ABSTRACT
Introduction
A new era of treatment for adults with treatment-resistant depression (TRD), which involves psychedelic substances, is dawning. Emerging evidence indicates that psychedelics can exert antidepressant effects through multiple neurobiological and psychological mechanisms. However, it remains to be seen if these new treatments will revolutionize the treatment of TRD.
Areas covered
The present review focuses on the efficacy of serotoninergic psychedelics psilocybin, lysergic acid diethylamide (LSD), N,N-dimethyltryptamine (DMT), ayahuasca, 5‐methoxy‐N,N‐dimethyltryptamine (5-MeO-DMT) and mescaline (3,4,5-trimethoxyphenethylamine), as well as 3,4-methylenedioxymethamphetamine (MDMA), for TRD. A systematic search was conducted for psilocybin in TRD as emerging trials had not yet been subject to review. A narrative review summarized findings on other psychedelics.
Expert opinion
Psychedelic therapy has created a paradigm shift in the treatment of TRD, as it can maximize therapeutic benefits and minimize potential risks. Psilocybin holds promise as a potential game-changer in the treatment of TRD, with initial evidence suggesting a rapid antidepressant effect sustained for some responders for at least 3 months. Nevertheless, further adequately powered, double-blind, comparator-controlled trials are required to explore and clarify the mechanisms of action and long-term effects of psychedelics in TRD. Psychedelics also hold promise for other psychiatric conditions, such as bipolar depression and post-traumatic stress disorder.
1. Introduction
1.1. Major depressive disorder and treatment resistance
Major depressive disorder (MDD) is a psychiatric disorder characterized by a cluster of symptoms including low mood, anhedonia, hopelessness, feelings of guilt, lack of energy, poor concentration, suicidal ideation and changes in sleep and appetite. To meet the criteria of a major depressive episode, these symptoms need to persist for at least 2 weeks [Citation1]. The 12-month prevalence of MDD is estimated to be around 5–6% [Citation2,Citation3]. MDD is associated with a considerable disease burden and is one of the leading causes of years lived with disability [Citation4,Citation5]. The total cost of depression in the United States was estimated to be more than $200 billion in 2010 [Citation6].
There are various treatment options for MDD, including psychological therapies and pharmacological treatments. The National Institute for Clinical Excellence (NICE) guidelines recommend cognitive behavioral therapy and selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNIRs), as the first line of treatment for depression [Citation7]. The remission rate following the first course of an SSRI (i.e. citalopram) is only 36.8%, whilst the remission rates are even lower in the second (30.6%), third (13.7%) and fourth course (13%) of treatment [Citation8]. Although definitions may vary, treatment-resistant depression (TRD) is commonly defined as failure to respond to two or more adequate in dose and duration (at least 6 weeks) treatment courses for different classes of antidepressants [Citation9]. TRD is associated with significant burden [Citation10] and higher healthcare costs than non-TRD[Citation11]. TRD also presents high suicide risk [Citation12], with TRD patients reporting greater lifetime suicidal behavior and worse quality of life compared to those with non-TRD [Citation13].
The treatment of TRD presents significant challenges. Current options for TRD include combinations of different antidepressants and augmentation strategies (e.g. addition of mood stabilizers, antipsychotics or thyroid hormone) [Citation14,Citation15]. These treatment options are associated with further side effects and complications. For example, in real-world settings, psychotropic drugs are associated with a higher severity of total and psychic side effects in TRD and non-responders when compared to responders [Citation16].
A significant proportion of people with TRD continue to experience symptoms despite numerous courses of different treatments [Citation17]. This highlights the need for novel treatment options in TRD, with the use of psychedelics being an emerging area of research in TRD. Recent meta-analyses have showed that psychedelics, and psilocybin in particular, produce rapid antidepressant effects in people with depression [Citation18–20], although research in TRD is still limited.
1.2. Psychedelic substances
Psychedelics are a group of psychoactive substances that are associated with altered state of consciousness and perception [Citation21]. There are two main categories of psychedelics; classic serotoninergic that primarily act on 5-HT2A receptors, including psilocybin, N,N-dimethyltryptamine (DMT), ayahuasca, lysergic acid diethylamide (LSD), and atypical, such as 3,4-methylenedioxymethamphetamine (MDMA), that act on various receptors.
Psilocybin is the psychoactive alkaloid of some species of mushrooms, also known as ‘magic mushrooms’ and is considered a prodrug of psilocin [Citation22]. Psilocybin exerts its effects through agonism of the serotonin 5-HT2A receptors [Citation23–25]. The 5-HT2A receptors are necessary for the psychedelic experience, as the subjective effects of psilocybin seem to be blocked by 5-HT2A antagonists [Citation26]. Psilocybin also has affinity, albeit to a smaller extent, for several other serotonin receptors such as 5-HT1A and 5-HT2C [Citation27,Citation28]. Psilocybin has a short duration of action with the half-life of a 25 mg psilocybin estimated to be 108 min (range 66–132 min). The peak subjective effects occur 60–90 min after intake and last 4–6 h [Citation29–31].
Ayahuasca has been historically used ceremonially, particularly in communities indigenous to the Amazon basin [Citation32]. Ayahuasca is a psychoactive brew of two plants, Banisteriopsis caapi and Psychotria viridis, which contain β-carboline alkaloids (harmine, tetrahydroharmine, and harmaline) and DMT, respectively [Citation33,Citation34]. These β-carboline alkaloids are monoamine oxidase-A (MAO-A) inhibitors, whereas DMT is orally psychoactive only when it is ingested along with MAO inhibitors [Citation34]. DMT is a classic serotoninergic psychedelic which is mainly an agonist of the 5-HT1A and 5-HT2A receptors [Citation21,Citation23].
5‐methoxy‐N,N‐dimethyltryptamine (5-MeO-DMT) is a short-acting serotoninergic psychedelic which primarily acts as an agonist of the 5-HT1A and 5-HT2A receptors, but also has lower binding affinity for dopamine receptors and norepinephrine transporters [Citation21,Citation23,Citation35].
LSD is primarily a 5-HT2A receptor partial agonist and a 5-HT1A receptor agonist but has also been shown to bind to 5-HT2C receptors [Citation36–38]. The use of LSD and psilocybin as recreational drugs became popular in the middle of the last century but were classed as a Schedule I drug in 1967, which prevented research in psychedelics [Citation39].
Mescaline (3,4,5-trimethoxyphenethylamine) is a naturally occurring alkaloid, which has been used for millennia and is mainly found in the peyote cactus in Mexico [Citation39,Citation40]. Mescaline is an agonist of the 5HT2A and 5-HT2C receptors [Citation41]. However, mescaline is a low potent psychedelic and a dose of about 300 mg is needed for a full-scale psychedelic experience [Citation42].
3,4-Methylenedioxymethamphetamine (MDMA), also known as ‘Ecstasy,’ has been used for recreational purposes since the 1980s [Citation43]. MDMA has high affinity for 5-HT receptors, and also binds to histamine, muscarinic and adrenergic receptors. It stimulates the release of monoamines, including serotonin, norepinephrine and dopamine [Citation44,Citation45]. In humans, 5-HT2A/C antagonist ketanserin significantly reduced the psychedelic effects (e.g. perceptual changes) of MDMA [Citation46].
1.3. Antidepressant effects of psychedelics
Psychedelics are increasingly administered in human studies across various psychiatric disorders with promising findings [Citation18,Citation20,Citation47]. A systematic review that included 19 studies found that 79.2% (n = 335) of patients with broadly defined unipolar mood disorder (i.e. MDD and dysthymia) showed improvement following psychedelic therapy with LSD or mescaline [Citation48]. The doses of LSD (20–1500 µg) and mescaline (200–400 mg) as well as the therapeutic paradigms differed between studies. Psychedelics are thought to exert antidepressant effects in two ways: a) directly through serotoninergic agonism and b) indirectly through various mechanisms, including changes in other neurotransmission systems, neuroplasticity and modulation of inflammatory markers, cortisol, and brain activity. These mechanisms have also been implicated in the pathophysiology of depression [Citation49–52].
1.3.1. Neurobiological mechanisms
1.3.1.1. Neurotransmission.
Classic serotoninergic psychedelics, such as psilocybin, LSD, mescaline and DMT (which is also included in ayahuasca) are thought to produce both direct and indirect antidepressant effects.
