1. Introduction
Although recent years have seen a shift toward the use of targeted cancer therapy, chemotherapy remains the backbone of treatment of most advanced or metastatic cancers, including hematological malignancies such as acute leukemia, multiple myeloma (MM) and lymphoma. Curative treatment of acute leukemia still consists of high doses of multi-drug chemotherapy regimens, and high dose melphalan followed by autologous stem cell transplantation remains the standard of care for younger patients with MM. However, the flip side of chemotherapeutics is their lack of specificity to target cancer cells, which may lead to severe side effects, such as myelosuppression, cardiomyopathy, toxic enteritis, and risk of severe infections. Therefore, new, potent, and less toxic treatment modalities are an important goal for future cancer therapy.
2. Liposomal drug delivery systems
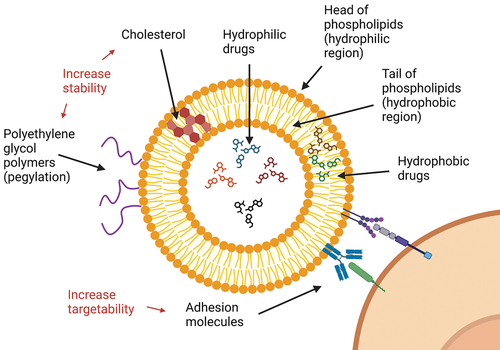
Drug-delivery systems have been developed to optimize the pharmacokinetics and pharmacodynamics of chemotherapeutics to increase their therapeutic efficacy. Different technologies for drug delivery exist, but liposomal formulations are the most widespread. Liposomes are generally bilayer phospholipid vesicles; thus, they may be loaded with both hydrophilic and hydrophobic drugs (). Furthermore, other compounds such as cholesterol or polyethylene glycol can be included to alter physiochemical properties and increase liposome stability and circulation time (). Anthracyclines, such as doxorubicin and daunorubicin, are cytotoxic antibiotics widely used to treat various malignancies, and hence can be used as a model for reviewing the clinical benefits of liposomal formulations. Although safety profiles are generally comparable, liposomal formulations of doxorubicin are in fact associated with a significant reduction in cardiotoxicity for treatment of patients with breast cancer [Citation1,Citation2]. Nonetheless, even though preclinical data on liposome-formulated chemotherapeutics may be promising, they often fail to translate into improved therapeutic efficacy in clinical studies [Citation3]. Thus, the rationale for developing new liposomal formulations of existing drugs may currently seem somewhat uncertain.
3. The hematological experience
3.1. Multiple myeloma
Introduced in the 1990s, pegylated liposomal doxorubicin (PLD) is approved for the use in patients with MM in combination with the proteasome inhibitor bortezomib. A randomized phase III trial initially showed a minor clinical benefit of 2.8 months increased time-to-progression for PLD plus bortezomib, compared to bortezomib alone [Citation4], although long-term follow up failed to show any survival benefits [Citation5]. When combined with vincristine and dexamethasone, single-dose PLD showed similar efficacy, however less myelotoxicity compared to four-day continuous infusion of conventional doxorubicin [Citation6]. Although the latter treatment regimen is currently scarcely used to treat patients with MM, it serves as an example of the possible benefits of liposomal doxorubicin. PLD has also been tested in combination with other drugs such as carfilzomib [Citation7], pomalidomide [Citation8], cyclophosphamide [Citation9], lenalidomide [Citation10], and thalidomide [Citation11]. However, randomized trials for these combinations are lacking. According to search in the database ClinicalTrials.gov (‘multiple myeloma’ + ‘liposomal doxorubicin’ at 27.06.23), there is currently only one ongoing or recruiting randomized clinical trial on the use of PLD, investigating the effects compared to cyclophosphamide in combination with the XPO1 inhibitor selinexor and dexamethasone (NCT04877275). Thus, the outlook for PLD in future treatment of MM remains dim.
