ABSTRACT
Introduction
Available treatments for colorectal cancer are limited. However, in the last few years several advances and new treatment options became available and expanded the continuum of care in metastatic colorectal cancer (mCRC).
Areas covered
Fruquintinib, a tyrosine kinase inhibitor, has been shown to be effective in heavily pretreated mCRC progressing to trifluridine-tipiracil (FTD/TPI) or regorafenib or both. Preclinical studies have shown that fruquintinib inhibits with high selectivity VEGFR 1-2-3, leading to a blockade in angiogenesis process, but also acts, with weak inhibition, on RET, FGFR-1, and c-kit kinases. Fruquintinib demonstrated good efficacy and tolerance in chemorefractory mCRC in two phase III trial: FRESCO and FRESCO 2. These results led to FDA approval of fruquintinib for pretreated mCRC patients who received prior fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
Expert opinion
Fruquintinib is a valid therapeutic option for heavily pretreated mCRC patients. However, an optimal sequence of treatments is yet to be defined. In this review, we propose an algorithm for later lines of treatment to integrate fruquintinib as a standard of care together with the new therapeutic combinations that recently showed clinical benefit for chemorefractory mCRC, in both molecularly selected (e.g. KRASG12C or HER2 amplification) and in non-oncogenic driven patients.
1. Introduction
Colorectal cancer (CRC) is the third most frequent cancer worldwide, with about 1.9 million new cases per year (10% of all new cases of cancer). It ranks second in mortality in the world, with about 900 000 deaths per year [Citation1,Citation2]. Limited availability of treatment options is one of the main reasons explaining high mortality rate among patients with advanced disease. This is why developing effective and tolerable therapies is paramount.
Despite screening programs, about 20% of CRC are diagnosed in metastatic stage, and 20–50% of patients with initially localized disease will relapse [Citation3]. Fluoropyrimidine-based chemotherapy in combination with anti-vascular endothelial growth factor (VEGF) and/or anti-epidermal growth factor receptor (EGFR) agents is deemed as the main strategy for the treatment of advanced CRC [Citation3]. Several studies demonstrated the efficacy of biological agents and target therapy mostly based on mutational status [Citation4,Citation5] and sideness [Citation6]. For mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) colorectal cancer (4–5% of all mCRC patients), immunotherapy represents the main treatment option [Citation3]. For the microsatellite stable (MSS) population, EGFR-inhibitors, such as cetuximab and panitumumab, are used in combination with chemotherapy for the left-sided, RAS/BRAF wild-type CRC. On the other hand, in right-sided RAS/BRAF wild-type tumors, as well as in RAS/BRAF mutated CRC, first-line treatment involves the combination of antiangiogenic drugs, such as bevacizumab, associated with chemotherapy [Citation3].
Continuum of care represents the paradigm for the management of mCRC [Citation7]. A comprehensive treatment strategy with the integration of sequential chemotherapies, biological agents, surgery, loco-regional treatments, treatment breaks, and best supportive care is a prerequisite to achieve the best outcomes. Nowadays, approximately 27–53% of mCRC patients are suitable to receive at least three lines of therapy and 12–27% are even able to receive at least four lines of therapy [Citation8–11]. It is, therefore, necessary to find new effective and safe therapeutic strategies for patients with heavily pretreated mCRC. Fruquintinib (Box 1), a highly selective oral tyrosine kinase inhibitor of vascular endothelial growth factor receptors (VEGFRs) 1, 2, and 3, has recently gained FDA approval for use in mCRC with disease progression during or after chemotherapy, anti-VEGF biological therapy, anti-EGFR biological therapy, if RAS wild-type, and at least one of FDT/TPI or regorafenib [Citation12]. It appears to be a good treatment option in fourth or later lines setting.
In this review, we will analyze the mechanism of action of fruquintinib and the VEGF pathway; thus, we revised results from preclinical studies to phase III trials, that led to the FDA approval of fruquintinib, and how this might change current clinical practice. We will also take a look at the future by evaluating additional possible therapeutic options for fruquintinib.
1.1. Mechanism of action
Fruquintinib (6-[6,7-dimethoxyquinazolin-4-yloxy]-N,2-dimethylbenzofuran-3-carboxamide) is a tyrosine kinase inhibitor (TKI), with a high selective action on VEGFR-1, 2 and 3, receptors which are related to tumor angiogenesis [Citation7,Citation13].
The angiogenesis process is implicated in tumor vascularization and therefore in its growth. The VEGF/VEGFR axis () plays a key role in this process [Citation14]. The VEGF family is composed of five glycoproteins named VEGF-A; VEGF-B; VEGF-C; VEGF-D; and Placental growth factor (PlGF). These proteins bind three tyrosine kinase receptors: VEGFR-1, 2 and 3. The structure of these receptors can be divided into three regions: an extracellular, a transmembrane, and an intracellular tyrosine kinase domain. VEGF binding to their respective receptors (VEGFRs) results in phosphorylation of their intercellular domain, leading to the activation of a cascade of signals such as the PI3K/AKT, MAPK, PKC, RAF/RAS, and ERK pathways [Citation7,Citation15].
Figure 1. VEGF/VEGFRs pathway Legend: Original imagine edited by biorender. PlGF: Placental growth factor; VEGF: vascular endothelial growth factor; VEGFRs: vascular endothelial growth factor receptors.
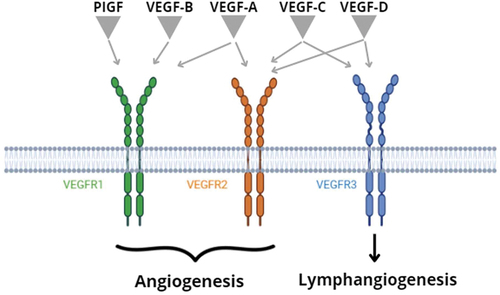
The VEGFR-1 is expressed in endothelial, myelomonocytic, and tumor cells and is activated by VEGF-A, VEGF-B, and PlGF. It has a negative role in physiological vasculogenesis competing with VEGFR-2 in binding to VEGF-A. On the other hand, it has an important function in tumor angiogenesis and progression, by the activation of the chemotaxis of inflammatory cells, the recruitment of medullary progenitor cells at the lesion site and the secretion of inflammatory cytokines and growth factors [Citation16,Citation17].