Agonism of the 5-HT1A and 5HT2A receptors may exert a direct antidepressant effect through the desensitization of these receptors, which is a hypothesized mechanism of SSRIs [Citation36,Citation53,Citation54]. Nevertheless, the desensitization hypothesis in depression has received some criticism [Citation55].
Serotoninergic psychedelics can also exert antidepressant effects indirectly through changes in other neurotransmission systems. For instance, psilocybin and LSD are thought to produce antidepressant effects through changes in glutamate and dopamine levels in the prefrontal cortex [Citation36,Citation56–58]. It should be noted that hallucinogenic 5-HT2A receptor agonists may present a unique ability to modulate 5-HT2A receptor signaling pathways (G protein-coupled receptors), compared to non-hallucinogenic agents [Citation24,Citation59,Citation60]. MDMA, similarly to antidepressant agents, increases the levels of serotonin, dopamine and norepinephrine [Citation44,Citation45], which can produce antidepressant effects.
1.3.1.2. Neuroplasticity.
Serotoninergic psychedelics, such as psilocybin, LSD and DMT can induce changes in pyramidal neurons in the prefrontal cortex, including glutamate release and a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) activation. This can enhance brain-derived neurotrophic factor (BDNF) and mammalian target of rapamycin (mTOR) signaling, and thus promote synaptogenesis [Citation61–63]. BDNF promotes neurogenesis [Citation64] and is thought to play a role in the pathophysiology of depression and suicidality [Citation50,Citation65]. Neuroplasticity induced by psychedelics may also be mediated by intracellular 5-HT2A receptors [Citation66].
Compared to placebo, ayahuasca also increased BDNF levels in people with TRD and healthy controls [Citation67], which was correlated with reduction in MADRS scores (rho = −0.55, p < .05). It has also been suggested that serotoninergic psychedelics may possess a unique ability to promote plasticity compared to other serotoninergic agents [Citation66].
1.3.1.3. Cortisol levels and inflammation.
Dysregulation in the serotonin system is associated with alterations to cortisol levels. It is hypothesized that, because psychedelics such as psilocybin can result in a spike in cortisol levels, the spike may activate the executive control network, resulting in increased control over emotional processing and negative thoughts [Citation68]. This may potentially result in a mild antidepressant effect. Galvao et al. [Citation69] report that although at baseline participants with TRD presented blunted awakening salivary cortisol response, 48 h following ayahuasca their cortisol response was comparable to the control group. However, cortisol levels were not associated with severity of depressive symptoms [Citation69]. Similar increases in cortisol levels have also been observed in healthy adults following the administration of other psychedelics, such as psilocybin [Citation70], LSD [Citation71], MDMA [Citation72], 5-MeO-DMT [Citation73], as well as fluoxetine and citalopram [Citation74,Citation75]. Nevertheless, cortisol levels are not always associated with response to antidepressants, and further research is therefore necessary [Citation76,Citation77].
Although research is still limited, psychedelics seem to possess anti-inflammatory properties, which may be attributed to agonism of the 5HT2A receptors [Citation78]. Ayahuasca was found to reduce C-reactive protein (CRP) levels following administration in people with TRD and healthy controls [Citation79]. Interestingly, these changes in CRP were correlated with reduction in MADRS scores (rho = 0.57, p < .05).
1.3.1.4. Modulation of brain activity.
In people with TRD, psilocybin decreased cerebral blood flow (CBF) in temporal cortex regions, including the amygdala. Decreased amygdala CBF correlated with reduction in depressive symptoms (r = 0.59, p = 0.01) [Citation80]. This is in line with existing research which indicates increased activity in the amygdala in depression [Citation81]. Psilocybin was associated with increased ventromedial prefrontal cortex resting-state functional connectivity (RSFC) with the bilateral inferior-lateral parietal cortex, which predicted treatment response at 5 weeks [Citation80]. In people with recurrent depression, ayahuasca was associated with increased blood perfusion in brain regions implicated in mood regulation, such as the left nucleus accumbens, right insula, and left subgenual area [Citation82].
1.3.2. Psychological mechanisms of psychedelics
Historically, the psychological state associated with the psychedelic experience was believed to ‘model psychosis’ and was therefore approached with both interest and suspicion [Citation83]. Newly emerging research has supported the hypothesis that the immediate and enduring psychological effects of psychedelics are associated with improvements in depressive symptomology [Citation84].
It is believed that components of the psychological state induced by psychedelics allow psychotherapeutic patients to engage in new thinking patterns, facilitating the therapeutic process and outcomes [Citation85]. Other potential mechanisms include emotional breakthroughs [Citation86], enhanced emotional empathy [Citation87,Citation88], ego dissolution [Citation89] and increased feelings of trust [Citation87]. These may further differentially facilitate the therapeutic process, as well as directly targeting symptoms of TRD.
Another psychological outcome of interest for TRD is strongly held negative biases. For example, psychedelics are associated with a decrease in the processing of negative emotional stimuli [Citation87,Citation90]. Carhart-Harris and Friston [Citation91] proposed an influential formulation for the actions of psychedelics in psychiatric disorders known as relaxed beliefs under psychedelics (REBUS) [Citation91]. The formulation unifies neurobiological and psychological processes and argues that, when administered in sufficient doses, psychedelics have the potential to dysregulate the neurological systems encoding beliefs and habits [Citation91]. It is argued that this could facilitate their psychological therapeutic effectiveness in psychiatric disorders characterized by rigid ruminations, such as TRD [Citation92]. However, REBUS remains a largely hypothetical model and requires further investigation and clarification [Citation93].
For centuries, psychedelics have been used to experience a self-transcendent and mystical or spiritual state [Citation94]. This state was reported as having substantial personal and psychological meaning in recipients lives when induced by psilocybin [Citation95]. A previous systematic review suggested that mystical experiences were a significant predictor of improved therapeutic outcomes [Citation20]. However, mystical experiences are difficult to measure and subject to a range of biases, as they often rely on self-reports from recipients participating in religious and spiritual events, arguably reducing their credibility. It has been hypothesized that mystical experiences could simply act as a biomarker of 5-HT2A receptor activation. Therefore, any association could be correlational rather than causal and therefore not necessary for enduring therapeutic effects [Citation96]. Nevertheless, further research is needed to explore these psychological mechanisms in TRD populations.
2. Rationale
Over the past decade, there has been a resurgence of clinical research into the antidepressant effects of psychedelics and especially psilocybin. There are a few studies that have explored the effects of psilocybin in MDD, with initial evidence from systematic reviews suggesting that psilocybin is effective and well-tolerated [Citation20,Citation48,Citation84]. Although only a few published studies have assessed the efficacy of psychedelics in TRD, early evidence is promising.
This review will focus on classic serotoninergic psychedelics, as well as MDMA due to its high affinity for 5-HT, as newly emerging research indicates that serotoninergic psychedelics could hold great promise for targeting TRD. Given the emerging nature of research on LSD, MDMA and ayahuasca in TRD, our preliminary literature search indicated that the volume of relevant, high-quality studies currently available may not be sufficient to support the rigorous criteria of a full systematic review. As such, we opted for a more exploratory literature review approach to provide a comprehensive overview of the existing findings.
Psilocybin for TRD has received special attention, with the first randomized controlled trial (RCT) published less than a year ago showing very promising findings [Citation47]. Since there are no systematic reviews on the efficacy of psilocybin in TRD, we conducted a systematic search to summarize existing research.
3. Systematic review on psilocybin
3.1. Methods
Full details of the search are reported here, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines [Citation97]. A protocol of the review was pre-registered on International prospective register of systematic reviews (PROSPERO; CRD42023429969).
3.1.1. Eligibility criteria
Studies were eligible for inclusion if (i) adult human participants were assessed, (ii) the study design was a randomized controlled or open label trial, (iii) study participants had TRD, defined using an evidence-based and operational definition of no improvement despite two or more adequate courses, in terms of dose and duration (at least 6 weeks), of different classes of antidepressants [Citation9,Citation98].
3.1.2. Search strategy
Key search terms were entered into the following databases: MEDLINE, EMBASE, and PsycINFO (all dates from inception to June 2023). The reference lists of key papers were also hand searched to identify any further studies eligible for screening. The following search terms were used: (‘psilocybin’) AND (‘Treatment-resistant depress*’ OR ‘Treatment resistant depress*’ OR ‘TRD’ OR ‘Major depressive disorder’ OR ‘depress*’). English papers with an available title and abstract were screened independently by two reviewers (RHT and MK) using Rayyan, an open-source review management software [Citation99]. Reviewers were blinded to one another’s selections and then unblinded to identify and discuss disparities and reach a consensus with the support of the senior author (AHY). Where full-text papers could not be identified, authors were contacted. This process was then repeated for the full-text screenings and data extraction. Data extracted included study design, dosage, outcomes, and population.