3.2. Acute myelogenous leukemia
PLD has also been tested for treatment of patients with acute myelogenous leukemia (AML). However, in combination with bortezomib for treatment of unfit patients, only minimal response was achieved [Citation12]. Although the use of PLD indeed was found to increase remission rates and survival when combined with cladribine, cytarabine, and G-CSF (mean overall survival 30.7 vs 14.9 months), this is currently only supported by a single-center retrospective study [Citation13]. The major effect of the addition of PLD in these patients also seemed to be limited to a shift from partial response to MRD+ complete remission.
CPX-351 is a new liposome-encapsulated formulation of cytarabine and daunorubicin that maintains the optimum antileukemic molar ratio of 5:1 in the plasma and bone marrow [Citation14]. These liposomes contain distearoylphosphatidylcholine, distearoylphosphatidylglycerol, and cholesterol in a 7:2:1 mole ratio, and have selective toxicity to leukemic cells compared to healthy hematopoietic progenitors in vitro [Citation15]. More importantly, CPX-351 markedly improved overall survival and remission rates compared with conventional treatment (47.7% vs. 33.3% remission rates) with similar toxicity in older patients with newly diagnosed secondary acute myeloid leukemia [Citation16]. There was also an improvement in the quality-of-life measure Q-TWiST [Citation17]. In contrast, a recent retrospective study questioned the clinical benefits of CPX-351 when compared to purine analogue-containing regimens [Citation18]. This finding warrants further investigation as the purine analogue-containing regimens generally have not found their place as a first-line treatment of AML even if they may produce slightly better outcomes. Thus, the role of CPX-351 in first-line treatment of AML is still best regarded as undetermined. Currently, multiple trials are ongoing for the use of CPX-351 in combination with different drugs such as gemtuzumab ozogamicin (NCT05599360), gilteritinib (NCT05024552), and venetoclax (NCT03826992), which may further clarify its place in treatment of AML.
3.3. Lymphoma
There are some data supporting the use of non-pegylated liposomal doxorubicin (NPLD) in lymphoma, but randomized clinical trials are scarce. In spite of the reduction in cardiotoxicity seen when using NPLD to treat patients with breast cancer, two randomized clinical trials comparing NPLD to conventional doxorubicin have failed to show any differences in left ventricular ejection fraction in patients treated for diffuse large B-cell lymphoma [Citation19,Citation20]. Although secondary endpoints in both studies, clinical outcomes were also similar. There are also several studies suggesting NPLD may be safe for treating patients with various types of lymphoma and severe cardiac comorbidities where conventional treatment may not be advisable, however, randomized controlled trials are currently lacking.
4. Expert opinion
Several liposomal anticancer drugs have previously been approved by FDA and EMA, including formulations of daunorubicin, doxorubicin, cytarabine, and vincristine (). However, the clinical benefits remain limited and several drugs such as formulations of liposomal daunorubicin [Citation21], liposomal cytarabine [Citation22], and liposomal vincristine [Citation23], have all since been discontinued for various reasons, including lack of proof of therapeutic efficacy. Thus, there are few success stories for liposomal anticancer drugs in hematology. Moreover, the use of drug-delivery systems in general also remains limited for treatment of all cancers, with some exceptions such as the albumin nanoparticle-based solution of paclitaxel. The theoretical benefits of drug-delivery systems such as increased cancer cell drug uptake and increased in vivo drug stability seem to translate poorly into improved clinical outcome. Although liposomal doxorubicin is associated with less cardiotoxicity, severe cardiomyopathy remains a rare side effect even for conventional doxorubicin, which may further explain why the use of the liposomal formulation is quite limited.
Table 1. List of liposomal drugs in hematology. Abbreviations: ALL, acute lymphoblastic leukemia; AML. Acute myelogenous leukemia; MM, multiple myeloma; RCTs, randomized controlled trials.
Thus, for liposomal formulations to increase therapeutic efficacy, new complex properties of liposomes need to be introduced. One example is the fixing of the optimum molar ratio between different drugs, exemplified by CPX-351. Still, the improvements in clinical outcomes are for the time being supported by a single phase III randomized controlled trial investigating only a subset of AML. More importantly, incremental cost per patient remains an issue [Citation24]. Thus, in order to confirm superiority compared to conventional treatment, larger studies on induction treatment of all major AML subsets are necessary.
