Lemborexant, may be recommended for the management of insomnia and EDS in many neurodegenerative and psychiatric diseases [Citation125]. Such drugs have not yet been investigated in PD specifically, but demonstrate a good safety profile for non-PD patients [Citation126,Citation127].
As mentioned, specific attention needs to be given to avoiding dopamine D3 agonists, such as ropinirole and pramipexole, in the Park Sleep group with a narcoleptic subtype, which can be identified by a high ESS score of 10 or above () [Citation6,Citation106]. Alerting agents such as modafinil may be specifically required and is rated as ‘possibly useful’ in addition to caffeine, which is currently considered ‘investigational’ [Citation110].
Figure 3. Algorithm for managing excessive daytime sleepiness in Park Sleep subtype.
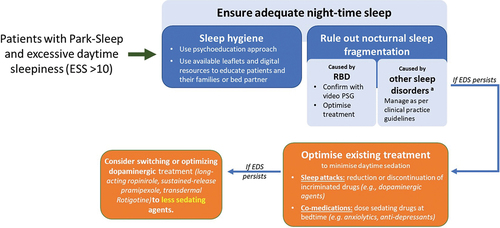
A specific point should be made about those who declare snoring, as these patients should have ESS performed and subsequently PSG, if required. Such patients have a high risk of developing OSA with there being effective treatment available [Citation128,Citation129]. These patients left untreated are at high risk of sleep attacks during the day impacting their work and QoL.
Special mention should be given to continuous delivery therapies in PD, with current focus for advanced stages of PD, given sleep disturbance is significantly more bothersome and an independent predictor of disease progression [Citation130,Citation131]. Notably, levodopa-carbidopa intestinal gel (LCIG) and continuous subcutaneous apomorphine infusion (CSAi) have shown in clinical trials to have significantly improved sleep disturbances, such as improved nighttime control of motor symptoms, improvement in daytime sleepiness, and reduced RBD features [Citation132–135]. Rotigotine (RTG) transdermal patch, a non-ergot dopamine agonist, provides continuous delivery, and has been shown in open-label studies to improve RBD and may be specifically indicated [Citation136,Citation137].
Lifestyle modification, such as advice on driving, swimming, and other occupations would need to be provided as part of the holistic management strategies [Citation138]. In addition, there is emerging evidence of the use of non-pharmacological therapies in the Park Sleep subtype group, as are depicted in Such therapies include sleep hygiene education, exercise, and interestingly, cognitive behavior therapy (CBT) for insomnia, parasomnia, or affective symptoms [Citation139–141]. In clinical trials, CBT for insomnia in PD has shown significant improvement in sleep efficiency, reducing nocturnal wakefulness, and even improving some functional performance [Citation142–145].
Figure 4. A pictorial summary of potential mind-body approaches to address sleep dysfunction as observed in Park Sleep…
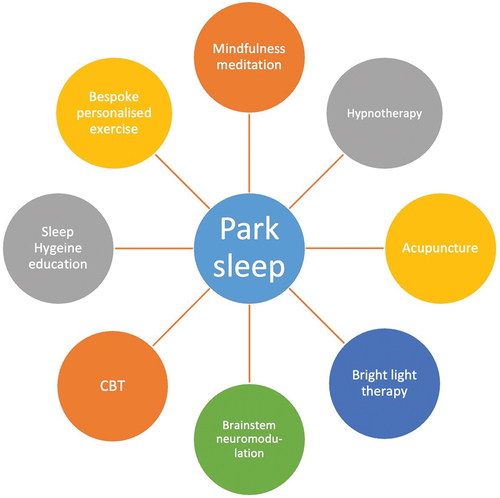
Other non-pharmacological strategies include approaches using mind-body interventions, such as mindfulness meditation [Citation146], exercise [Citation147], and hypnotherapy [Citation148] and are further potential routes being investigated. Of note, clinical hypnosis is a possible mental strategy with the ability to better sleep efficiency, being administered either from a trained hypnotherapist, applying post-hypnotic suggestions for a better sleep whenever patient goes to bed or by using an audio recording [Citation148,Citation149]. These mind-body interventions continue to be explored further in the field of PD treatment.
5. Conclusion
In conclusion, the Park Sleep subtype is a clinically relevant concept underpinning the clinical and pathological heterogeneities of PD. Its recognition, as part of the dashboard described by previously [Citation150], highlights the critical need to tailor treatment for the relevant PD populations, and the avoidance of medications such as pramipexole and ropinirole is practical in this subgroup to protect the safety and wellbeing of the patient. Much work needs to be done regarding tests and potential biomarkers, however at this time PSG, if available, remains a useful surrogate, alongside clinical assessments, to ascertain the diagnosis of certain features of this subtype. The treatment of sleep disorders in PD remains a challenge faced by clinicians, due to their complex and often intricate nature; however, a wide range of both pharmacological and non-pharmacological interventions exist. Further research is now important to enrich our clinical phenotyping of PD in the clinic so that clinical practice as well as research is enabled to address the natural history of this important non-motor subtype of PD.
6. Expert opinion
PD is a pathologically and phenotypically heterogeneous, multisystem disorder affecting the central and peripheral nervous system and is not a single ‘disease’ as traditionally viewed. Multiple neurotransmitters are involved, and apart from dopaminergic neurotransmission, the other key involvement is seen in the cholinergic, noradrenergic, and serotonergic systems. Clinically, preferentially greater pathological involvement of these non-dopaminergic systems may give rise to non-motor symptom-predominant presentations at diagnosis, termed non-motor subtypes of PD.