Instead, the VEGFR-2 plays a central role in both physiologic and pathologic angiogenesis. It is expressed mainly on endothelial cells of vessels, but also in other contests like the hematopoietic cells and the tumor cells. It presents a higher affinity for VEGF-A ligands, despite a lower affinity for VEGF-C and VEGF-D. By the activation of the PI3K-Akt, MAPK, and the calcium signaling pathways, VEGFR-2 can impact on tubulogenesis and can regulate endothelial cell growth, differentiation, migration and prevent cell apoptosis [Citation17–19]. These pathways allow tumor survival even in a hypoxic environment and have been shown to contribute to the development of a more aggressive tumor type [Citation20].
Differently, the VEGFR-3 is a regulator of lymphatic endothelial cell function and lymphangiogenesis. It is located on lymphatic endothelial cells but also on tumor cells. Through binding to VEGF-C and D ligands, VEGFR-3 influences the differentiation, the migration, and the survival of lymphatic endothelial cells. These mechanisms result in increased lymphatic vessels in tumor margin, allowing easier lymphatic dissemination of the cancer [Citation17,Citation21,Citation22].
Fruquintinib has demonstrated a potent inhibition of VEGFR-1, 2 and 3 receptors and thus results in a negative effect on tumor growth with the blockade of angiogenesis and in its dissemination through the reduction of lymphangiogenesis. It should also be mentioned that fruquintinib showed weak inhibition of RET, FGFR-1, and c-KIT kinases [Citation23]. In colorectal cancer RET induces an inhibition of cellular apoptosis [Citation24]; FGFR-1 activates the proliferation of tumor cells in the early stage and their diffusion in the later stage [Citation25]; and c-KIT promotes cancer cell proliferation, motility, and adhesion [Citation26]. Therefore, the inhibition of RET, FGFR-1, and c-KIT kinases, although weak, contributes to enhancing the effect of Fruquintinib in blocking tumor growth.
1.2. Angiognesis in colorectal cancer
Angiogenesis is the process of new blood vessel formation from preexisting vascular bed, essential in the growth and development of normal tissues as well as tumors. It is a highly regulated and complex process involving a variety of proangiogenic and antiangiogenic factors and multiple-signaling pathways that intertwine and regulate each other [Citation27].
Although many different stimuli are implicated in angiogenesis, signaling induced by VEGF is considered rate limiting, which regulates key processes throughout the angiogenic cascade, such as endothelial cell migration and proliferation, capillary tube formation and neovascular survival. VEGFRs are the specific transmembrane receptors for VEGF, and their activation, especially of VEGFR-2, have been demonstrated the major stimulator of angiogenesis; in contrast, inhibition of the VEGFR function results in regression of preformed tumor vessels and could subsequently lead to extensive tissue necrosis [Citation28].
Increased angiogenesis plays a pivotal role in tumor growth, progression, and metastasis across many solid tumors, including colorectal cancer. Therefore, targeting VEGF pathway represents a successful strategy in the treatment of mCRC.
Bevacizumab, in combination with standard chemotherapy, was the first FDA-approved biological agent targeting the VEGF pathway for CRC treatment. This approval initially applied exclusively to first and second-line treatment in bevacizumab-naïve patients. Eventually, both preclinical and clinical studies demonstrated the consistent expression of VEGF during tumor growth and throughout tumor progression. As a result, further studies have investigated whether sustained VEGF inhibition, along with secondary and tertiary cytotoxic regimens, could be beneficial in some patients with solid tumors [Citation29–32].
Results from ML18147 and BEBYP studies showed that bevacizumab continued beyond disease progression, while switching chemotherapy, is beneficial for patients with mCRC who were previously treated with bevacizumab in the first line setting, with an absolute benefit in progression-free survival (PFS) compared to chemotherapy alone of 1.8 and 1.6 months, respectively in the two studies [Citation33,Citation34]. Besides, available evidence suggests that VEGF-targeted agents should generally be preferred over EGFR antibodies in second-line therapy. This assumption is supported by two randomized phase 2 trials PRODIGE 18 (2017) and SPIRITT (2015) comparing bevacizumab continuation versus switching to EGFR antibodies in second-line, with no reported benefit for switching antibodies [Citation35,Citation36].
The biological concept supporting the continuation of angiogenesis inhibition after disease progression was corroborated by two other trials, such as VELOUR and RAISE [Citation37,Citation38]. In the VELOUR Phase III trial, aflibercept, an antiangiogenic recombinant fusion protein blocking VEGFA, VEGFB, and PlGF, improved outcomes when added to FOLFIRI treatment for patients progressing after oxaliplatin-based first-line treatment. Aflibercept demonstrated improvement in outcomes irrespective of whether patients had received bevacizumab in their previous treatment or not. In the RAISE trial, ramucirumab, a human monoclonal antibody that binds to the extracellular domain of VEGFR-2 with high affinity and selectivity, in combination with FOLFIRI, significantly prolonged overall survival (OS) and PFS of patients with mCRC whose disease progressed during first-line treatment. Notably, ramucirumab is still not approved in Italy.
The choice among three effective agents and the absence of prospective head-to-head trials comparing bevacizumab with the two alternatives make it particularly challenging to determine the optimal antiangiogenic agent in the second-line setting.
The antiangiogenic activity proves to be an effective tool also in further lines of treatment, as either regorafenib or bevacizumab associated with the antimetabolite FTD/TPI is recommended in the third line regardless of previous treatments. In the CORRECT trial, regorafenib, an orally administered multikinase inhibitor with potent inhibitory activity on VEGFR-1, VEGFR-2, and VEGFR-3, proved beneficial for patients with mCRC progressing after undergoing all approved standard treatments, including bevacizumab [Citation39]. Finally, findings from the phase III SUNLIGHT trial suggest that the continuation of bevacizumab, in combination with FTD/TPI, proves to be an effective treatment option for refractory mCRC patients. This applies irrespective of mutational status and prior bevacizumab treatment, demonstrating an absolute improvement in OS and PFS compared with FTD/TPI monotherapy of 3.3 and 3.2 months, respectively [Citation40].
In this treatment landscape, the FRESCO-2 clinical trial provides further evidence for the therapeutic relevance of angiogenesis inhibition in highly pretreated patients with refractory mCRC and supports the notion that persistently inhibiting the VEGF pathway yields a survival benefit regardless of different lines of treatment and previous therapies [Citation29].