A long-sought-after property of drug-delivery systems is cancer cell selectivity. The reduced cardiotoxicity of liposomal doxorubicin is believed to stem from lower myocardial penetration of the drug, with a subsequently altered molecular response to the cellular damage [Citation25]. In the case of curative treatment of AML with intensive chemotherapy, myelosuppression with the following risk of severe infections is the main cause of treatment-related mortality. Thus, it would be hugely beneficial for patients if drug-delivery systems could impair the toxic effects of anticancer drugs on healthy hematopoietic stem and progenitor cells. However, as these cells reside alongside leukemic cells in the bone marrow, drug selectivity is more complex than avoiding distribution of drug into specific tissues. One possible solution may be binding of leukemia-specific adhesion molecules or antibodies to the liposomes to create immunoliposomes (). However, there still is a major challenge in identifying a common target molecule. This will need to cover all subsets of AML cells with the interindividual variation of different patients, in addition to the intraindividual variation of the specific patients, which includes leukemic stem cells and other subclones. In addition, the target molecule must be expressed at low levels or lacking in healthy hematopoietic stem and progenitor cells. For that reason, the use of chemotherapy in any form may never be a targeted therapy in hematological malignancies, and the use of liposomal formulations may thus always be limited.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Ghasemi K, Vaseghi G, Mansourian M. Pharmacological interventions for preventing anthracycline-induced clinical and subclinical cardiotoxicity: a network meta-analysis of metastatic breast cancer. J Oncol Pharm Pract. 2021 Mar;27(2):414–427. doi: 10.1177/1078155220965674
- Ansari L, Shiehzadeh F, Taherzadeh Z, et al. The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials. Cancer Gene Ther. 2017 May;24(5):189–193. doi: 10.1038/cgt.2017.9
- Petersen GH, Alzghari SK, Chee W, et al. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J Control Release. 2016 Jun 28;232:255–264. doi: 10.1016/j.jconrel.2016.04.028
- Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007 Sep 1;25(25):3892–3901. doi: 10.1200/JCO.2006.10.5460
- Orlowski RZ, Nagler A, Sonneveld P, et al. Final overall survival results of a randomized trial comparing bortezomib plus pegylated liposomal doxorubicin with bortezomib alone in patients with relapsed or refractory multiple myeloma. Cancer. 2016 Jul 1;122(13):2050–2056. doi: 10.1002/cncr.30026
- Rifkin RM, Gregory SA, Mohrbacher A, et al. Pegylated liposomal doxorubicin, vincristine, and dexamethasone provide significant reduction in toxicity compared with doxorubicin, vincristine, and dexamethasone in patients with newly diagnosed multiple myeloma: a phase III multicenter randomized trial. Cancer. 2006 Feb 15;106(4):848–858. doi: 10.1002/cncr.21662
- Schroeder MA, Fiala MA, Huselton E, et al. A phase I/II trial of Carfilzomib, pegylated liposomal doxorubicin, and dexamethasone for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res. 2019 Jul 1;25(13):3776–3783. doi: 10.1158/1078-0432.CCR-18-1909
- Cohen A, Spektor TM, Stampleman L, et al. Safety and efficacy of pomalidomide, dexamethasone and pegylated liposomal doxorubicin for patients with relapsed or refractory multiple myeloma. Br J Haematol. 2018 Jan;180(1):60–70. doi: 10.1111/bjh.14992
- Becker PS, Gooley TA, Green DJ, et al. A phase 2 study of bortezomib, cyclophosphamide, pegylated liposomal doxorubicin and dexamethasone for newly diagnosed multiple myeloma. Blood Cancer J. 2016 May 13;6(5):e422. doi: 10.1038/bcj.2016.31