Non-motor subtypes segregate into several clinical categories, and Park Sleep has been postulated to be a clinical subtype often associated with a dominant underlying serotonergic deficit, which can be imaged in vivo and may become a potential biomarker. Such patients may also have significant orexin cell loss in the arousal area of the raphe in the brainstem and clinically may develop ‘sleep attacks’ like narcolepsy without cataplexy in addition to generalized excessive somnolence.
Clinical characterization can be aided by assessments with scales such as NMSQuest and ESS in the clinic and can be confirmed by using PSG. Awareness is important as such patients may be very sensitive to dopamine D3 agonists, such as ropinirole and pramipexole, and should mostly be advised not to drive or not undertake activities such as swimming alone. In addition, alerting agents such as modafinil are often required for tailored subtype-specific therapy. In our advanced PD subgroup, Park Sleep subtype tends to be more problematic, so infusion therapies such as CSAi and LCIG are shown to be promising therapeutics in advanced PD populations with sleep disturbances. As further options for advanced therapies in PD are explored, it is essential to ensure that the impact of sleep disturbances in people with PD is assessed, given the high burden as the disease progresses. Other potential treatments including Opicapone, a Catechol-O-methyl transferase (COMT) inhibitor, which is emerging on the market with the potential to provide benefit in Park Sleep manifestations in PD, are showing increasing recognition of Park Sleep subtype in PD treatment.
Non-pharmacological therapy in Park Sleep subtype is shown to be a useful agent to adopt in the clinical management of these subtypes of patients. Therapies that follow a body-mind wellness route, such as mindfulness meditation and exercise, are a growing area of management in these groups.
Abbreviations
| PD | = | Parkinson’s disease |
| NMS | = | Non-motor symptoms |
| QoL | = | Quality of Life |
| EDS | = | Excessive daytime sleepiness |
| REM | = | Rapid eye movement |
| RBD | = | REM sleep behavior disorder |
| OSA | = | Obstructive sleep apnea |
| H&Y | = | Hoehn & Yahr |
| UPDRS | = | Unified PD Rating Scale |
| NREM | = | Non-REM |
| PLMD | = | Periodic limb movement disorder |
| RGC | = | Retinal ganglion cells |
| PSG | = | Polysomnography |
| EEG | = | Electrophysiology |
| DaT | = | Dopamine transporter |
| DaTScan | = | Dopamine transporter single photon emission computed tomography |
| 123I-FP-CIT | = | 123I–2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)-nortropane |
| iRBD | = | Idiopathic RBD |
| ESS | = | Epworth sleepiness scale |
| PET | = | Positron emission tomography |
| MRI | = | Magnetic Resonance Imaging |
| NM | = | Neuromelanin |
| SERT | = | Serotonergic |
| CRP | = | C-reactive protein |
| IL-6 | = | Interleukin-6 |
| CSF | = | Cerebral spinal fluid |
| GFAP | = | Glial fibrillary acidic protein |
| SNP | = | Single nucleotide polymorphism |
| MSLT | = | Multiple sleep latency test |
| NMSQuest | = | Non-motor symptom questionnaire |
| FDG | = | [1 8 F] Fluorodeoxyglucose |
| GM | = | Grey matter |
| MIBG | = | 123I-labeled meta-iodobenzylguanidine |
| DLB | = | Dementia with Lewy Bodies |
| RNA | = | Ribonucleic acid |
| SERT | = | DASB serotonergic transporter activity. |
| MWT | = | Maintenance of wakefulness test |
| OSLER | = | Oxford sleep resistance |
| ICSD-3 | = | International Classification of Sleep Disorders version-3 |
| LCIG | = | Levodopa-carbidopa intestinal gel (LCIG) |
| CSAi | = | Continuous subcutaneous Apomorphine infusion |
| RTG | = | Rotigotine |
| CBT | = | Cognitive behavioral therapy |
| CPAP | = | Continuous positive airway pressure |
Article highlights
The Park Sleep subtype is characterized by excessive daytime somnolence (EDS), along with insomnia as a secondary phenomenon, which may co-exist with RBD.
Tests such as polysomnography and the Multiple Sleep Latency Test (MSLT) may be useful in the recognition of anormal sleepiness and RBD, alongside simple screening scales including the non-motor symptoms questionnaire and the ESS.
Clinical recognition of the Park Sleep subtype is critical, particularly in cases with a propensity to sudden onset of sleep, where Dopamine D3 agonists should be avoided
The treatment of sleep disorders in PD is challenging, due to their complex nature, and management involving both pharmacological and non-pharmacological strategies may be the most efficacious
Further research will enhance our clinical phenotyping of PD in the clinic, and as such, will enrich our understanding of the Park Sleep subtype, its biomarkers and management.
Declaration of interest
K R Chaudhuri reports receiving royalties from Oxford (book), Cambridge publishers (book), MAPI institute (KPPS, PDSS 2). He also reports being on the Committee Chair at MDS (unpaid), EAN (unpaid), receiving grants from Bial, receiving academic grants from EU Horizon 2020, Parkinson’s UK, NIHR, Parkinson’s Foundation, and Wellcome Trust. He is also on the advisory board for AbbVie, UCB, GKC, Bial, Cynapsus, Lobsor, Stada, Zambon, Profile Pharma, Synovion, Roche, Theravance, Scion, Britannia, Acadia, and 4D Pharma.