3.1.3. Analysis
Due to a paucity of clinical research, a quantitative meta-analysis was not considered appropriate. The synthesis without meta-analysis (SWiM) reporting guidelines [Citation100] were used to guide and promote clear and comprehensive reporting of the narrative synthesis.
3.1.4. Risk of bias
Risk of bias (RoB) for included studies was independently assessed by RHT, DT, and MK, and any discrepancies were resolved by consensus with the senior author (AHY). Due to the present review including both randomized and non-randomized controlled studies, two RoB tools were employed, each designed to assess for studies using a different study design. The ROBINS-I tool [Citation101] was used to evaluate RoB in non-randomized controlled studies, and the Revised Cochrane Risk of Bias Tool was utilized to assess randomized trials [Citation102]. Studies assessed using the ROBINS-I tool received an overall rating of ‘Low risk,’ ‘Moderate risk,’ ‘Serious risk’ or ‘Critical risk’ of bias. Studies assessed using the Revised Cochrane Risk of Bias Tool received an overall rating of ‘Low risk,’ ‘Some concerns’ or ‘High risk.’
3.2. Results
3.2.1. Study selection
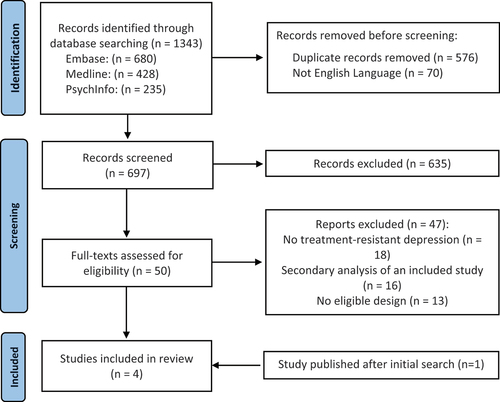
The search resulted in 1343 records. As demonstrated in the PRISMA flow chart (), after duplicates were removed, the titles and abstracts of 697 studies were screened for inclusion.
From these, four articles reporting on three studies were eligible for inclusion. Specifically, Carhatt-Harris et al. [Citation103] is an updated and extended 6-month follow-up to Carhart-Harris et al. [Citation30], with an increased sample size.
3.2.2. Study characteristics
provides a summary of the key study characteristics.
Table 1. Characteristics and findings of psilocybin studies on treatment-resistant depression.
All trials included participants with a diagnosis of TRD as confirmed using medical records or a Mini-International Neuropsychiatric Interview. All trials defined TRD as no response to two or more antidepressant medications within the current episode for more than 6 weeks [Citation30,Citation103] or 8 weeks or more [Citation47,Citation104]. Only one trial [Citation104] included participants who were currently taking an SSRI.
3.2.3. Risk of bias
The RoB assessment is presented in the appendix (Table S1). All studies received a judgment of ‘moderate risk’, other than Goodwin et al. [Citation47] which received a judgment of ‘Some concerns’. However, due to a paucity of research, trials with varying study designs were included in the present review, and therefore different risk of bias assessment tools were used, limiting comparability of overall RoB judgments. Goodwin et al. [Citation47] recruited a larger sample and employed double-blinding and a control condition. Therefore, the present review emphasizes the outcomes of this trial.
3.2.4. Outcomes
The objective of the present review was to systematically identify, collate, and critically analyze the methods and outcomes of all published clinical trials administering psilocybin to patient groups with TRD. The primary outcome was a change in depressive symptom score as assessed using pre-/post-measurement on standard instruments such as the Montgomery-Åsberg Depression Rating Scale or equivalent. Three studies were identified and included. Only one trial [Citation47] was a randomized controlled trial. All other trials were open label [Citation30,Citation103,Citation104]. All trials included some form of psychological support.
3.3. Findings on psilocybin
A summary of findings for psilocybin trials in TRD is presented in . As the first psilocybin in TRD trial, Carhart and colleagues [Citation30] recruited only 12 participants to their single-arm, open label feasibility pilot study. Due to the small sample size, the results use the Hedges’ g formula to report effect sizes. Change in severity of depression was measured using the 16-item Quick Inventory of Depressive Symptoms (QIDS-SR16) from baseline to week 1 (marked reduction, Hedges’ g = 3.1), week 5 (marked reduction, Hedges’ g = 2.7) and 3 months (sustained reduction, Hedges’ g = 2.0). A follow-up of this study with more participants reported a sustained reduction at 3 months (Cohen’s d = 1.5) and 6 months (Cohen’s d = 1.4) [Citation103]. This trial was limited by the small sample size, the open-label design and the absence of a control condition. However, it was designed to act as a proof-of-principle study and did provide preliminary support for the efficacy and safety of psilocybin.
Goodwin et al. [Citation47] is the largest trial included in the present review, recruiting 233 adults. The trial employed a double-blind study design, with participants blinded to their random allocation to one of the three treatment arms. Each group received a different dose of psilocybin: 25 mg (n = 79), 10 mg (n = 75), and an active control group who received 1 mg (n = 79). Changes in the severity of depression were assessed from baseline to week 3 using the Montgomery–Åsberg Depression Rating Scale (MADRS) total scores. A 25 mg dose of psilocybin but not a 10 mg dose, reduced depression scores significantly more than a 1 mg dose (95% confidence interval [CI]: −10.2 to − 2.9; p < 0.001). Across treatment arms, the least-squares mean change at week 3 were − 12.0 points in the 25-mg group, −7.9 in the 10-mg group, and − 5.4 in the 1-mg group. Although the study used a double-blind trial design, the quality of blinding in psychedelic studies can be challenged by the acute subjective psychedelic experience. This trial though is strengthened by its multi-site design, running across 12 countries, therefore resulting in a diverse sample.
Finally, Goodwin et al. [Citation104] (n = 19) administered a 25 mg dose of psilocybin as adjunctive to an SSRI. Mean change from baseline to week 3 MADRS total score was − 14.9 (95% CI: −20.7 to − 9.2). The trial is limited by its open-label design, small sample size, and the absence of a control group. Despite these limitations, findings are meaningful: it is the first trial to administer psilocybin in combination with SSRIs to those with TRD and therefore provides preliminary evidence of SSRI withdrawal not being a prerequisite for psilocybin’s antidepressant effects.
4. Findings on other psychedelic substances
4.1. DMT and ayahuasca
In an exploratory open-label phase 1 study, D’Souza et al. [Citation105] administered 2 intravenous doses of DMT (0.1 mg/kg followed by 0.3 mg/kg) at least 48 h apart to 7 people with TRD. Participants reported a significant reduction in HAMD-17 scores 1 day after DMT, but only following the 0.3 mg/kg session.
One double-blind RCT has explored the efficacy of ayahuasca in TRD [Citation106]. This RCT included 29 participants who received either a single dose of 1 ml/kg of placebo or ayahuasca containing 0.36 mg/kg of DMT. The placebo was designed to mimic the taste and color of ayahuasca. Findings indicate that ayahuasca was effective in reducing depression severity at day 7 compared to placebo (Cohen’s d = 0.98). Nevertheless, remission rates did not reach statistical significance (ps>.05). The characteristics and findings of the studies are presented in .
Table 2. Characteristics and findings of psychedelic studies on treatment-resistant depression.
Although these studies report significant reductions in depressive symptoms, they present various methodological limitations, including open-label design, small sample size and short follow-up, hence any findings should be interpreted with caution.
4.2. 5-MeO DMT
Reckweg et al. [Citation107] conducted a combined phase 1 and 2 open-label trial to assess the antidepressant effects of 5-MeO-DMT in a vaporized formulation (GH001) in people with TRD. In phase 1, participants ( = 8) received 12 mg and 18 mg of GH001, while in phase 2 ( = 8) participants received of up to three increasing doses of GH001 (6 mg, 12 mg, and 18 mg) at least 3 h apart. Findings indicate a significant decrease in MADRS scores at 2 h, 1 day and 7 days following GH001 administration in all groups, whilst 87.5% ( = 7) of participants in phase 2 study showed remission (MADRS ≤10) at day 7. In phase 1, the remission rates at day 7 were 50% ( = 4) in the 12 mg group and 25% ( = 2) in the 18 mg group (see ).