- Baz RC, Shain KH, Hussein MA, et al. Phase II study of pegylated liposomal doxorubicin, low-dose dexamethasone, and lenalidomide in patients with newly diagnosed multiple myeloma. Am J Hematol. 2014 Jan;89(1):62–67. doi: 10.1002/ajh.23587
- Sher T, Ailawadhi S, Miller KC, et al. A steroid-independent regimen of bortezomib, liposomal doxorubicin and thalidomide demonstrate high response rates in newly diagnosed multiple myeloma patients. Br J Haematol. 2011 Jul;154(1):104–110. doi: 10.1111/j.1365-2141.2011.08703.x
- Tomlinson BK, Tuscano JM, Abedi M, et al. A phase II study of bortezomib in combination with pegylated liposomal doxorubicin for acute myeloid leukemia. Am J Hematol. 2019 Nov;94(11):E291–e294. doi: 10.1002/ajh.25605
- Yao H, Zhang C, Tan X, et al. Efficacy and toxicity of CLAG combined with pegylated liposomal doxorubicin in the treatment of refractory or relapsed acute myeloid leukemia. Cancer Med. 2023 Jun;12(11):12377–12387. doi: 10.1002/cam4.5938
- Tardi P, Johnstone S, Harasym N, et al. In vivo maintenance of synergistic cytarabine: daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009 Jan;33(1):129–139. doi: 10.1016/j.leukres.2008.06.028
- Kim HP, Gerhard B, Harasym TO, et al. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol. 2011 Jul;39(7):741–750. doi: 10.1016/j.exphem.2011.04.001
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018 Sep 10;36(26):2684–2692. doi: 10.1200/JCO.2017.77.6112
- Cortes JE, Lin TL, Uy GL, et al. Quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) analysis of CPX-351 versus 7 + 3 in older adults with newly diagnosed high-risk/secondary AML. J Hematol Oncol. 2021 Jul 13;14(1):110. doi: 10.1186/s13045-021-01119-w
- Benitez LL, Perissinotti AJ, Rausch CR, et al. Multicenter comparison of high-dose cytarabine-based regimens versus liposomal daunorubicin and cytarabine (CPX-351) in patients with secondary acute myeloid leukemia. Leuk Lymphoma. 2021 Sep;62(9):2184–2192. doi: 10.1080/10428194.2021.1907378
- Fridrik MA, Jaeger U, Petzer A, et al. Cardiotoxicity with rituximab, cyclophosphamide, non-pegylated liposomal doxorubicin, vincristine and prednisolone compared to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in frontline treatment of patients with diffuse large B-cell lymphoma: a randomised phase-III study from the Austrian cancer drug therapy working group [arbeitsgemeinschaft medikamentöse tumortherapie AGMT](NHL-14). Eur J Cancer. 2016 May;58:112–121. doi: 10.1016/j.ejca.2016.02.004
- Sancho JM, Fernández-Alvarez R, Gual-Capllonch F, et al. R-COMP versus R-CHOP as first-line therapy for diffuse large B-cell lymphoma in patients ≥60 years: results of a randomized phase 2 study from the Spanish GELTAMO group. Cancer Med. 2021 Feb;10(4):1314–1326. doi: 10.1002/cam4.3730
- Latagliata R, Breccia M, Fazi P, et al. Liposomal daunorubicin versus standard daunorubicin: long term follow-up of the GIMEMA GSI 103 AMLE randomized trial in patients older than 60 years with acute myelogenous leukaemia. Br J Haematol. 2008 Dec;143(5):681–689. doi: 10.1111/j.1365-2141.2008.07400.x
- Glantz MJ, LaFollette S, Jaeckle KA, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999 Oct;17(10):3110–3116. doi: 10.1200/JCO.1999.17.10.3110
- O’Brien S, Schiller G, Lister J, et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome–negative acute lymphoblastic leukemia. J Clin Oncol. 2013 Feb 20;31(6):676–683. doi: 10.1200/JCO.2012.46.2309
- Bewersdorf JP, Patel KK, Goshua G, et al. Cost-effectiveness of liposomal cytarabine/daunorubicin in patients with newly diagnosed acute myeloid leukemia. Blood. 2022;139(11):1766–1770. doi: 10.1182/blood.2021014401
- Gyöngyösi M, Lukovic D, Zlabinger K, et al. Liposomal doxorubicin attenuates cardiotoxicity via induction of interferon-related DNA damage resistance. Cardiovasc Res. 2020 Apr 1;116(5):970–982. doi: 10.1093/cvr/cvz192