C Falup-Pecurariu reports receiving royalties from Elsevier, Springer Verlag, honoraria from AbbVie, and International Parkinson Disease and Movement Disorders Society, outside of the present work.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Todorova A, Jenner P, Ray Chaudhuri K. Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Pract Neurol. 2014 Oct;14(5):310–322. doi: 10.1136/practneurol-2013-000741
- Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011 Feb 15;26(3):399–406. doi: 10.1002/mds.23462
- Politis M, Wu K, Molloy S, et al. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord. 2010 Aug 15;25(11):1646–1651. doi: 10.1002/mds.23135
- Sauerbier A, Jenner P, Todorova A, et al. Non motor subtypes and Parkinson’s disease. Parkinsonism Related Disord. 2016 /Jan/1/;22:S41–S46. doi: 10.1016/j.parkreldis.2015.09.027
- Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord. 2016 Aug;31(8):1095–1102. doi: 10.1002/mds.26510
- Titova N, Chaudhuri KR. Personalized medicine in Parkinson’s disease: time to be precise. Mov Disord. 2017 Aug;32(8):1147–1154. doi: 10.1002/mds.27027
- Titova N, Chaudhuri KR. Non-motor Parkinson disease: new concepts and personalised management. Med J Aust. 2018 May 21;208(9):404–409. doi: 10.5694/mja17.00993
- Rodriguez-Sanchez F, Rodriguez-Blazquez C, Bielza C, et al. Identifying Parkinson’s disease subtypes with motor and non-motor symptoms via model-based multi-partition clustering. Sci Rep. 2021 Dec 8;11(1):23645. doi: 10.1038/s41598-021-03118-w
- Erro R, Vitale C, Amboni M, et al. The heterogeneity of early Parkinson’s disease: a cluster analysis on newly diagnosed untreated patients. PLoS One. 2013;8(8):e70244. doi: 10.1371/journal.pone.0070244
- Wasserman D, Bindman D, Nesbitt AD, et al. Striatal dopaminergic deficit and sleep in idiopathic rapid eye movement behaviour disorder: an explorative study. Nat Sci Sleep. 2021;13:1–9. DOI:10.2147/NSS.S267037
- Mantovani S, Smith SS, Gordon R, et al. An overview of sleep and circadian dysfunction in Parkinson’s disease. J Sleep Res. 2018 Jun;27(3):e12673. doi: 10.1111/jsr.12673
- Stefani A, Högl B. Sleep in Parkinson’s disease. Neuropsychopharmacology. 2020 Jan;45(1):121–128. doi: 10.1038/s41386-019-0448-y
- Hunt J, Coulson EJ, Rajnarayanan R, et al. Sleep and circadian rhythms in Parkinson’s disease and preclinical models. Mol Neurodegener. 2022 Jan 9;17(1):2. doi: 10.1186/s13024-021-00504-w
- Trenkwalder C. Sleep dysfunction in Parkinson’s disease. Clin Neurosci. 1998;5(2):107–114.
- Mu J, Chaudhuri KR, Bielza C, et al. Parkinson’s Disease subtypes identified from cluster analysis of motor and non-motor symptoms. Front Aging Neurosci. 2017;9:301. doi: 10.3389/fnagi.2017.00301
- Keir LHM, Breen DP. New awakenings: current understanding of sleep dysfunction and its treatment in Parkinson’s disease. J Neurol. 2020 Jan;267(1):288–294. doi: 10.1007/s00415-019-09651-z
- Chaudhuri KR, Leta V, Bannister K, et al. The noradrenergic subtype of Parkinson disease: from animal models to clinical practice. Nat Rev Neurol. 2023 Jun;19(6):333–345. doi: 10.1038/s41582-023-00802-5
- Pérez-Carbonell L, Mignot E, Leschziner G, et al. Understanding and approaching excessive daytime sleepiness. Lancet. 2022 Sep 24;400(10357):1033–1046. doi: 10.1016/S0140-6736(22)01018-2
- Abbott RD, Ross G, White L, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65(9):1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d
- Diederich NJ, Vaillant M, Mancuso G, et al. Progressive sleep ‘destructuring’in Parkinson’s disease. A polysomnographic study in 46 patients. Sleep Med. 2005;6(4):313–318. doi: 10.1016/j.sleep.2005.03.011
- O’Suilleabhain PE, Dewey, Jr RB Jr. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson disease. Arch Neurol. 2002;59(6):986–989. doi: 10.1001/archneur.59.6.986
- Kumar S, Bhatia M, Behari M. Excessive daytime sleepiness in Parkinson’s disease as assessed by Epworth Sleepiness Scale (ESS). Sleep Med. 2003;4(4):339–342. doi: 10.1016/S1389-9457(03)00105-9
- Pal S, Bhattacharya K, Agapito C, et al. A study of excessive daytime sleepiness and its clinical significance in three groups of Parkinson’s disease patients taking pramipexole, cabergoline and levodopa mono and combination therapy. J Neural Transm. 2001;108(1):71–77. doi: 10.1007/s007020170098
- Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010 Jan;1184(1):15–54. doi: 10.1111/j.1749-6632.2009.05115.x
- Zhang X, Sun X, Wang J, et al. Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson’s disease: a meta and meta-regression analysis. Neurol Sci. 2017;38(1):163–170. doi: 10.1007/s10072-016-2744-1
- Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration—an update. Nat Rev Neurol. 2018;14(1):40–55. doi: 10.1038/nrneurol.2017.157
- Arnaldi D, Antelmi E, Louis EKS, et al. Idiopathic REM sleep behavior disorder and neurodegenerative risk: to tell or not to tell to the patient? How to minimize the risk? Sleep Med Rev. 2017;36:82–95. DOI:10.1016/j.smrv.2016.11.002
- Chaudhuri KR, Leta V, Bannister K, et al. The noradrenergic subtype of Parkinson’s disease. Nat Rev Neurol. 2023;19:333–345.
- American psychiatric association D, association AP. Diagnostic and statistical manual of mental disorders: DSM-5.American Psychiatric Association.2013;5:5. Washington, DC: American Psychiatric Association.