2. Preclinical results and pharmacodynamics
One of the primary drawbacks of small-molecule VEGFR inhibitors, such as sunitinib, sorafenib, regorafenib, and pazopanib, is their very low selectivity. This means increased toxicity, making administration of high doses and the combination of these drugs with other chemotherapeutic agents, difficult. On the contrary, fruquintinib represents a new generation of small-molecule VEGFR inhibitors with impressive selectivity in the inhibition of VEGFR 1, 2 and 3.
The potent in vitro activity against VEGFR of fruquintinib was confirmed in vivo following oral administration. Preclinical data reported by Sun et al. showed that at the maximum tolerated dose of once daily oral administration fruquintinib achieved complete VEGFR2 suppression (drug concentrations were maintained above that required to produce >85% inhibition of VEGFR2 phosphorylation in mouse) for 24 hours/day.
Fruquintinib was further evaluated in xenografts in combination with chemotherapeutic drugs. The combination with docetaxel achieved a 73% reduction in tumor growth in gastric carcinoma xenografts, surpassing the approximately 45% reduction obtained by the drugs when used alone. Similarly, in combination with oxaliplatin, it demonstrated a tumor growth inhibition of 68% in the colon cancer xenograft, outperforming the effects on the drugs in isolation [Citation41].
The oral bioavailability of fruquintinib reached a moderate level of approximately 42–53% in all the animal models. Fruquintinib exhibits intrinsic membrane permeability, allowing high drug exposure with low oral doses. It majorly distributes in gastrointestinal tract, liver, kidney, adrenal, and adipose and his metabolism is mostly hepatic. Despite its high lipophilicity, facilitating binding with adipose tissue, fruquintinib exhibits limited distribution to the brain, bone marrow and testes [Citation42]. A predictive study on fruquintinib pharmacokinetic properties in humans, determined the minimum effective oral (3 mg), predicting the area under the curve (AUC), corresponding to the minimum efficacy in animal models, such as BGC-823 ×enograft mice. With a half-life of 50 hours in humans, fruquintinib is administered once a day, reaching steady state after 10 days [Citation42].
Finally, promising results of other combinations of angiogenetic and immunotherapy [Citation43], as well as fruquintinib activity on VEGF, allowed researchers to evaluate fruquintinib not only in monotherapy but also in combination with other drugs, in particular with immunotherapy. Further studies evaluated the preclinical activity of fruquintinib in combination with sintilimab, an anti-PD1 antibody (MC38 and CT26). The combination of fruquintinib and sintilimab demonstrated stronger inhibition of tumor growth, accompanied by higher infiltration of CD8+ T lymphocytes, revealing that fruquintinib acts in synergy with sintilimab to stimulate the immune system against the tumor. It is worth noticing that mice treated with the combination did not exhibit any unexpected side effects, indicating good tolerance of the combination [Citation44]. Fruquintinib showed very promising preclinical results, leading investigators to assess its efficacy in clinical trials as well.
3. Phase III clinical trials
Two randomized phase III trials were crucial for the clinical development of fruquintinib in patients with chemo-refractory mCRC. The FRESCO trial was a randomized, double-blind, placebo-controlled, multicenter study conducted in China. From December 2014 to June 2017, a total of 416 patients were randomized (2:1) to receive fruquintinib (5 mg) or placebo, per os once daily, with the 3 weeks on/1 week off scheme. The study included patients with mCRC who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if RAS wild-type, an anti-EGFR therapy. The study population excluded patients who received VEGFR inhibitors (e.g. regorafenib, ramucirumab, or apatinib). Anti-VEGF therapy and KRAS mutational status were stratification factors. Disease characteristics were well balanced in both treatment arms, with a major part of patients having multi-organ metastasis (95.3% in the fruquintinib arm and 97.1% in the placebo arm) and the left colon as the primary tumor location (77.0% and 83.3%, respectively). Among patients receiving fruquintinib, 30.2% previously received bevacizumab and/or aflibercept, and 14.4% received cetuximab.
The primary endpoint was OS, and secondary endpoints included PFS, objective response rate (ORR) and disease control rate (DCR). The results demonstrated that fruquintinib was effective and safe in the treatment of mCRC. OS significantly improved in the fruquintinib group compared with the placebo group (9.3 months vs 6.6 months; HR = 0.65; p < 0.001) and the benefit was observed across nearly all subgroups (including patients who previously received more than three treatment lines, previous use of VEGF inhibitors, previous use of EGFR inhibitors, and regardless of KRAS status). PFS (3.7 months vs 1.8 months; p < 0.001), ORR (4.7% vs 0.0%; p = 0.01) and DCR (62.2% vs 12.3%; p < 0.001), were also significantly increased in the fruquintinib group compared with placebo. At the time of PD, 45.2% of patients received subsequent treatments (42.4% in the fruquintinib arm and 50.7% in the placebo arm). The overall incidence of adverse events (AEs) and serious AEs was higher in the fruquintinib treatment group than in the placebo group. The most frequently reported adverse events of grade 3 to 4 severity in the fruquintinib treatment group are reported in and they included hypertension, hand-foot skin (HFS) reaction, proteinuria, and diarrhea. Dose discontinuation was reported in 15.1% in the fruquintinib arm, and treatment interruption or dose reduction in 47.1% [Citation45]. Fruquintinib was approved in China in September 2018 as third-line therapy or later in mCRC [Citation46].
Table 1. Most commonly adverse events with fruquinitnib reported in phase III clinical trails.
When this study was conducted in China, the standard of care for the treatment of mCRC differed from the current standard of care in Western countries. In the FRESCO study [Citation45], prior therapy included only the standard first two lines of cytotoxic chemotherapy and no third-line treatment with FTD/TPI or regorafenib was available in China at that time. Moreover, only 30% of patients received prior therapy with a VEGF inhibitor (bevacizumab) or anti-EGFR antibodies. In addition, the study did not provide the evaluation of microsatellite instability status, which may influence prognosis and response to further treatments.