4.3. LSD
No studies that assessed the effects of LSD in TRD or MDD were found. Holze et al. [Citation108] recently published a randomized double-blind trial in 44 patients with anxiety, with or without a life-threatening ilness, which indicated significant reductions in the Hamilton Depression Scale 21-item version (HAM-D-21) (Cohen’s d = −1.1, p = .0004) and Beck Depression Inventory (BDI) scores (Cohen’s d = −.72, p=.02) 16 weeks following oral LSD administration (200 μg). In the pilot for this trial, which included people with anxiety associated with life-threatening diseases, the reported depression scores appear to be decreased 2 months following LSD administration (200 μg), although statistical significance was not assessed as depression was a secondary outcome [Citation109].
4.4. Mescaline
Research on the antidepressant effects of mescaline is scarce. A cross-sectional naturalistic study in 452 participants found that 86% (n = 184) of those with self-reported depression stated that their condition improved following mescaline use [Citation110]. However, this study presents important limitations, including cross-sectional design and absence of psychometric assessment and diagnosis validation of depression. The lack of high-quality studies does not allow us to comment on the potential usefulness of mescaline for TRD.
4.5. MDMA
Current evidence on the efficacy of MDMA in depression is limited, with existing RCTs focusing on post-traumatic stress disorder (PTSD) and distress in people with life-threatening conditions.
A phase 3 RCT [Citation111] administered doses ranging from 80-180 mg of MDMA combined with therapy session to 46 participants with severe PTSD. MDMA therapy significantly improved depression assessed using the BDI II ~18 weeks after baseline compared to placebo (Cohen’s d = 0.67, 95%CI: 0.22, 1.12). Similarly, a cross-over RCT in PTSD (n = 28) reported that participants who received a single dose of 40 mg-125 mg of MDMA had reduced depression scores at 12-month follow-up, albeit due to the cross-over design, there was no control group for comparison at this time-point [Citation112]. On the contrary, one small RCT (n = 18) found that although MDMA-assisted psychotherapy for distress related to life-threatening conditions improved depression scores, this effect was not superior to placebo [Citation113].
A couple non-RCT studies have also found significant associations between MDMA use and improvement in depressive symptoms. One small study reported that participants with high depression scores (n = 20) (i.e. 63 or more on the depression dimension of brief symptom inventory) experienced an immediate decrease in depressive symptoms following ecstasy use in a social gathering [Citation114]. In addition, although the direction of causality is undetermined, an observational study in a large sample of US adults (n = 213,437) also found that, like psilocybin, a lifetime use of MDMA is associated with reduced odds of depressive episodes [Citation115].
5. Importance of psychological therapy
Across all of the psychedelic substances discussed, contemporary administration of psychedelics usually involves psychotherapeutic support. It is argued that psychological support is integral to understanding the therapeutic efficacy of psychedelics. Components integral to the experience of psychedelic-assisted therapy include the therapist encouraging and guiding an appropriate and supportive state of mind (set) and environment (setting) [Citation116]. Non-pharmacological variables, including variables measuring components of set and setting, were found to predict how 261 healthy volunteers experienced psilocybin [Citation117].
The psychological effects of psychedelics, such as enhanced trust, feelings of closeness to others, and increased emotional empathy, may promote the delivery and efficacy of psychotherapy [Citation87,Citation88]. Similarly, pre-existing variables might mediate treatment effects and be important for tailoring therapy. Modlin et al. [Citation118] proposed that openness, motivation and affective tolerance, as well as therapeutic alliance and safety factors, can influence readiness to psilocybin therapy and subsequent treatment outcomes.
Secondary analysis of a recent double-blind randomized controlled trial administering psilocybin in a population with depression found that the strength of the therapeutic alliance predicted greater emotional-breakthrough and mystical-type experiences. A weaker alliance ahead of the second psilocybin session predicted higher final depression scores [Citation119]. Interestingly, this trial employed a new psychedelic therapy model, called ‘Accept-Connect-Embody’ (ACE), aiming to use the development of a non-directive, open, and safe set and setting to allow the emergence of unconscious psychological phenomena, such as memories and emotions.
Common features of psychotherapy include the exploration and gaining of meaning, emotional skills, and bonds. It has been argued that these are central to the often vulnerable and emotionally heightened experience of a psychedelic experience. Therefore, many existing psychotherapies could be employed to effectively guide and facilitate the administration of psychedelics and bolster the therapeutic effects [Citation120]. However, since most psychedelic studies do not employ specific and formal psychotherapeutic approaches, further research is required before useful comparisons can be drawn between therapies.
6. Limitations of psychedelic research
Although preliminary evidence on the efficacy of psychedelics is promising, high quality RCTs in TRD are still scarce. To our knowledge, no RCTs have explored the efficacy of LSD, DMT, 5-MeO-DMT, mescaline and MDMA in TRD, whilst only one RCT on psilocybin for TRD had a large sample size (n = 233 [Citation47]). The RCT that assessed ayahuasca for TRD [Citation106] had a small sample size (n = 29) and a short follow-up period of only 7 days.
Most existing studies in psychedelics for TRD were open-label. Non-randomized open-label studies with no control group present various methodological limitations, including expectancy effects and selection bias. These may lead to an over-representation of participants with positive attitudes toward psychedelics and inflate effect sizes. Finally, none of the RCTs for TRD included an antidepressant as a comparator. Therefore, it is unclear whether psychedelics are superior to other antidepressants in TRD. However, a phase 2 RCT comparing the efficacy of psilocybin versus escitalopram in MDD (n = 59) found that change in depressive scores at 6 weeks was not significantly different between the two groups [Citation121]. It is also unclear whether the antidepressant effects of psychedelics in TRD are sustained long-term. Existing studies on psychedelics for TRD included a very brief follow-up period of a few days, with the exception of one RCT in psilocybin which had a 3-month follow-up [Citation47].
Integrity of blinding is also a crucial issue in psychedelics. Concealing the allocation group is almost impossible in psychedelic research, due to the pronounced subjective effects of these drugs; a study which involved psilocybin-assisted psychotherapy reports that 93.6% of participants correctly guessed their allocation group [Citation122]. However, this is not unique to psychedelics, as in most RCTs assessing psychological interventions masking participants to their allocation group is also impossible. Failure of blinding could lead to expectancy bias [Citation123,Citation124]. This challenge can be mitigated by having independent and blinded raters.
Finally, there has been substantial disagreement and uncertainty surrounding the optimal delivery of psychedelics and the role of both the psychedelic experience and psychotherapy for the efficacy of these substances. Further, the existing literature has varied considerably in its definitions and delivery of appropriate set, setting, dosage, and outcomes measured [Citation94]. This can complicate drawing clear conclusions and collating data appropriately.
7. Risks and safety
There is a distinction between the risks associated with recreational use of psychedelics and medical use in a controlled supervised environment. Current research suggests that the use of serotoninergic psychedelics in controlled environments is relatively safe and does not lead to dependence [Citation21,Citation48]. Bender and Hellerstein [Citation125] recently reviewed the second-wave clinical data of classical psychedelics to assess their risk-benefit profile. Across all data from all included classical psychedelics, no serious adverse events (SAEs) were reported across trials in classic psychedelics, with 11 of 14 included trials administering psilocybin.
Furthermore, two of the papers included in the present review [Citation30,Citation103] on psilocybin for TRD report that psilocybin was generally well tolerated, and no SAEs or unexpected adverse events (UAEs) occurred. Adverse reactions included transient headache, anxiety, nausea and thought disorder. Goodwin et al. [Citation47] report that SAEs on day 1 were reported by 4% of the participants in the 25-mg group, 8% of participants in the 10-mg group, and 1% in the 1 mg group . From day 2 up to week 3, SAEs reported by 9%, 7% and 1% of participants in the 25-mg 10-mg and 1-mg group, respectively. These included suicidal ideation, self-injury and hospitalization for depression. Goodwin et al. [Citation104] report that 2 participants had increased blood pressure which was considered severe and possibly related to psilocybin, and one participant experienced chest heaviness. These reactions were treated with clonidine. Although none of the included studies compared psilocybin with other medications, this increase in adverse events (AEs) may not be specific to psilocybin. For instance, a trial that compared psilocybin with escitalopram in depression reported the number of participants reporting AEs was similar in the two groups (87% and 83%) [Citation121]. Studies that included DMT, 5‐MeO‐DMT, LSD and ayahuasca also report mostly mild AEs [Citation105,Citation107,Citation109], with the exception of one SAE following LSD (anxiety and delusions) [Citation108] and another SAE following DMT, which involved significant asymptomatic bradycardia and hypotension [Citation105]. Palhano-Fontes et al. [Citation106] mention that participants had a psychiatric evaluation following ayahuasca dosing and 4 of the 14 participants had to remain in the hospital ward for a week, without providing further details.