- Gros P, Videnovic A. Sleep and circadian rhythm disorders in Parkinson’s disease. Curr Sleep Medicine Rep. 2017;3(3):222–234. doi: 10.1007/s40675-017-0079-y
- Zhu K, van Hilten JJ, Marinus J, et al. The course of insomnia in Parkinson’s disease. Parkinsonism Related Disord. 2016;33:51–57. doi: 10.1016/j.parkreldis.2016.09.010
- Kay DB, Tanner JJ, Bowers D. Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 2018;8(6):e00967. doi: 10.1002/brb3.967
- Crosta F, Desideri G, Marini C. Obstructive sleep apnea syndrome in Parkinson’s disease and other parkinsonisms. Funct Neurol. 2017;32(3):137. doi: 10.11138/FNeur/2017.32.3.137
- Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3
- Kaminska M, Lafontaine AL, Kimoff RJ. The interaction between obstructive sleep apnea and parkinson’s disease: possible mechanisms and implications for cognitive function. Parkinsons Dis. 2015;2015:1–11. doi: 10.1155/2015/849472
- Redline S, Kapur VK, Sanders MH, et al. Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment. Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):369–374. doi: 10.1164/ajrccm.161.2.9904031
- Sobreira-Neto MA, Pena-Pereira MA, Sobreira EST, et al. Obstructive sleep apnea and Parkinson’s disease: characteristics and associated factors. Arq Neuropsiquiatr. 2019;77(9):609–616. doi: 10.1590/0004-282x20190098
- Schulte EC, Winkelmann J. When Parkinson’s disease patients go to sleep: specific sleep disturbances related to Parkinson’s disease.J Neurol. 2011 [2011/May/1];258(2):328–335. DOI:10.1007/s00415-011-5933-0.
- Andrade AG, Bubu OM, Varga AW, et al. The relationship between obstructive sleep apnea and Alzheimer’s disease. J Alzheimers Dis. 2018;64(s1):S255–S270. doi: 10.3233/JAD-179936
- Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88(4):640–651. doi: 10.1016/j.bcp.2013.12.024
- Bucks RS, Olaithe M, Rosenzweig I, et al. Reviewing the relationship between OSA and cognition: where do we go from here? Respirology. 2017;22(7):1253–1261. doi: 10.1111/resp.13140
- Zhang X, Molsberry SA, Pavlova M, et al. Association of sleepwalking and REM sleep behavior disorder with Parkinson disease in men. JAMA Netw Open. 2021 Apr 1;4(4):e215713. doi: 10.1001/jamanetworkopen.2021.5713
- Terzaghi M, Minafra B, Zangaglia R, et al. NREM sleep arousal-related disorders reflect cognitive impairment in Parkinson’s disease. Sleep Med. 2020 [2020/Nov/1/];75:491–496.
- Messina A, Bitetti I, Precenzano F, et al. Non-Rapid eye movement sleep parasomnias and migraine: a role of orexinergic projections [hypothesis and theory]. Front Neurol. 2018 [2018-Feb-28];9. doi: 10.3389/fneur.2018.00095
- Juszczak GR, Swiergiel AH Serotonergic hypothesis of sleepwalking. Med Hypotheses. 2005;64(1):28–32. 2005/01/01/. doi: 10.1016/j.mehy.2004.06.013
- Endo T, Matsumura R, Tokuda IT, et al. Bright light improves sleep in patients with Parkinson’s disease: possible role of circadian restoration. Sci Rep. 2020;10(1):7982. 2020/05/14. doi: 10.1038/s41598-020-64645-6
- Breen DP, Vuono R, Nawarathna U, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014 May;71(5):589–595. doi: 10.1001/jamaneurol.2014.65
- Coomans CP, Ramkisoensing A, Meijer JH. The suprachiasmatic nuclei as a seasonal clock. Front Neuroendocrinol. 2015 Apr;37:29–42. doi: 10.1016/j.yfrne.2014.11.002
- La Morgia C, Ross-Cisneros FN, Sadun AA, et al. Retinal ganglion cells and circadian rhythms in alzheimer’s disease, parkinson’s disease, and beyond. Front Neurol. 2017;8:162. doi: 10.3389/fneur.2017.00162
- Högl B, Stefani A. REM sleep behavior disorder (RBD): update on diagnosis and treatment. Somnologie (Berl). 2017;21(Suppl 1):1–8. doi: 10.1007/s11818-016-0048-6
- Cesari M, Heidbreder A, St Louis EK, et al. Video-polysomnography procedures for diagnosis of rapid eye movement sleep behavior disorder (RBD) and the identification of its prodromal stages: guidelines from the International RBD study group. Sleep. 2022 Mar 14;45(3). doi: 10.1093/sleep/zsab257
- Otaiku AI Association of sleep abnormalities in older adults with risk of developing Parkinson’s disease. Sleep. 2022 Nov 9;45(11). doi: 10.1093/sleep/zsac206
- Cesari M, Christensen JAE, Muntean ML, et al. A data-driven system to identify REM sleep behavior disorder and to predict its progression from the prodromal stage in Parkinson’s disease. Sleep Med. 2021 Jan;77:238–248.
- Chahine LM, Kauta SR, Daley JT, et al. Surface EMG activity during REM sleep in Parkinson’s disease correlates with disease severity. Parkinsonism Relat Disord. 2014 Jul;20(7):766–771. doi: 10.1016/j.parkreldis.2014.04.011
- Dijkstra F, de Volder I, Viaene M, et al. Polysomnographic predictors of sleep, motor, and cognitive dysfunction progression in Parkinson’s disease. Curr Neurol Neurosci Rep. 2022 Oct;22(10):657–674. doi: 10.1007/s11910-022-01226-2
- Skorvanek M, Feketeova E, Kurtis MM, et al. Accuracy of rating scales and clinical measures for screening of rapid eye movement sleep behavior disorder and for predicting conversion to Parkinson’s disease and other synucleinopathies [review]. Front Neurol. 2018 [2018-May-25];9. doi: 10.3389/fneur.2018.00376
- Rodrigues Brazète J, Gagnon JF, Postuma RB, et al. Electroencephalogram slowing predicts neurodegeneration in rapid eye movement sleep behavior disorder. Neurobiol Aging. 2016 Jan;37:74–81.