Hence, in order to solve geographic differences in clinical practice, a global phase III trial was conducted and involved 153 sites in the United States, Europe, Japan, and Australia. The FRESCO II study was a randomized, double-blind, placebo-controlled, multicenter trial assessing the treatment with fruquintinib compared to placebo in heavily pretreated patients with mCRC. The FRESCO II trial included patients with mCRC who received all current standard approved cytotoxic and targeted therapies and progressed on or were intolerant to FTD/TPI or regorafenib, or both. From September 2020 to December 2021, 687 patients were randomly assigned (2:1) to receive fruquintinib (5 mg) or placebo orally once daily on days 1–21 in 28-day cycles. Stratification factors were previous FTD/TPI or regorafenib, or both, RAS mutation status, and duration of metastatic disease. A major part of patients received >3 treatment lines (72%), including an anti-VEGF agent in approximately 96% of cases. The study met its primary demonstrating that fruquintinib significantly prolonged OS compared with placebo (7.4 months vs. 4.8; HR = 0.66; 95% CI, 0.55–0.80; p < 0.001). The study also met key secondary endpoints, such as PFS (3.7 months vs. 1.8; HR = 0.32; 95% CI: 0.27, 0.39; p < 0.001), confirmed ORR (1.5% vs. 0%; p = 0.059), and DCR (55.5% vs. 16.1%; p < 0.001). OS subgroup analysis confirmed the benefit received from fruquintinib across all subgroups, including patients who received both FTD/TPI and regorafenib (HR 0.6). In the safety analysis, G ≥ 3 AEs were observed in a slightly higher percentage in the fruquintinib arm than in the placebo arm. The most commonly reported G3–4 AEs in the fruquintinib arm were hypertension, asthenia, and HFS reaction (). The safety profile of fruquintinib in this study was consistent with previously reported safety. Treatment-related AEs of grade 3 to 4 severity occurred in 62.7% of patients in the fruquintinib group and 50.4% in the placebo group. AEs leading to discontinuation occurred in 20% of patients who received fruquintinib, compared to 21% of patients who received placebo [Citation12]. These studies support the use of fruquintinib as a new treatment opportunity for patients with advanced refractory mCRC.
4. How to integrate fruquintinib in clinical practice
The Chinese FRESCO study paved the way for the antiangiogenic fruquintinib as new treatment option for chemorefractory mCRC. As already explained above, because neither regorafenib nor FDT/TPI was available in China when FRESCO trial was conducted, placebo was used as control, instead of comparing fruquintinib directly with regorafenib or FTD/TPI [Citation45].
Subsequently, a meta-analysis was conducted to compare efficacy and safety of these three agents in the chemorefractory setting [Citation47]. Five phase 3 trials were identified: CORRECT [Citation39] and CONCUR [Citation48] for regorafenib, RECOURSE [Citation49] and TERRA [Citation50] for FTD/TPI, and FRESCO [Citation45] for fruquintinib. No statistically significant differences in OS were observed between regorafenib and FDT/TPI (HR 0.945, 95% CI 0.677–1.320, p = 0.753), regorafenib and fruquintinib (HR 1.056, 95% CI 0.690–1.621, p = 0.814), or FTD/TPI and fruquintinib (HR 1.117, 95% CI 0.740–1.685, p = 0.610). However, fruquintinib was superior in PFS compared with FTD/TPI (HR 1.756, 95% CI 1.079–2.857, p = 0.023). None of the three agents was better in terms of all grade AEs or grade 3–5 AEs. Nevertheless, subgroup analysis of AEs showed different toxicity profiles between the three drugs, being grade ≥3 hematological toxicities more frequent with FTD/TPI, while liver function abnormalities, HFS, grade ≥3 hypertension and proteinuria were more common with regorafenib and fruquintinib. The indirect comparison between regorafenib and fruquintinib showed that both grade 3–5 AEs for hypertension and proteinuria were lower with regorafenib than with fruquintinib, while the incidence of HFS was much higher with regorafenib [Citation47].
Several other studies have retrospectively compared regorafenib and FTD/TPI, showing similar efficacy outcomes but different toxicity profiles [Citation51–54]. OS was similar among patients who received regorafenib after FDT/TPI and patients who received FDT/TPI after regorafenib (HR 0.98, 95% CI 0.83–1.14) [Citation52]. Additionally, FTD/TPI-related AEs proved to be more manageable compared with those of regorafenib [Citation53].
Moreover, the dose modifications should be noticed since they could partly reflect the tolerance of treatments. In the previously mentioned meta-analysis, regorafenib had a more frequent dose interruption or reduction compared to FTD/TPI (RD 0.4802, 95%CI 0.400–0.561, p < 0.001) and fruquintinib (RD 0.2469, 95%CI 0.148–0.346, p < 0.001) [Citation47]. These data suggest that FTD/TPI could be the more tolerable and manageable agent, followed by fruquintinib and then regorafenib, with no significant differences in terms of OS between the three drugs.
Finally, the phase 3 SUNLIGHT trial changed the treatment paradigm for the third-line setting, achieving unprecedented results in this context. The combination of FTD/TPI and bevacizumab determined a significant improvement in OS and PFS compared with FTD/TPI monotherapy (median OS 10.8 months vs 7.5 months, HR 0.61, 95% CI 0.49–0.77, p < 0.001; median PFS 5.6 months vs 2.4 months, HR 0.44, 95% CI 0.36–0.54, p < 0.001). The benefit was observed regardless of prior anti-VEGF, further supporting the importance of continuing angiogenesis beyond progression. Moreover, the combination did not significantly increase the rate of severe AEs (72.4% vs 69.5%) [Citation40].
The results of the global FRESCO-2 study support the clinical relevance of angiogenesis inhibition even in the more advanced lines of treatment, considering that 73% of patients had received more than three previous lines of therapy for metastatic disease, including previous treatments with either FTD/TPI (52%), regorafenib (8%), or both (39%) [Citation12]. Subgroup analyses showed that fruquintinib improved OS and PFS irrespective of number of prior lines of treatment or previous types of anti-cancer treatment (anti-VEGF, anti-EGFR, regorafenib, FTD/TPI or both) [Citation55]. This finding also suggests that there is no complete refractoriness in patients who have already received another anti-VEGFR agent (regorafenib). The higher target selectivity of fruquintinib compared with other approved anti-VEGF or anti-VEGFR therapies could explain its efficacy regardless of previous exposure to regorafenib. These data support the introduction of fruquintinib as a novel treatment option for patients with refractory mCRC, irrespective of prior number of lines of treatment or type of prior anti-cancer treatment.