The potential long-term risks associated with psychedelics are poorly understood and warrant further research. There is also very little research exploring and reporting on the adverse reactions and risks associated with psychedelics and serious psychiatric disorders. For instance, it is not yet well determined whether psilocybin therapy for depression can increase the risk of mania or psychosis [Citation126]. It is also unclear to what extent the increased suicidality and self-harming reported in studies [Citation47] is attributed to psychedelics or TRD per se.
A potential adverse effect is hallucinogen persisting perception disorder (HPPD). HPPD is a rare DSM-5 disorder in which a person who has had a prior exposure to a hallucinogen drug experiences a total or partial recurrence of visual hallucinations or perceptual disturbances long after the exposure [Citation1]. Although DSM-5 suggests a prevalence of 4.2% among individuals who use psychedelics, HPPD has not yet been documented in contemporary psychedelic studies. HPPD may be partially associated with preexisting psychiatric comorbidity, such as individual or family history of anxiety [Citation127]. As newly emerging clinical trials diversify patient groups and administer psychedelics to those with more serious psychiatric symptomatology, it will be important to consider a possible increase in the occurrence of these more serious and longer enduring AEs.
Nevertheless, there are notable risks when psychedelics are used as recreational drugs in uncontrolled settings without a therapist. For instance, in online survey data from 1993, 39% of individuals who had tried psilocybin mushrooms outside of clinical settings rated the experience among the top five most challenging of their life and 10.7% reported having put themselves or others at risk of physical harm [Citation128].
Baseline traits and biomarkers are being studied and could be used to prevent adverse reactions [Citation129–131]. The evidence suggests that targeting psychological processes and administering psychedelics in a controlled environment alongside psychological therapy are critical components of psychedelics research and can further minimize potential risks.
8. Conclusions
The present paper reviewed serotoninergic psychedelics, with the main focus on psilocybin for TRD. Preliminary evidence provided by one double-blind RCT and two open label studies suggest that psilocybin produces significant and sustained antidepressant effects in TRD. One RCT on ayahuasca also reported promising findings, although based on a small sample and short follow-up. Although initial findings are encouraging, further adequately powered RCTs with longer follow-ups are required to establish the potential efficacy of psychedelics for TRD.
9. Expert opinion
9.1. Key findings and weaknesses
Over the past decade, there has been a resurgence of psychedelics in psychiatric research, with psychedelics representing a new era in the treatment for TRD.
Psilocybin in particular may become a new treatment option for TRD, with preliminary research suggesting rapid antidepressant effects that sustained for at least 3 months and remission incidence of 29% at 3 weeks [Citation47]. These findings are very encouraging considering that the participants included had not responded to at least 2 antidepressant agents. Two open-label feasibility trials have also reported meaningful results. Furthermore, antidepressants may take several weeks to produce a significant effect [Citation132], thus rapid antidepressant agents such as psilocybin could have real advantages for TRD. Due to the lack of evidence on other serotoninergic psychedelics (i.e. ayahuasca, DMT, 5-MeO-DMT, mescaline, MDMA), psilocybin currently seems to be the most promising one for TRD. Nevertheless, existing studies present various methodological limitations including blinding concerns and expectancy effects. However, these are not unique to psychedelics, as in most studies involving psychological interventions participants are also aware of their allocation group. Evidence on other serotoninergic psychedelics, apart from psilocybin, for TRD is limited. One small sample RCT reported that ayahuasca had significant antidepressant effects in people with TRD, whilst double-blind RCTs on LSD, DMT, 5-MeO-DMT, MDMA and mescaline for TRD have yet to be published. In addition, the psychological, pharmacological and neurocognitive mechanisms of psychedelics for TRD are yet to be clearly delineated. Further research is needed to explore the long-term effects of psychedelics on TRD.
Psychological variables, such as mind-set, setting and openness also seem to influence the psychedelic experience [Citation117,Citation129–131] and targeting those may improve treatment outcomes. Due to the importance of psychological factors, psychedelic studies often involve psychotherapeutic support [Citation30,Citation47,Citation104,Citation111]. The emphasis on psychedelic-assisted psychotherapy constitutes a paradigm shift in depression research, and it could maximize the therapeutic benefits of psychedelics and mitigate risks.
It could be argued that the main effects of psychedelics are induced by the integrated psychological support. Nevertheless, in most studies, psychological support only includes sessions that aim to prepare participants, as well as guide them during and after their psychedelic experience. Therefore, this alone is unlikely to be responsible for the observed antidepressant effects.
Psychedelic use for TRD also presents limitations that need to be considered. Psilocybin is known to be a very short-acting substance, the half-life of which is estimated between 66–132 min [Citation31]. Given the lack of longitudinal research, it is possible that relatively frequent psilocybin administrations would be required to maintain the antidepressant effect. Long-term clinical issues remain to be investigated and existing pharmacological options such as antidepressants require daily administration. Although in psychedelic studies participants are often asked to discontinue antidepressant treatments, preliminary findings are suggesting that this is not required [Citation104]. Finally, ayahuasca has a diverse pharmacological profile, due to being a brew of different psychoactive substances including DMT and β-carboline alkaloids that are MAO-A inhibitors. This makes it challenging to control its consistency and potential drug interactions. MAO inhibitors can interact with other psychotropic substances, such as SSRIs [Citation133] and result in adverse reactions. This can render the therapeutic use of ayahuasca more problematic and challenging to research compared to other classic psychedelics, such as psilocybin.
Although initial outcomes look promising and attitudes are shifting, the potential effectiveness of psychedelics may depend on individual traits. For example, personality traits and temperament may mediate effects. More specifically, it has been reported that openness to experience predicted well-being changes following psychedelic use, whilst positive mind-set was associated with less challenging psychedelic experiences [Citation130,Citation131]. Targeting these may optimize preparation for psychedelic therapy and subsequent treatment outcomes. Similarly, adopting a personalized approach to psychedelic therapy may also be more beneficial for participants who do not possess these supporting characteristics.
9.2. Future research and implications
Although preliminary findings on psilocybin are encouraging, only three studies were included in the present systematic review, limiting conclusions. Further large-scale, double-blind RCTs as well as mechanistic studies are needed to establish the efficacy, safety, long-term outcomes, and mechanisms of psychedelics. Future studies should also compare the efficacy of psychedelics with antidepressants in TRD. Targeting psychological factors, such as openness and mind-set, can improve psychedelic experience and subsequent outcomes.
As presented in , it is exciting to observe numerous ongoing RCTs on psilocybin for TRD, which will update and extend the preliminary but promising outcomes presented in this review, as well as improving our understanding of psychedelic action.
Table 3. Currently registered psilocybin studies on treatment-resistant depression.
Beyond antidepressant effects, there are anxiolytic and anti-addictive effects are further explored. Although we did not have the scope to discuss further effects in the present paper, psychedelic therapy holds promise for other psychiatric conditions including PTSD [Citation111], anorexia [Citation134], addiction [Citation135], borderline personality disorder [Citation136] and bipolar depression.
Although bipolar depression can be difficult to treat, the associated risk of possible onset of mania and hypomania has resulted in caution. A recent study, published as an abstract, reported preliminary findings indicating that participants with bipolar II TRD responded to 25 mg of psilocybin with no UAEs and no recorded onset of mania or hypomania [Citation137]. At the three-week follow-up point, 11 of 14 participants (78.6%) met remission criteria (MADRS ≤10). Although the details of the full trial are yet to be published, and the study was small with an open-label design, these results are compelling, demonstrating feasibility and safety, and warrant further attention.
Australia recently legalized the medical use of psilocybin for TRD and MDMA for PTSD, whilst in Oregon U.S.A. the first psilocybin services centers are expected to open this year. Legalization for medical purposes is a turning point which can shift attitudes and accelerate research. It is anticipated that psychedelic research will broaden over the next decade, and the emergence of new evidence may result in shifting clinical guidelines and legalization of psychedelics for mental health in more countries.
Article highlights
Psychedelics may represent a new era of treatment in psychiatric disorders.
Treatment-resistant depression (TRD) is prevalent and associated with a significant burden, highlighting a demand for novel treatments.