- Ferini-Strambi L, Fasiello E, Sforza M, et al. Neuropsychological, electrophysiological, and neuroimaging biomarkers for REM behavior disorder. Expert Rev Neurother. 2019 Nov;19(11):1069–1087. doi: 10.1080/14737175.2019.1640603
- Li Y, Kang W, Yang Q, et al. Predictive markers for early conversion of iRBD to neurodegenerative synucleinopathy diseases. Neurology. 2017 Apr 18;88(16):1493–1500. doi: 10.1212/WNL.0000000000003838
- Iranzo A, Santamaría J, Valldeoriola F, et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2017 Sep;82(3):419–428. doi: 10.1002/ana.25026
- Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011 Sep;10(9):797–805. doi: 10.1016/S1474-4422(11)70152-1
- Happe S, Baier PC, Helmschmied K, et al. Association of daytime sleepiness with nigrostriatal dopaminergic degeneration in early Parkinson’s disease. J Neurol. 2007 Aug;254(8):1037–1043. doi: 10.1007/s00415-006-0483-6
- Yousaf T, Pagano G, Niccolini F, et al. Excessive daytime sleepiness may be associated with caudate denervation in Parkinson disease. J Neurol Sci. 2018 Apr 15;387:220–227. doi: 10.1016/j.jns.2018.02.032
- Eisensehr I, Linke R, Tatsch K, et al. Alteration of the striatal dopaminergic system in human narcolepsy. Neurology. 2003 Jun 10;60(11):1817–1819. doi: 10.1212/01.WNL.0000069608.84542.46
- Ge J, Wu P, Peng S, et al. Assessing cerebral glucose metabolism in patients with idiopathic rapid eye movement sleep behavior disorder. J Cereb Blood Flow Metab. 2015;35(12):2062–2069. doi: 10.1038/jcbfm.2015.173
- Liguori C, Ruffini R, Olivola E, et al. Cerebral glucose metabolism in idiopathic REM sleep behavior disorder is different from tau-related and α-synuclein-related neurodegenerative disorders: A brain [18F]FDG PET study. Parkinsonism Relat Disord. 2019 Jul;64:97–105.
- Diaz-Galvan P, Miyagawa T, Przybelski SA, et al. Brain glucose metabolism and nigrostriatal degeneration in isolated rapid eye movement sleep behaviour disorder. Brain Commun. 2023;5(1):fcad021. doi: 10.1093/braincomms/fcad021
- Yang C, Chang J, Liang X, et al. Gray matter alterations in parkinson’s disease with rapid eye movement sleep behavior disorder: a meta-analysis of voxel-based morphometry studies. Front Aging Neurosci. 2020;12:213. doi: 10.3389/fnagi.2020.00213
- Rahayel S, Gaubert M, Postuma RB, et al. Brain atrophy in Parkinson’s disease with polysomnography-confirmed REM sleep behavior disorder. Sleep. 2019 Jun 11;42(6). doi: 10.1093/sleep/zsz062
- Salsone M, Cerasa A, Arabia G, et al. Reduced thalamic volume in Parkinson disease with REM sleep behavior disorder: volumetric study. Parkinsonism Relat Disord. 2014 Sep;20(9):1004–1008. doi: 10.1016/j.parkreldis.2014.06.012
- Jiang X, Wu Z, Zhong M, et al. Abnormal gray matter volume and functional connectivity in parkinson’s disease with rapid eye movement sleep behavior disorder. Parkinsons Dis. 2021;2021:8851027. doi: 10.1155/2021/8851027
- Pyatigorskaya N, Gaurav R, Arnaldi D, et al. Magnetic resonance imaging biomarkers to assess substantia nigra damage in idiopathic rapid eye movement sleep behavior disorder. Sleep. 2017 Nov 1;40(11). doi: 10.1093/sleep/zsx149
- Sommerauer M, Fedorova TD, Hansen AK, et al. Evaluation of the noradrenergic system in Parkinson’s disease: an 11C-MeNER PET and neuromelanin MRI study. Brain. 2018 Feb 1;141(2):496–504. doi: 10.1093/brain/awx348
- Wilson H, Giordano B, Turkheimer FE, et al. Serotonergic dysregulation is linked to sleep problems in Parkinson’s disease. NeuroImage Clin. 2018;18:630–637. doi: 10.1016/j.nicl.2018.03.001
- Mizrahi-Kliger AD, Feldmann LK, Kühn AA, et al. Etiologies of insomnia in Parkinson’s disease – lessons from human studies and animal models. Exp Neurol [ 2022/04/01/]. 2022;350:113976. doi: 10.1016/j.expneurol.2022.113976
- Prieto GA. Abnormalities of Dopamine D(3) receptor signaling in the diseased brain. J Cent Nerv Syst Dis. 2017;9:1179573517726335. doi: 10.1177/1179573517726335
- Yang P, Perlmutter JS, Benzinger TLS, et al. Dopamine D3 receptor: A neglected participant in Parkinson disease pathogenesis and treatment? Ageing Res Rev. 2020 Jan;57:100994.