Currently, there are few clinical and molecular predictive biomarkers of response to regorafenib, FTD/TPI, or (even less) to fruquintinib. In previous studies, patients with high ECOG, old age, a shorter time from initial diagnosis of metastatic disease, an initial dose <160 mg, >3 metastatic sites, liver metastases, and KRAS mutation have shown a lower probability of benefit from regorafenib [Citation56–59], while patients with HFS within the first month of treatment have achieved a longer survival time [Citation59,Citation60]. A similar differential benefit was observed for fruquintinib in a post-hoc analysis of the FRESCO trial [Citation61]. Potential factors associated with treatment response to antiangiogenics and multitarget agents include LDH, hERG1/aHIF-2α expression, the circulating angiopoietin-2 level [Citation62–65]. On the other hand, high ECOG and age ≥65 years still yielded efficacy with FTD/TPI [Citation49,Citation57], while KRASG12C mutations are biomarkers for reduced OS benefit of FTD/TPI treatment [Citation66]. However, these findings should be cautiously interpreted and need to be prospectively confirmed since almost all of them came from subgroup analyses of retrospective studies, while others came from post-hoc analyses of prospective trials, both with potential biases.
Further clinical research will be needed to define the optimal sequence of treatments for patients with mCRC who have progressed to the first lines of therapies and who could potentially benefit from further lines of treatment, such as FTD/TPI with or without bevacizumab, regorafenib, fruquintinib, but also targeted therapies in patients molecularly selected for the presence of oncogenic drivers. At this regard, BRAF plus EGFR inhibition, dual HER2 blockade, dual KRAS-G12C plus EGFR inhibition, and anti-EGFR rechallenge have shown promising activity in chemorefractory mCRC with BRAFV600E mutation, KRASG12C mutation, HER2 amplification, and BRAF/RAS wild-type status, respectively. Nevertheless, the use of most targeted therapies is supported by limited evidence, predominantly derived from phase II non-randomized trials [Citation67–69]. This limitation is chiefly attributed to the low prevalence of these actionable alterations (for example, the prevalence of KRASG12C mutation or HER2 amplification/over-expression is only 3% [Citation70,Citation71]).
Until 2023 the only exception was represented by the BEACON trial enrolling mCRC patients with BRAFV600E mutations, which occur in 10% of cases [Citation72]. This phase III trial showed the superiority of the anti-BRAF encorafenib plus cetuximab over chemotherapy in patients who have received at least one prior line of treatment, with a median OS was 9.3 vs 5.9 months (HR 0.61, 95% CI 0.48–0.77) and an ORR of 19.5% vs 1.8%. In addition, the CodeBreaK 300 open-label, phase III trial published in 2023 compared the efficacy of the KRASG12C inhibitor sotorasib plus panitumumab with the standard of care (SOC) in 160 patients with chemorefractory KRASG12C-mutant mCRC [Citation73]. Enrolled patients were randomized in a 1:1:1 ratio to receive sotorasib at a dose of 960 mg or 240 mg once daily plus panitumumab, or the investigator’s choice of FTD/TPI or regorafenib (SOC). The primary endpoint was PFS, which was met at the primary analysis. Both sotorasib plus panitumumab arms demonstrated statistically significant superior PFS compared to SOC, with the 960 mg dose demonstrating a more clinically meaningful benefit. Indeed, the median PFS was 5.6 months and 3.9 months in the 960-mg sotorasib – panitumumab and 240-mg sotorasib – panitumumab groups, respectively, as compared with 2.2 months in the SOC arm. The HR for disease progression or death was 0.49 (95% CI 0.30–0.80; p = 0.006) for high-dose sotorasib plus panitumumab and 0.58 (95% CI 0.36–0.93; p = 0.03) for low-dose sotorasib plus panitumumab, respectively. The ORR was 26.4%, 5.7%, and 0%, respectively. The percentage of patients with AEs of grade 3 or higher was similar in the sotorasib – panitumumab groups (35.8% and 30.2%), while was higher in the SOC arm (43.1%). OS data are not mature, and longer follow-up is needed to determine the effects of treatment on this endpoint. Some concerns, however, remain about the use of PFS for a study in the refractory setting, which can be justified by the low prevalence of KRASG12C mutation. Furthermore, as the CodeBreaK 300 trial was designed before the publication of the SUNLIGHT trial results, whether sotorasib 960 mg plus panitumumab is still superior (especially in terms of OS) to FTD/TPI plus bevacizumab in the KRASG12C-mutated mCRC population remains unknown. Consequently, both combinations can be deemed reasonable third-line options in this patient population and can potentially be used in sequential approaches in the late-line setting. Finally, in May 2023, were published the results of the MOUNTAINEER study, a phase II trial that enrolled patients with chemotherapy refractory, HER2-positive, RAS wild-type metastatic colorectal cancer. The study demonstrated the efficacy of the combination of tucatinib and trastuzumab in this setting with a median PFS of 8·2 months, a median OS of 24·1 months, an ORR of 38·1% and a median response duration of 12·4 months. The trial shown also a good safety profile. The most commonly reported adverse event was diarrhea (63% of patients), mostly low-grade, followed by fatigue (44%) and nausea (35%). Only the 6% of the patients discontinued the treatment because of adverse events. Based on these promising results, the combination of trastuzumab and tucatinib could represent a new therapeutic strategy in pretreated HER2-positive mCRC [Citation67].
A Comparison of patients and disease characteristics as well as results of these trials is reported in .
Table 2. Comparison of patients and disease characteristics and results between FRESCO-2, SUNLIGHT, code break 300 and MONTAINEER trials.
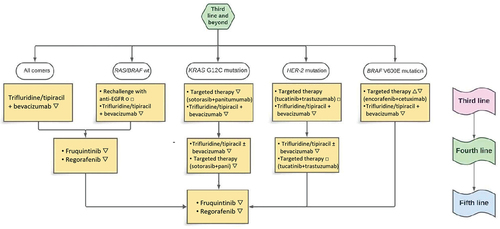
4.1. Treatment algorithm proposal
It is well established and seen in clinical practice that the proportion of patients receiving therapy and survival rates decline with each successive line of treatment in mCRC. As an increasing number of mCRC patients undergo at least three lines of therapy, it becomes imperative to establish a strategic approach for selecting the most appropriate treatment in this scenario. Preservation of quality of life is crucial in patients with mCRC in the third and later-lines; therefore, the prevention and management of adverse events are essential for the most appropriate continuum of care in this patient population.
An optimal sequence of treatments for patients with mCRC who have progressed to the first two lines of therapies and who could potentially benefit from another line of treatment is yet to be established. Currently, the most suitable third-line treatment is represented by the combination of bevacizumab and FTD/TPI, as demonstrated in the SUNLIGHT trial [Citation40]. The doublet achieved improvements in OS, PFS and disease control and delayed worsening of global health status, without increasing serious adverse events or toxicities leading to treatment discontinuation. All international guidelines recommend this combination as the preferred treatment option for the refractory setting. The current standard of care could be further modified by the recent results reported for late-line therapy of the disease [Citation74]. However, it should be considered that only three of the recently published studies are phase III trials [Citation12,Citation40,Citation73].