Preliminary evidence suggests that psilocybin is effective and safe in TRD.
Evidence on the efficacy of LSD, DMT, 5-MeO-DMT, ayahuasca, mescaline, and MDMA in TRD is limited.
Psychological support is an important component of treatment with psychedelics which serves to maximize benefits and mitigate potential adverse reactions.
Further research is needed to confirm efficacy and to understand the mechanisms and long-term effects of psychedelics in TRD.
Declaration of interest
M Kalfas, R H Taylor, and D Tsapekos were supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed in the research are those of the authors.
A H Young reports paid lectures and advisory boards for the following companies with drugs used in affective and related disorders: AstraZeneca (AZ), Compass Pathways, Eli Lilly, Lundbeck, Sunovion, Servier, LivaNova, and Janssen. No shareholdings in pharmaceutical companies. Lead Investigator for Embolden Study (AZ), BCI Neuroplasticity study and Aripiprazole Mania Study. Investigator initiated studies from AZ, Eli Lilly, Lundbeck, Wyeth, Janssen.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (15.9 KB)PRISMA_2020_checklist.docx
Download MS Word (34.3 KB)Acknowledgments
For the purposes of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Accepted Author Manuscript version arising from this submission.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14656566.2023.2281582
Additional information
Funding
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. ISBN:978-0-89042-576-3
- Bromet E, Andrade LH, Hwang I et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9(1):1–16. doi: 10.1186/1741-7015-9-90
- Hasin DS, Goodwin RD, Stinson FS, et al. Epidemiology of major depressive disorder: results from the National epidemiologic survey on alcoholism and related conditions. Arch Gen Psychiatry. 2005;62(10):1097–1106. doi: 10.1001/archpsyc.62.10.1097
- Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLOS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547
- World Health Organization. Depression and other common mental disorders: global health estimates 2017. [cited Jul 21 2023]. Available from: https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf
- Greenberg PE, Fournier A-A, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):5356. doi: 10.4088/JCP.14m09298
- National Institute for Clinical Excellence. Depression in adults: treatment and management 2022. [cited Jul 21 2023]. Available from: https://www.nice.org.uk/guidance/ng222
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905
- Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62:10–17.
- Johnston KM, Powell LC, Anderson IM, et al. The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord. 2019;242:195–210. doi: 10.1016/j.jad.2018.06.045
- Shah D, Allen L, Zheng W, et al. Economic burden of treatment-resistant depression among adults with chronic non-cancer pain conditions and major depressive disorder in the US. PharmacoEconomics. 2021 Jun;39(6):639–651. doi: 10.1007/s40273-021-01029-2
- Bergfeld IO, Mantione M, Figee M, et al. Treatment-resistant depression and suicidality. J Affect Disord. 2018;235:362–367. doi: 10.1016/j.jad.2018.04.016
- Corral R, Alessandria H, Agudelo Baena LM, et al. Suicidality and quality of life in treatment-resistant depression patients in Latin America: secondary interim analysis of the TRAL study. Front Psychiatry. 2022;13:812938. doi: 10.3389/fpsyt.2022.812938
- Scott F, Hampsey E, Gnanapragasam S, et al. Systematic review and meta-analysis of augmentation and combination treatments for early-stage treatment-resistant depression. J Psychopharmacol. 2023 Mar;37(3):268–278. doi: 10.1177/02698811221104058
- Nuñez NA, Joseph B, Pahwa M, et al. Augmentation strategies for treatment resistant major depression: a systematic review and network meta-analysis. J Affect Disord. 2022;302:385–400. doi: 10.1016/j.jad.2021.12.134
- Panariello F, Kasper S, Zohar J, et al. Characterisation of medication side effects in patients with mostly resistant depression in a real-world setting. World J Biol Psychiatry. 2023 Jun;24(5):439–448. doi: 10.1080/15622975.2022.2134588
- Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;369–388. doi: 10.2147/PPA.S29716
- Perez N, Langlest F, Mallet L, et al. Psilocybin-assisted therapy for depression: a systematic review and dose-response meta-analysis of human studies. Eur Neuropsychopharmacol. 2023 Aug 7;76:61–76. doi: 10.1016/j.euroneuro.2023.07.011
- Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022 Jan 1;296:26–34. doi: 10.1016/j.jad.2021.09.041
- Ko K, Kopra EI, Cleare AJ, et al. Psychedelic therapy for depressive symptoms: a systematic review and meta-analysis. J Affect Disord. 2022;322:194–204. doi: 10.1016/j.jad.2022.09.168
- Nichols DE, Barker EL. Psychedelics. Pharmacol Rev. 2016;68(2):264–355. doi: 10.1124/pr.115.011478
- Passie T, Seifert J, Schneider U, et al. The pharmacology of psilocybin. Addict Biol. 2002;7(4):357–364
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61(3):364–381. doi: 10.1016/j.neuropharm.2011.01.017
- González-Maeso J, Weisstaub NV, Zhou M, et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53(3):439–452. doi: 10.1016/j.neuron.2007.01.008
- Madsen MK, Fisher PM, Burmester D, et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacol. 2019;44(7):1328–1334. doi: 10.1038/s41386-019-0324-9
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, et al. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9(17):3897–3902. doi: 10.1097/00001756-199812010-00024
- Erkizia-Santamaría I, Alles-Pascual R, Horrillo I, et al. Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed Pharmacother. 2022;154:113612. doi: 10.1016/j.biopha.2022.113612
- Rickli A, Moning OD, Hoener MC, et al. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol. 2016;26(8):1327–1337. doi: 10.1016/j.euroneuro.2016.05.001
- Brown RT, Nicholas CR, Cozzi NV, et al. Pharmacokinetics of escalating doses of oral psilocybin in healthy adults. Clin Pharmacokinet. 2017;56(12):1543–1554. doi: 10.1007/s40262-017-0540-6
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;3(7):619–627. doi: 10.1016/S2215-0366(16)30065-7
- MacCallum CA, Lo LA, Pistawka CA, et al. Therapeutic use of psilocybin: practical considerations for dosing and administration. Front Psychiatry. 2022;13:1040217. doi: 10.3389/fpsyt.2022.1040217
- Goldin D, Salani D. Ayahuasca: what healthcare providers need to know. J Addict Nurs. 2021;32(2):167–173. doi: 10.1097/JAN.0000000000000405
- Callaway JC, Raymon LP, Hearn WL, et al. Quantitation of N, N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J Anal Toxicol. 1996;20(6):492–497
- McKenna DJ. Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacol Ther. 2004;102(2):111–129. doi: 10.1016/j.pharmthera.2004.03.002