- Janzen A, Vadasz D, Booij J, et al. Progressive olfactory impairment and cardiac sympathetic denervation in REM sleep behavior disorder. J Parkinsons Dis. 2022;12(6):1921–1935. doi: 10.3233/JPD-223201
- Knudsen K, Fedorova TD, Hansen AK, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018 Jul;17(7):618–628. doi: 10.1016/S1474-4422(18)30162-5
- Fernández-Santiago R, Iranzo A, Gaig C, et al. MicroRNA association with synucleinopathy conversion in rapid eye movement behavior disorder. Ann Neurol. 2015 May;77(5):895–901. doi: 10.1002/ana.24384
- Ferrie JE, Kivimäki M, Akbaraly TN, et al. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II study. Am J Epidemiol. 2013 Sep 15;178(6):956–961. doi: 10.1093/aje/kwt072
- Li L, Zhao Z, Ma J, et al. Elevated plasma melatonin levels are correlated with the non-motor symptoms in parkinson’s disease: a cross-sectional study [original research]. Front Neurosci. 2020 [2020-May-19];14:14.
- Videnovic A, Noble C, Reid KJ, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71(4):463–469. doi: 10.1001/jamaneurol.2013.6239
- Rossi M, Candelise N, Baiardi S, et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020;140(1):49–62. doi: 10.1007/s00401-020-02160-8
- Iranzo A, Fairfoul G, Ayudhaya ACN, et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 2021;20(3):203–212. doi: 10.1016/S1474-4422(20)30449-X
- Wang X-T, Liu F-T, Bi Y-L, et al. Associations of sleep characteristics with alpha-synuclein in cerebrospinal fluid in older adults. Ann Clin Transl Neurol. 2020;7(10):2026–2034. doi: 10.1002/acn3.51204
- Wang X-T, Yu H, Liu F-T, et al. Associations of sleep disorders with cerebrospinal fluid α-synuclein in prodromal and early Parkinson’s disease. J Neurol. 2022;269(5):2469–2478. 2022/05/01. doi: 10.1007/s00415-021-10812-2
- Ripley B, Overeem S, Fujiki N, et al. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology. 2001;57(12):2253–2258. doi: 10.1212/WNL.57.12.2253
- Drouot X, Moutereau S, Nguyen J, et al. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology. 2003;61(4):540–543. doi: 10.1212/01.WNL.0000078194.53210.48
- Overeem S, Van Hilten J, Ripley B, et al. Normal hypocretin-1 levels in Parkinson’s disease patients with excessive daytime sleepiness. Neurology. 2002;58(3):498–499. doi: 10.1212/WNL.58.3.498
- Yasui K, Inoue Y, Kanbayashi T, et al. CSF orexin levels of Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal degeneration. J Neurolog Sci. 2006;250(1):120–123. 2006/12/01/. doi: 10.1016/j.jns.2006.08.004
- Ogawa T, Kajiyama Y, Ishido H, et al. Decreased cerebrospinal fluid orexin levels not associated with clinical sleep disturbance in Parkinson’s disease: A retrospective study. PLoS One. 2022;17(12):e0279747. doi: 10.1371/journal.pone.0279747
- Takahashi Y, Kanbayashi T, Hoshikawa M, et al. Relationship of orexin (hypocretin) system and astrocyte activation in Parkinson’s disease with hypersomnolence. Sleep Biol Rhythms. 2015;13(3):252–260. doi: 10.1111/sbr.12112
- Li M, Wang L, Liu JH, et al. Relationships between rapid eye movement sleep behavior disorder and neurodegenerative diseases: clinical assessments, biomarkers, and treatment. Chin Med J (Engl). 2018 Apr 20;131(8):966–973. doi: 10.4103/0366-6999.229886
- Bjørnarå KA, Pihlstrøm L, Dietrichs E, et al. Risk variants of the α-synuclein locus and REM sleep behavior disorder in Parkinson’s disease: a genetic association study. BMC neurol. 2018;18(1):1–5. doi: 10.1186/s12883-018-1023-6
- Toffoli M, Dreussi E, Cecchin E, et al. SNCA 3′ UTR genetic variants in patients with Parkinson’s disease and REM sleep behavior disorder. Neurol Sci. 2017;38(7):1233–1240. doi: 10.1007/s10072-017-2945-2
- Pont-Sunyer C, Iranzo A, Gaig C, et al. Sleep disorders in parkinsonian and nonparkinsonian LRRK2 mutation carriers. PLoS One. 2015;10(7):e0132368. doi: 10.1371/journal.pone.0132368
- Pont-Sunyer C, Tolosa E, Caspell-Garcia C, et al. The prodromal phase of leucine-rich repeat kinase 2-associated Parkinson disease: clinical and imaging studies. Mov Disord. 2017 May;32(5):726–738. doi: 10.1002/mds.26964
- Limousin N, Konofal E, Karroum E, et al. Restless legs syndrome, rapid eye movement sleep behavior disorder, and hypersomnia in patients with two parkin mutations. Mov Disord. 2009 Oct 15;24(13):1970–1976. doi: 10.1002/mds.22711
- Wang H, Lane JM, Jones SE, et al. Genome-wide association analysis of self-reported daytime sleepiness identifies 42 loci that suggest biological subtypes. Nat Commun. 2019;10(1):3503. 2019/08/13. doi: 10.1038/s41467-019-11456-7
- Nuzum ND, Loughman A, Szymlek-Gay EA, et al. To the gut microbiome and beyond: the brain-first or body-first hypothesis in Parkinson’s disease. Front Microbiol. 2022;13:791213. doi: 10.3389/fmicb.2022.791213
- Heintz‐Buschart A, Pandey U, Wicke T, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2018;33(1):88–98. doi: 10.1002/mds.27105
- Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019 Mar 1;142(3):744–759. doi: 10.1093/brain/awz030
- Aurora RN, Zak RS, Maganti RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med. 2010 Feb 15;6(1):85–95. doi: 10.5664/jcsm.27717
- Haq IZ, Naidu Y, Reddy P, et al. Narcolepsy in Parkinson’s disease. Expert Rev Neurotherapeutics. 2010;10(6):879–884. 2010/06/01. doi: 10.1586/ern.10.56
- Frucht S, Rogers JD, Greene PE, et al. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology. 1999 Jun 10;52(9):1908–1910. doi: 10.1212/WNL.52.9.1908
- Marras C, Chaudhuri KR, Titova N, et al. Therapy of Parkinson’s disease subtypes. Neurotherapeutics. 2020 Oct;17(4):1366–1377. doi: 10.1007/s13311-020-00894-7
- Neutel D, Peralta R, Pires J, et al. End of OSLER test sessions in Parkinson’s disease do not correspond to true sleep onset: results from an exploratory study [original research]. Front Neurol. 2015 [2015-Sep-24];6:6.