Targeted therapies in chemorefractory mCRC with BRAFV600E mutation, KRASG12C mutation, HER2 amplification and BRAF/RAS wild type status could represent a good treatment option in patients with tumor-related symptoms and high tumor burden, taking into account that the reported response rates (spanning from 19.5% to 45.3%) [Citation67,Citation68,Citation72,Citation75] widely exceeded those observed with either fruquintinib, regorafenib and FTD/TPI with or without bevacizumab (1–10%). Noteworthy, the definitive feasibility of incorporating the combination therapy comprising sotorasib, panitumumab and the standard-of-care in KRASG12C mutant tumors awaits the forthcoming overall survival results. Based on the results obtained, efforts are being made in recent years to anticipate the use of target therapies in early phases of metastatic disease. In fact, ongoing trial are examining the efficacy of target therapies in first-line in combination with chemotherapy for tumors with mutation of BRAF V600E (NCT04607421), KRAS G12C (Codebreak 301), and HER2 (NCT0523651).
In the context of therapeutic selection in the fourth-line setting, a prudent strategy involves tailoring the treatment based on the divergent toxicity profiles. Indirect comparative analysis conducted through retrospective studies and meta-analyses reported no differences in efficacy and all-grade AEs or grade 3–5 AEs among regorafenib, FTD/TPI, and fruquintinib. A persistent constraint within our optimal treatment sequencing lies in the absence of data regarding the impact of fruquintinib subsequent to the currently established standard of care regimen comprising FTD/TPI and Bevacizumab. Consequently, the efficacy of sustained angiogenic inhibition remains uncertain, and it remains unclear whether such inhibition will confirm or not the findings of the FRESCO-2 trial, particularly given that 52% of patients in said trial exclusively received FTD/TPI as their preceding third-line treatment.
A proposed treatment algorithm is here proposed ().
5. Future prospectives
With the approval of new agents, the landscape of mCRC is likely to become more complex, particularly for the advanced lines. Despite the availability of many treatment options for the chemorefractory setting, the prognosis of these patients is still poor, and the optimum third-line treatment for mCRC remains controversial. In this setting, a multicenter real-world observational study will investigate the combination of FTD/TPI with regorafenib or fruquintinib in terms of efficacy and safety for third-line and beyond mCRC (NCT05993702).
On the other hand, several clinical trials are currently evaluating fruquintinib in earlier lines of treatment in different combinations to define the optimal treatment sequence for patients with mCRC (). For the second line, a phase II trial (NCT05634590) and a single-center phase Ib/II trial (NCT05522738) are investigating the combination of fruquintinib and chemotherapy (FOLFOX or FOLFIRI, depending on the previous chemotherapy regimen) in RAS mutant mCRC. A phase II study (NCT05555901) will evaluate the efficacy and safety of fruquintinib plus FOLFIRI or bevacizumab plus FOLFIRI as second-line treatment in mCRC to investigate the best treatment sequence. This study will enroll approximately 272 patients at several centers in China. For the first line, a prospective, open-label, multicenter, single-arm phase 2 study (NCT05004441) is evaluating the combination of fruquintinib and mFOLFOX6 or FOLFIRI, in previously untreated unresectable mCRC. After eight cycles, patients with evidence of stable disease (SD) are followed by maintenance therapy (fruquintinib plus capecitabine) until disease progression or intolerable toxicity. In addition, a phase II study (NCT04866108) will assess the combination of fruquintinib and capecitabine as first-line therapy for patients unsuitable for platinum and irinotecan-based chemotherapy. Given the proven importance of sustained inhibition of angiogenesis in CRC, many phase Ib/II are currently evaluating the use of fruquintinib as maintenance therapy after first-line treatment, as single agent (NCT04296019), in combination with capecitabine (NCT05016869, NCT05451719, NCT04733963 and also alternating fruquintinib and bevacizumab plus capecitabine (NCT05659290).
Table 3. Ongoing trial with Fruquintinib.
Until now, immune checkpoint inhibitors (ICIs) have shown efficacy only in a subset of patients with mCRC who are dMMR or MSI-H. However, ICIs seem to have synergistic antitumor effects with anti-angiogenic therapy in MSS mCRC, through its immunomodulatory effects [Citation76]. The combination of regorafenib and nivolumab has already shown a manageable safety profile and an encouraged antitumor activity in patients with MSS mCRC who have received at least two previous lines of chemotherapy (ORR of 36% and a mPFS of 7.9 months) [Citation77]. There is already evidence that combining fruquintinib with immunotherapy, such as sintilimab, an anti PD-1 inhibitors, should reverse the immunosuppressive tumor microenvironment and improve the antitumor immune response. Indeed, this combination exhibited a strong inhibition of tumor growth, reduced angiogenesis, reprogramed the vascular structure, and enhanced the infiltration of CD8+ T cells, CD8+ TNFa+, T cells, and CD8+, compared to fruquintinib and sintilimab alone [Citation78]. Based on this evidence, the combination of fruquintinib and sintilimab, an anti PD-1, is currently under investigation in a phase II trial (NCT04695470) in chemorefractory mCRC. Similarly, a prospective clinical trial is currently evaluating the combination of fruquintinib and camrelizumab, an antiPD-1 monoclonal antibody, in a Chinese population in advanced mCRC after failure of standard treatments (NCT04866862). Furthermore, a dose-escalation phase Ib trial is evaluating the safety of GB226 (an anti-PD-1), in combination with fruquintinib (NCT03977090).
For patients with a limited number of metastases, radical local treatment plus systemic therapy remains one of the best choices to achieve long-term tumor control. The normalization of vascular flow by anti-angiogenesis drugs could reverse hypoxia and the tumor’s acidic microenvironment, improving the radiosensitivity of cancer cells [Citation79]. A single-arm prospective phase II trial evaluated the combination of stereotactic ablative radiotherapy (SBRT) combined with fruquintinib and tislelizumab in mCRC, showing encouraging results of this triple combination therapy [Citation80]. Similarly, another phase II trial is currently recruiting patients to evaluate safety and efficacy of hypofractionated radiotherapy combined with sintilimab and fruquintinib in MSS mCRC (NCT05292417).