- Halberstadt AL, Nichols DE, Geyer MA. Behavioral effects of α, α, β, β-tetradeutero-5-MeO-DMT in rats: comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor. Psychopharmacol (Berl). 2012;221:709–718. doi: 10.1007/s00213-011-2616-6
- De Gregorio D, Enns JP, Nuñez NA, et al. D-lysergic acid diethylamide, psilocybin, and other classic hallucinogens: mechanism of action and potential therapeutic applications in mood disorders. Prog Brain Res. 2018;242:69–96. doi: 10.1016/bs.pbr.2018.07.008
- Egan CT, Herrick-Davis K, Miller K, et al. Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacol (Berl). 1998;136(4):409–414
- Passie T, Halpern JH, Stichtenoth DO, et al. The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther. 2008;14(4):295–314. doi: 10.1111/j.1755-5949.2008.00059.x
- Rucker JJ, Iliff J, Nutt DJ. Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology. 2018;142:200–218. doi: 10.1016/j.neuropharm.2017.12.040
- Vamvakopoulou IA, Narine KA, Campbell I, et al. Mescaline: the forgotten psychedelic. Neuropharmacology. 2022;222:109294. doi: 10.1016/j.neuropharm.2022.109294
- Monte AP, Waldman SR, Marona-Lewicka D, et al. Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives. J Med Chem. 1997;40(19):2997–3008. PMID: 9301661. doi: 10.1021/jm970219x
- Cassels BK, Sáez-Briones P. Dark classics in chemical neuroscience: mescaline. ACS Chem Neurosci. 2018;9(10):2448–2458. doi: 10.1021/acschemneuro.8b00215
- Green AR, Mechan AO, Elliott JM, et al. The pharmacology and clinical pharmacology of 3, 4-methylenedioxymethamphetamine (Mdma,“ecstasy”). Pharmacol Rev. 2003;55(3):463–508. doi: 10.1124/pr.55.3.3
- Battaglia G, Brooks BP, Kulsakdinun C, et al. Pharmacologic profile of MDMA (3, 4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149(1–2):159–163
- Dunlap LE, Andrews AM, Olson DE. Dark classics in chemical neuroscience: 3, 4-methylenedioxymethamphetamine. ACS Chem Neurosci. 2018;9(10):2408–2427. doi: 10.1021/acschemneuro.8b00155
- Liechti ME, Saur MR, Gamma A, et al. Psychological and physiological effects of MDMA (“ecstasy”) after pretreatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23(4):396–404. doi: 10.1016/S0893-133X(00)00126-3
- Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. 2022;387(18):1637–1648. doi: 10.1056/NEJMoa2206443
- Rucker JJ, Jelen LA, Flynn S, et al. Psychedelics in the treatment of unipolar mood disorders: a systematic review. J Psychopharmacol. 2016;30(12):1220–1229. doi: 10.1177/0269881116679368
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–449. doi: 10.2147/ndt.s5700
- Vrshek-Schallhorn S, Doane LD, Mineka S, et al. The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychol Med. 2013;43(3):483–493 doi: 10.1017/S0033291712001213
- Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013:135–151. doi: 10.1007/7854_2012_211
- Blier P. Altered function of the serotonin 1A autoreceptor and the antidepressant response. Neuron. 2010;65(1):1–2. doi: 10.1016/j.neuron.2009.12.028
- Van Oekelen D, Luyten WH, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72(22):2429–2449. doi: 10.1016/s0024-3205(03)00141-3
- Commons KG, Linnros SE. Delayed antidepressant efficacy and the desensitization hypothesis. ACS Chem Neurosci. 2019;10(7):3048–3052. doi: 10.1021/acschemneuro.8b00698
- Carhart-Harris RL, Erritzoe D, Williams T, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 2012;109(6):2138–2143. doi: 10.1073/pnas.1119598109
- Ling S, Ceban F, Lui LM, et al. Molecular mechanisms of psilocybin and implications for the treatment of depression. CNS Drugs. 2022;36(1):17–30. doi: 10.1007/s40263-021-00877-y
- Pilc A, Machaczka A, Kawalec P, et al. Where do we go next in antidepressant drug discovery? A new generation of antidepressants: a pivotal role of AMPA receptor potentiation and mGlu2/3 receptor antagonism. Expert Opin Drug Discov. 2022 Oct;17(10):1131–1146. doi: 10.1080/17460441.2022.2111415
- Liu X, Zhu H, Gao H, et al. Gs signaling pathway distinguishes hallucinogenic and nonhallucinogenic 5-HT2AR agonists induced head twitch response in mice. Biochem Biophys Res Commun. 2022;598:20–25. doi: 10.1016/j.bbrc.2022.01.113
- López-Giménez JF, González-Maeso J. Hallucinogens and serotonin 5-HT 2A receptor-mediated signaling pathways. Curr Top Behav Neurosci. 2018;45–73. doi: 10.1007/7854_2017_478
- Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci. 2021;42(11):929–942. doi: 10.1016/j.tips.2021.08.003
- Becker AM, Holze F, Grandinetti T, et al. Acute effects of psilocybin after escitalopram or placebo pretreatment in a randomized, double‐blind, placebo‐controlled, crossover study in healthy subjects. Clin Pharmacol Ther. 2022;111(4):886–895. doi: 10.1002/cpt.2487
- De Vos CM, Mason NL, Kuypers KP. Psychedelics and neuroplasticity: a systematic review unraveling the biological underpinnings of psychedelics. Front Psychiatry. 2021;12:724606. doi: 10.3389/fpsyt.2021.724606
- Liu PZ, Nusslock R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 2018;12:52. doi: 10.3389/fnins.2018.00052
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971
- Vargas MV, Dunlap LE, Dong C, et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science. 2023;379(6633):700–706. doi: 10.1126/science.adf0435
- Almeida R, Galvão A, Da Silva FS, et al. Modulation of serum brain-derived neurotrophic factor by a single dose of ayahuasca: observation from a randomized controlled trial. Front Psychol query. 2019;10:1234. doi: 10.3389/fpsyg.2019.01234
- De Veen BT, Schellekens AF, Verheij MM, et al. Psilocybin for treating substance use disorders? Expert Rev Neurother. 2017;17(2):203–212. doi: 10.1080/14737175.2016.1220834
- Galvao ACDM, De Almeida RN, Silva EADS, et al. Cortisol modulation by ayahuasca in patients with treatment resistant depression and healthy controls. Front Psychiatry. 2018;9:185. doi: 10.3389/fpsyt.2018.00185
- Hasler F, Grimberg U, Benz MA, et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose–effect study. Psychopharmacol (Berl). 2004;172(2):145–156. doi: 10.1007/s00213-003-1640-6
- Strajhar P, Schmid Y, Liakoni E, et al. Acute effects of lysergic acid diethylamide on circulating steroid levels in healthy subjects. J Neuroendocrinol. 2016;28(3). doi: 10.1111/jne.12374
- Dela Torre R, Farre M, Roset P, et al. Pharmacology of MDMA in humans. Annals Of The New York Academy Of Sciences. 2000;914(1):225–237
- Uthaug MV, Lancelotta R, Szabo A, et al. Prospective examination of synthetic 5-methoxy-N, N-dimethyltryptamine inhalation: effects on salivary IL-6, cortisol levels, affect, and non-judgment. Psychopharmacol (Berl). 2020;237(3):773–785. doi: 10.1007/s00213-019-05414-w
- Seifritz E, Baumann P, Müller MJ, et al. Neuroendocrine effects of a 20-mg citalopram infusion in healthy males. Neuropsychopharmacology. 1996;14(4):253–263
- Von Bardeleben U, Steiger A, Gerken A, et al. Effects of fluoxetine upon pharmacoendocrine and sleep-EEG parameters in normal controls. Int Clin Psychopharmacol. 1989;4 Suppl 1:1–5.