- BENNETT L, STRADLING J, DAVIES R. A behavioural test to assess daytime sleepiness in obstructive sleep apnoea. J Sleep Res. 1997;6(2):142–145. doi: 10.1046/j.1365-2869.1997.00039.x
- Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord. 2019 Feb;34(2):180–198. doi: 10.1002/mds.27602
- Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov Disord. 2003;18(3):287–293. doi: 10.1002/mds.10390
- Högl B, Saletu M, Brandauer E, et al. Modafinil for the treatment of daytime sleepiness in Parkinson’s disease: a double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep. 2002 Dec;25(8):905–909. doi: 10.1093/sleep/25.8.62
- Ondo WG, Fayle R, Atassi F, et al. Modafinil for daytime somnolence in Parkinson’s disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry. 2005 Dec;76(12):1636–1639. doi: 10.1136/jnnp.2005.065870
- Rodrigues TM, Castro Caldas A, Ferreira JJ. Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: systematic review and meta-analysis. Parkinsonism Relat Disord. 2016 Jun;27:25–34. doi: 10.1016/j.parkreldis.2016.03.002
- Knie B, Mitra MT, Logishetty K, et al. Excessive daytime sleepiness in patients with Parkinson’s disease. CNS Drugs. 2011 Mar;25(3):203–212. doi: 10.2165/11539720-000000000-00000
- Ando R, Choudhury ME, Yamanishi Y, et al. Modafinil alleviates levodopa-induced excessive nighttime sleepiness and restores monoaminergic systems in a nocturnal animal model of Parkinson’s disease. J Pharmacol Sci. 2018;136(4):266–271. 2018/04/01/. doi: 10.1016/j.jphs.2018.03.005
- Van Vliet SA, Vanwersch RA, Jongsma MJ, et al. Neuroprotective effects of modafinil in a marmoset Parkinson model: behavioral and neurochemical aspects. Behav Pharmacol. 2006;17(5–6):453–462. doi: 10.1097/00008877-200609000-00011
- Jenner P, Zeng B-Y, Smith L, et al. Antiparkinsonian and neuroprotective effects of modafinil in the mptp-treated common marmoset. Exp Brain Res. 2000;133(2):178–188. doi: 10.1007/s002210000370
- Aguirre J, Cintra A, Hillion J, et al. A stereological study on the neuroprotective actions of acute modafinil treatment on 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced nigral lesions of the male black mouse. Neurosci lett. 1999;275(3):215–218. doi: 10.1016/S0304-3940(99)00706-5
- Hu X, Li J, Wang X, et al. Neuroprotective Effect of Melatonin on Sleep Disorders Associated with Parkinson’s Disease. Antioxidants. 2023;12(2):396. doi: 10.3390/antiox12020396
- Liguori C, Placidi F, Izzi F, et al. Pitolisant for treating narcolepsy comorbid with Parkinson’s disease. Sleep Med [ 2020/05/01/]. 2020;69:86–87. doi: 10.1016/j.sleep.2020.01.020
- Yang J, Gao J. Solriamfetol for the treatment of excessive daytime sleepiness associated with narcolepsy. Expert Rev Clin Pharmacol. 2019 Aug;12(8):723–728. doi: 10.1080/17512433.2019.1632705
- Videnovic A, Amara AW, Comella C, et al. Solriamfetol for excessive daytime sleepiness in parkinson’s disease: phase 2 proof-of-concept trial. Mov Disord. 2021;36(10):2408–2412. doi: 10.1002/mds.28702
- Iturburu A, Pallares Vela E, Cruz C, et al. Solriamfetol for the use of narcolepsy: a systematic review. Cureus. 2022 May;14(5):e24937. doi: 10.7759/cureus.24937
- Muehlan C, Vaillant C, Zenklusen I, et al. Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opin Drug Metab Toxicol. 2020 Nov;16(11):1063–1078. doi: 10.1080/17425255.2020.1817380
- Cheng JY, Filippov G, Moline M, et al. Respiratory safety of lemborexant in healthy adult and elderly subjects with mild obstructive sleep apnea: A randomized, double-blind, placebo-controlled, crossover study. J Sleep Res. 2020 Aug;29(4):e13021. doi: 10.1111/jsr.13021
- Moline M, Zammit G, Yardley J, et al. Lack of residual morning effects of lemborexant treatment for insomnia: summary of findings across 9 clinical trials. Postgrad Med. 2021 Jan;133(1):71–81. doi: 10.1080/00325481.2020.1823724
- Meng L, Benedetti A, Lafontaine AL, et al. Obstructive sleep apnea, CPAP therapy and Parkinson’s disease motor function: A longitudinal study. Parkinsonism Relat Disord. 2020 Jan;70:45–50.