The results of these analyses should help to enhance the current understanding of the combination of these three different strategies in this disease setting. Based on several research works about the gut microbiome and its link to cancer, a single-arm, phase II study was designed to evaluate the efficacy and safety of fecal microbiota transplantation (FMT) plus sintilimab and fruquintinib as later-line treatment in advanced CRC (NCT05279677).
In recent years, many new targeted therapies have been investigated in the treatment of mCRC, and several clinical trials are currently underway to evaluate the use of fruquintinib in combination with new specific molecular targeted therapies. For example, in HER2-positive/mutated mCRC patients, a single-arm trial is investigating the combination of fruquintinib and disitamabivedotin, an anti-HER2 antibody–drug conjugate with a monomethylauristatin E-cleavable linker, after the failure of conventional prior therapy (NCT05661357).
6. Conclusion
In the last years, the number of patients with mCRC who receive at least three lines of therapy is increasing. Consequently, there is a need to find new effective and safe therapeutic strategies for pretreated mCRC. Fruquintinib, a tyrosine kinase inhibitor with high selectivity for VEGFR 12–3, demonstrated good efficacy and tolerance in pretreated mCRC patients who received prior fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy in two phase III trial: FRESCO [Citation45] (OS 9.3 months; PFS 3.7 months; ORR 4.7%) and FRESCO 2 [Citation12] (OS 7.4 months; PFS 3.7 months; ORR 1.5%). Subgroup analyses and meta-analysis have also highlighted that the efficacy of fruquintinib appears to be independent of previous exposure to VEGF inhibitors, with toxicity profiles comparable to the treatments currently used in the third line. Thus, Fruquintinib is a valid therapeutic option for heavily pretreated mCRC patients. However, ongoing trial are evaluating fruquintinib in previous line of treatment and in combination with chemotherapy, immunotherapy, and targeted therapy, that may further modify the standard of care of the mCRC.
7. Expert opinion
Upfront testing of molecular markers (MMR/allRAS/BRAF) enables personalized medicine in the first-line therapy of mCRC [Citation3]. Moreover, in patients whose tumors show BRAFV600E mutation, KRASG12C mutation or HER2 gene amplification, current treatment options also incorporate the corresponding appropriate molecular-targeted drugs in later lines of treatment [Citation68,Citation72,Citation73]. Recently, the combination of tucatinib and trastuzumab or sotorasib and panitumumab, respectively for the HER2-amplified or KRASG12C mutated mCRC showed clinically meaningful benefit in pretreated mCRC patients [Citation67,Citation73]. In fact, ongoing trial are examining the efficacy of target therapies in first line in combination with chemotherapy for tumors with mutation of BRAF V600E (NCT04607421), KRAS G12C (Codebreak 301), and HER2-amplified (NCT0523651). This highlights the importance of an early and routine use of Next-Generation Sequencing (NGS) approach to sequence the cancer genome and find potentially targetable mutations.
During the past year, practice-changing data have primarily involved late-line treatment of patients with mCRC, while keeping initial therapies unmodified. Both the SUNLIGHT and FRESCO-2 trials have contributed to enrich the treatment landscape in later lines for patients with mCRC, highlighting the importance of sustained inhibition of angiogenesis in the advanced stages [Citation12,Citation40].
Among new therapeutic strategies, fruquintinib stands out as a novel and effective treatment option in the continuum of care of mCRC after failure of most available chemotherapies and targeted drugs, including other antiangiogenic agents. As reported, fruquintinib demonstrates good efficacy and an acceptable tolerance profile in pretreated mCRC patients regardless of previous treatments. However, after failure of the first two lines of therapy, treatment options for patients with both molecularly selected and unselected mCRC are limited and the optimal sequence is yet to be defined. This led us to propose a treatment algorithm for pretreated patients for refractory mCRC with fruquintinib as a new standard of care together with new combinations of treatments recently studied in mCRC. Specifically, considering the overall risk – benefit profile of each available option, we propose FTD/TPI plus bevacizumab as the preferred third-line therapy in most patients, whereas both fruquintinib and regorafenib could be considered for fourth-line treatment and beyond.
To conclude, mCRC remains a clinically challenging disease. Despite notable progress achieved through the latest therapeutic strategies in treating patients with mCRC, invariable resistances and lack of predictive biomarkers to current targeted therapies remain a major issue in clinical settings. In the near future, to improve the clinical outcomes of patients with mCRC, research and clinical investigations should focus on promising strategies. These may include better-designed drugs and the incorporation in clinical practice of novel tools such as liquid biopsy, which can optimize the integration of EGFR inhibitors as a rechallenge treatment in mCRC [Citation75]. ctDNA samples are easy to acquire, capture the heterogeneity within the tumor, and characterize the evolution of CRC genetics over the course of disease progression. In addition, the adoption of new biomarkers in clinical practice seems to be essential to further strengthen therapeutic strategies in MSS mCRC patients.
Box 1: Drug Summary
Drug name: Fruquintinib.
Indication: Fourth-line treatment and beyond for mCRC patients regardless of previous treatments [Citation13].
Pharmacology description/mechanism of action: Fruquintinib is a tyrosine kinase inhibitor (TKI), with a high selective action on VEGFR-1, 2 and 3, receptors which are related to tumor angiogenesis [Citation7,Citation13].
Chemical structure: 6-[6,7-dimethoxyquinazolin-4-yloxy]-N,2-dimethylbenzofuran-3-carboxamide [Citation7].
Pivotal trial(s): FRESCO and FRESCO-2 [Citation12,Citation45].
Abbreviations
| AEs | = | adverse events |
| AUC | = | area under the curve |
| CRC | = | Colorectal cancer |
| DCR | = | disease control rate |
| dMMR | = | mismatch repair-deficient |
| EGFR | = | epidermal growth factor receptor |
| FMT | = | fecal microbiota transplantation |
| FTD/TPI | = | trifluridine-tipiracil |
| HFS | = | hand-foot skin |
| ICIs | = | immune checkpoint inhibitors |
| mCRC | = | metastatic colorectal cancer |
| MSI-H | = | microsatellite instability-high |
| MSS | = | microsatellite stable |
| ORR | = | objective response rate |
| OS | = | overall survival |
| PFS | = | progression free survival |
| PlGF | = | Placental growth factor |
| SD | = | stable disease |
| SBRT | = | stereotactic ablative radiotherapy |
| SOC | = | standard of care |
| TKI | = | tyrosine kinase inhibitor |
| VEGF | = | vascular endothelial growth factor |
| VEGFRs | = | vascular endothelial growth factor receptors |
Article highlights
In the last decade the number of patients with mCRC that undergo at least three lines of therapy is increasing. So, it becomes imperative to establish a new strategic approach in order to expand the continuum of care for pretreated mCRC.