- Deuschle M, Hamann B, Meichel C, et al. Antidepressive treatment with amitriptyline and paroxetine: effects on saliva cortisol concentrations. J Clin Psychopharmacol. 2003;23(2):201–205. doi: 10.1097/00004714-200304000-00014
- Schüle C. Neuroendocrinological mechanisms of actions of antidepressant drugs. J Neuroendocrinol. 2007;19(3):213–226. doi: 10.1111/j.1365-2826.2006.01516.x
- Flanagan TW, Nichols CD. Psychedelics as anti-inflammatory agents. Int Rev Psychiatry. 2018;30(4):363–375. doi: 10.1080/09540261.2018.1481827
- Galvão-Coelho NL, de Menezes Galvão AC, de Almeida RN, et al. Changes in inflammatory biomarkers are related to the antidepressant effects of ayahuasca. J Psychopharmacol. 2020;34(10):1125–1133. doi: 10.1177/0269881120936486
- Carhart-Harris RL, Roseman L, Bolstridge M, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-13282-7
- Drevets WC, Videen TO, Price JL, et al. A functional anatomical study of unipolar depression. J Neurosci. 1992;12(9):3628–3641
- Sanches RF, de Lima Osório F, Dos Santos RG, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol. 2016;36(1):77–81. doi: 10.1097/JCP.0000000000000436
- Friesen P. Psychosis and psychedelics: historical entanglements and contemporary contrasts. Transcult Psychiatry. 2022;59(5):592–609. doi: 10.1177/13634615221129116
- Muttoni S, Ardissino M, John C. Classical psychedelics for the treatment of depression and anxiety: a systematic review. J Affect Disord. 2019;258:11–24. doi: 10.1016/j.jad.2019.07.076
- Swanson LR. Unifying theories of psychedelic drug effects. Front Pharmacol. 2018;9:172. doi: 10.3389/fphar.2018.00172
- Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the emotional breakthrough inventory. J Psychopharmacol. 2019;33(9):1076–1087. doi: 10.1177/0269881119855974
- Dolder PC, Schmid Y, Müller F, et al. LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology. 2016;41(11):2638–2646. doi: 10.1038/npp.2016.82
- Pokorny T, Preller KH, Kometer M, et al. Effect of psilocybin on empathy and moral decision-making. Int J Neuropsychopharmacol. 2017;20(9):747–757. doi: 10.1093/ijnp/pyx047
- Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018:36:393–430. doi: 10.1007/7854_2017_474
- Kometer M, Schmidt A, Bachmann R, et al. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72(11):898–906. doi: 10.1016/j.biopsych.2012.04.005
- Carhart-Harris RL, Friston KJ, Barker EL. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev. 2019;71(3):316–344. doi: 10.1124/pr.118.017160
- Nutt D, Erritzoe D, Carhart-Harris R. Psychedelic psychiatry’s brave new world. Cell. 2020;181(1):24–28. doi: 10.1016/j.cell.2020.03.020
- van Elk M, Yaden DB. Pharmacological, neural, and psychological mechanisms underlying psychedelics: a critical review. Neurosci Biobehav Rev. 2022;104793. doi: 10.1016/j.neubiorev.2022.104793
- Garcia-Romeu A, Richards WA. Current perspectives on psychedelic therapy: use of serotonergic hallucinogens in clinical interventions. Int Rev Psychiatry. 2018;30(4):291–316. doi: 10.1080/09540261.2018.1486289
- Griffiths RR, Richards WA, McCann U, et al. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacol (Berl). 2006;187(3):268–283. doi: 10.1007/s00213-006-0457-5
- Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563–567. doi: 10.1021/acsptsci.0c00192
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;88:105906. doi: 10.1136/bmj.n71
- Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry. 2017 Jan 1;74(1):9–10. doi: 10.1001/jamapsychiatry.2016.2586
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10
- Campbell M. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020. l6890. doi: 10.1136/bmj.l6890
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.doi: 10.1136/bmj.i4919
- Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898
- Carhart-Harris RL, Bolstridge M, Day CM, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacol (Berl). 2018;235(2):399–408. doi: 10.1007/s00213-017-4771-x
- Goodwin GM, Croal M, Feifel D, et al. Psilocybin for treatment resistant depression in patients taking a concomitant SSRI medication. Neuropsychopharmacol. 2023;48(10):1–8. doi: 10.1038/s41386-023-01648-7
- D’Souza DC, Syed SA, Flynn LT, et al. Exploratory study of the dose-related safety, tolerability, and efficacy of dimethyltryptamine (DMT) in healthy volunteers and major depressive disorder. Neuropsychopharmacol. 2022;47(10):1854–1862. doi: 10.1038/s41386-022-01344-y
- Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655–663. doi: 10.1017/S0033291718001356
- Reckweg JT, van Leeuwen CJ, Henquet C, et al. A phase 1/2 trial to assess safety and efficacy of a vaporized 5-methoxy-N, N-dimethyltryptamine formulation (GH001) in patients with treatment-resistant depression. Front Psychiatry. 2023;14:1133414. doi: 10.3389/fpsyt.2023.1133414
- Holze F, Gasser P, Müller F, et al. Lysergic acid diethylamide–assisted therapy in patients with anxiety with and without a life-threatening illness: a randomized, double-blind, placebo-controlled phase II study. Biol Psychiatry. 2023;93(3):215–223. doi: 10.1016/j.biopsych.2022.08.025
- Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):513–520. doi: 10.1097/NMD.0000000000000113
- Agin-Liebes G, Haas TF, Lancelotta R, et al. Naturalistic use of mescaline is associated with self-reported psychiatric improvements and enduring positive life changes. ACS Pharmacol Transl Sci. 2021;4(2):543–552. doi: 10.1021/acsptsci.1c00018
- Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2023;27(6):1025–1033. doi: 10.1038/s41591-021-01336-3
- Ot’alora GM, Grigsby J, Poulter B, et al. 3, 4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295–1307. doi: 10.1177/0269881118806297
- Wolfson PE, Andries J, Feduccia AA, et al. MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: a randomized pilot study. Sci Rep. 2020;10(1):20442. doi: 10.1038/s41598-020-75706-1
- Majumder I, White JM, Irvine RJ. Antidepressant‐like effects of ecstasy in subjects with a predisposition to depression. Addict Behav. 2012;37(10):1189–1192. doi: 10.1016/j.addbeh.2012.05.022
- Jones GM, Nock MK. Lifetime use of MDMA/ecstasy and psilocybin is associated with reduced odds of major depressive episodes. J Psychopharmacol. 2022;36(1):57–65. doi: 10.1177/02698811211066714
- Hartogsohn I. Constructing drug effects: a history of set and setting. Drug Sci Policy Law. 2017;3:2050324516683325. doi: 10.1177/2050324516683325
- Studerus E, Gamma A, Kometer M, et al. Prediction of psilocybin response in healthy volunteers. PLoS One. 2012;7(2):e30800. doi: 10.1371/journal.pone.0030800
- Modlin NL, Miller TM, Rucker JJ, et al. Optimizing outcomes in psilocybin therapy: considerations in participant evaluation and preparation. J Affect Disord. 2023;326:18–25. doi: 10.1016/j.jad.2023.01.077
- Murphy R, Kettner H, Zeifman R, et al. Therapeutic alliance and rapport modulate responses to psilocybin assisted therapy for depression. Front Pharmacol. 2022;12:788155. doi: 10.3389/fphar.2021.788155
- Nayak S, Johnson MW. Psychedelics and psychotherapy. Pharmacopsychiatry. 2021;54(4):167–175. doi: 10.1055/a-1312-7297
- Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384(15):1402–1411. doi: 10.1056/NEJMoa2032994
- Bogenschutz MP, Ross S, Bhatt S, et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry. 2022;79(10):953–962. doi: 10.1001/jamapsychiatry.2022.2096
- Muthukumaraswamy SD, Forsyth A, Lumley T. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev Clin Pharmacol. 2021;14(9):1133–1152. doi: 10.1080/17512433.2021.1933434
- Butler M, Jelen L, Rucker J. Expectancy in placebo-controlled trials of psychedelics: if so, so what? Psychopharmacol (Berl). 2022;239(10):3047–3055. doi: 10.1007/s00213-022-06221-6
- Bender D, Hellerstein DJ. Assessing the risk–benefit profile of classical psychedelics: a clinical review of second-wave psychedelic research. Psychopharmacol (Berl). 2022;239(6):1–26. doi: 10.1007/s00213-021-06049-6
- Borissova A, Rucker JJ. The development of psilocybin therapy for treatment-resistant depression: an update. BJPsych Bull. 2023;26:1–7. doi: 10.1192/bjb.2023.25
- Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Behav Neurosci. 2018;36:333–360. doi: 10.1007/7854_2016_457
- Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268–1278. doi: 10.1177/0269881116662634
- Aday JS, Davis AK, Mitzkovitz CM, et al. Predicting reactions to psychedelic drugs: a systematic review of states and traits related to acute drug effects. ACS Pharmacol Transl Sci. 2021;4(2):424–435. doi: 10.1021/acsptsci.1c00014
- Haijen EC, Kaelen M, Roseman L, et al. Predicting responses to psychedelics: a prospective study. Front Pharmacol. 2018;9:897. doi: 10.3389/fphar.2018.00897
- Kopra EI, Ferris JA, Winstock AR, et al. Adverse experiences resulting in emergency medical treatment seeking following the use of magic mushrooms. J Psychopharmacol. 2022;36(8):965–973. doi: 10.1177/02698811221084063
- Gelenberg AJ, Chesen CL. How fast are antidepressants? J Clin Psychiatry. 2000;61(10):712–721. doi: 10.4088/jcp.v61n1002
- Callaway JC, Grob CS. Ayahuasca preparations and serotonin reuptake inhibitors: a potential combination for severe adverse interactions. J Psychoactive Drugs. 1998;30(4):367–369. doi: 10.1080/02791072.1998.10399712
- Peck SK, Shao S, Gruen T, et al. Psilocybin therapy for females with anorexia nervosa: a phase 1, open-label feasibility study. Nat Med. 2023;29(8):1–7. doi: 10.1038/s41591-023-02455-9
- Der Meer PB V, Fuentes JJ, Kaptein AA, et al. Therapeutic effect of psilocybin in addiction: a systematic review. Front Psychiatry. 2023;14:1134454. doi: 10.3389/fpsyt.2023.1134454
- Zeifman RJ, Wagner AC. Exploring the case for research on incorporating psychedelics within interventions for borderline personality disorder. J Contextual Behav Sci. 2020;15:1–11. doi: 10.1016/j.jcbs.2019.11.001
- Aaronson S, Miller T, Rudow S, et al. 96. An open label study of synthetic psilocybin in bipolar type II depression. Biol Psychiatry. 2023;93(9):S132–S133. doi: 10.1016/j.biopsych.2023.02.336