- Neikrug AB, Liu L, Avanzino JA, et al. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep. 2014 Jan 1;37(1):177–185. doi: 10.5665/sleep.3332
- Ou R, Hou Y, Wei Q, et al. Longitudinal evolution of non-motor symptoms in early Parkinson’s disease: a 3-year prospective cohort study. NPJ Parkinsons Dis. 2021 Jul 15;7(1):58. doi: 10.1038/s41531-021-00207-5
- Santos-García D, de Deus T, Cores C, et al. Predictors of global non-motor symptoms burden progression in Parkinson’s disease. Results from the COPPADIS cohort at 2-year follow-up. J Pers Med. 2021 Jun 30;11(7):626. doi: 10.3390/jpm11070626
- Chaudhuri KR, Antonini A, Pahwa R, et al. Effects of levodopa-carbidopa intestinal gel on dyskinesia and non-motor symptoms including sleep: results from a meta-analysis with 24-month follow-up. J Parkinsons Dis. 2022;12(7):2071–2083. doi: 10.3233/JPD-223295
- Antonini A, Poewe W, Chaudhuri KR, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord. 2017 Dec;45:13–20.
- Standaert DG, Aldred J, Anca-Herschkovitsch M, et al. DUOGLOBE: one-year outcomes in a real-world study of levodopa carbidopa intestinal gel for Parkinson’s disease. Mov Disord Clin Pract. 2021 Oct;8(7):1061–1074. doi: 10.1002/mdc3.13239
- De Cock VC, Dodet P, Leu-Semenescu S, et al. Safety and efficacy of subcutaneous night-time only apomorphine infusion to treat insomnia in patients with Parkinson’s disease (APOMORPHEE): a multicentre, randomised, controlled, double-blind crossover study. Lancet Neurol. 2022 May;21(5):428–437. doi: 10.1016/S1474-4422(22)00085-0
- Högl B, Oertel WH, Stiasny-Kolster K, et al. Treatment of moderate to severe restless legs syndrome: 2-year safety and efficacy of rotigotine transdermal patch. BMC neurol. 2010 Sep 28;10(1):86. doi: 10.1186/1471-2377-10-86
- Oertel W, Trenkwalder C, Beneš H, et al. Long-term safety and efficacy of rotigotine transdermal patch for moderate-to-severe idiopathic restless legs syndrome: a 5-year open-label extension study. Lancet Neurol. 2011 Aug;10(8):710–720. doi: 10.1016/S1474-4422(11)70127-2
- Lee J, Kim Y, Kim YL. Non-pharmacological therapies for sleep disturbances in people with Parkinson’s disease: A systematic review. J Adv Nurs. 2018 Apr 27;74(8):1741–1751. doi: 10.1111/jan.13694
- Taximaimaiti R, Luo X, Wang XP. Pharmacological and non-pharmacological treatments of sleep disorders in Parkinson’s disease. Curr Neuropharmacol. 2021;19(12):2233–2249. doi: 10.2174/1570159X19666210517115706
- Wahbeh H, Elsas SM, Oken BS. Mind-body interventions: applications in neurology. Neurology. 2008 Jun 10;70(24):2321–2328. doi: 10.1212/01.wnl.0000314667.16386.5e
- O’Regan D, Nesbitt A, Biabani N, et al. A novel group cognitive behavioral therapy approach to adult non-rapid eye movement parasomnias [brief research report]. Front Psychiatry. 2021 [2021-Jul-1];12:12.
- Humbert M, Findley J, Hernandez-Con M, et al. Cognitive behavioral therapy for insomnia in Parkinson’s disease: a case series. NPJ Parkinsons Dis. 2017;3(1):25. doi: 10.1038/s41531-017-0027-z
- Osawa C, Kamei Y, Nozaki K, et al. Brief cognitive behavioral therapy for insomnia in Parkinson’s disease: a case series study 1. Jpn Psychol Res. 2021;63(2):59–71. doi: 10.1111/jpr.12287
- Lebrun C, Gély-Nargeot MC, Bayard S. Insomnia comorbid to Parkinson’s disease part II: therapeutic approaches. Geriatr Psychol Neuropsychiatr Vieil. 2021 Apr 6. DOI:10.1684/pnv.2021.0927
- Patel S, Ojo O, Genc G, et al. A computerized cognitive behavioral therapy randomized, controlled, pilot trial for insomnia in Parkinson disease (ACCORD-PD). J Clin Mov Disord. 2017;4(1):16. doi: 10.1186/s40734-017-0062-2
- Son HG, Choi E-O. The effects of mindfulness meditation-based complex exercise program on motor and nonmotor symptoms and quality of life in patients with Parkinson’s disease. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12(2):145–153. doi: 10.1016/j.anr.2018.06.001
- Wang K, Li K, Zhang P, et al. Mind-body exercises for non-motor symptoms of patients with Parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2021;13:770920. doi: 10.3389/fnagi.2021.770920
- Elkins G, Sliwinski J, Bowers J, et al. Feasibility of clinical hypnosis for the treatment of Parkinson’s disease: a case study. Int J Clin Exp Hypn. 2013;61(2):172–182. doi: 10.1080/00207144.2013.753829
- Amara AW, Chahine LM, Videnovic A. Treatment of sleep dysfunction in Parkinson’s disease. Curr Treat Options Neurol. 2017;19(7):1–16. doi: 10.1007/s11940-017-0461-6
- Chaudhuri KR, Titova N, Qamar MA, et al. The dashboard vitals of Parkinson’s: not to be missed yet an unmet need. J Pers Med. 2022 Dec 2;12(12):1994. doi: 10.3390/jpm12121994