Fruquintinib is a tyrosine kinase inhibitor, with a high selective action on VEGFR-1, 2 and 3, and weak one on RET, FGFR-1, and c-kit kinases.
Fruquintinib showed efficacy and a good safety profile in phase III clinical trials (FRESCO and FRESCO 2).
An optimal treatment sequence for the late lines of therapy in mCRC has yet to be determined.
Fruquintinib and new combinations of treatments in both molecularly selected and in all-comers patients represent a potential option for heavily pretreated mCRC patients.
Declaration of interest
A Santoro reports honoraria and advisory board consultancy from BMS (Bristol Myers Squibb), Servier, Gilead, Pfizer, Eisai, Bayer, and MSD (Merck Sharp and Dohme); speaker honoraria from Takeda, BMS, Roche, Abb-Vie, Amgen, Celgene, Servier, Gilead, AstraZeneca, Pfizer, Lilly, Sandoz, Eisai, Novartis, Bayer, and MSD (all outside the submitted work). The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed receiving research funding from HutchMed, Enterome, Guardant Health, Natera, Eisai; and being on the Advisory Board of HutchMed, Takeda, Illumina, Personalis. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Argilés G, Tabernero J, Labianca R, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2020;31(10):1291–1305. doi: 10.1016/j.annonc.2020.06.022
- Mrozovski JM. Cancer colorectal. Actual Pharm. 2023;62(623):49–51. doi: 10.1016/j.actpha.2023.01.013
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up ☆. Ann Oncol. 2023;34(1):10–32. doi: 10.1016/j.annonc.2022.10.003
- Van Cutsem E, Lenz H-J, Köhne C-H, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700. doi: 10.1200/JCO.2014.59.4812
- Douillard J-Y, Oliner KS, Siena S, et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–1034. doi: 10.1056/nejmoa1305275
- Venook AP, Niedzwiecki D, Innocenti F et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (alliance). J Clin Oncol. 2016 May;34(15_suppl):3504–3504.
- Lavacchi D, Roviello G, Guidolin A, et al. Evaluation of fruquintinib in the continuum of care of patients with colorectal cancer. Int J Mol Sci. 2023;24(6):5840. doi: 10.3390/ijms24065840
- Abrams TA, Meyer G, Schrag D, et al. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106(2):1–10. doi: 10.1093/jnci/djt371
- Boyne DJ, Ngan E, Carbonell C, et al. Real-world study to assess patterns of treatment practices and clinical outcomes in metastatic colorectal cancer patients with RAS wild-type left-sided tumours in Canada. Curr Oncol. 2023;30(9):8220–8232. doi: 10.3390/curroncol30090596
- Rossini D, Germani MM, Lonardi S, et al. Treatments after second progression in metastatic colorectal cancer: a pooled analysis of the TRIBE and TRIBE2 studies. Eur J Cancer. 2022 Jul;170:64–72. doi: 10.1016/j.ejca.2022.04.019
- Aranda E, Polo E, Camps C, et al. Treatment patterns for metastatic colorectal cancer in Spain. Clin Transl Oncol. 2020;22(9):1455–1462. doi: 10.1007/s12094-019-02279-5
- Dasari A, Lonardi S, Garcia-Carbonero R, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. 2023;402(10395):41–53. doi: 10.1016/S0140-6736(23)00772-9
- Dasari A, Sobrero A, Yao J, et al. FRESCO-2: a global phase III study investigating the efficacy and safety of fruquintinib in metastatic colorectal cancer. Futur Oncol. 2021;17(24):3151–3162. doi: 10.2217/fon-2021-0202
- Mabeta P, Steenkamp V. The VEGF/VEGFR axis revisited: implications for cancer therapy. Int J Mol Sci. 2022;23(24). doi: 10.3390/ijms232415585
- Geindreau M, Ghiringhelli F, Bruchard M. Vascular endothelial growth factor, a key modulator of the anti-tumor immune response. Int J Mol Sci. 2021;22(9). doi: 10.3390/ijms22094871
- Lacal PM, Graziani GTherapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res. 2018 Oct;136:97–107. doi: 10.1016/j.phrs.2018.08.023
- Melincovici CS, Boşca, AB, Şuşman S et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol = Rev Roum Morphol Embryol. 2018;59(2):455–467.
- Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci. 2006;11(1):447–888, pp. 818–829. doi:10.2741/1839
- Zhong M, Li N, Qiu X, et al. TIPE regulates VEGFR2 expression and promotes angiogenesis in colorectal cancer. Int J Biol Sci. 2020;16(2):272–283. doi: 10.7150/ijbs.37906
- Rapisarda A, Melillo G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res. 2012;114:237–267. doi: 10.1016/B978-0-12-386503-8.00006-5
- Coso S, Zeng Y, Opeskin K, et al. Vascular endothelial growth factor receptor-3 directly interacts with phosphatidylinositol 3-kinase to regulate lymphangiogenesis. PloS One. 2012;7(6):e39558. doi: 10.1371/journal.pone.0039558
- Su J-L, Yen C-J, Chen P-S, et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007 Feb;96(4):541–545.
- Zhang Y, Zou JY, Wang Z, et al. Fruquintinib: a novel antivascular endothelial growth factor receptor tyrosine kinase inhibitor for the treatment of metastatic colorectal cancer. Cancer Manag Res. 2019;11:7787–7803. doi: 10.2147/CMAR.S215533
- Li AY, McCusker MG, Russo A, et al. RET fusions in solid tumors. Cancer Treat Rev. 2019 Dec;81:101911. doi: 10.1016/j.ctrv.2019.101911
- Katoh M. FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole-body homeostasis (review). Int J Mol Med. 2016 Jul;38(1):3–15. doi: 10.3892/ijmm.2016.2620
- Stankov K, Popovic S, Mikov M. C-KIT signaling in cancer treatment. Curr Pharm Des. 2014;20(17):2849–2880. doi: 10.2174/13816128113199990593
- Troiani T, Martinelli E, Orditura M, et al. Beyond bevacizumab: new anti-VEGF strategies in colorectal cancer. Expert Opin Investig Drugs. 2012;21(7):949–959. doi: 10.1517/13543784.2012.689287
- Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–1105. doi: 10.1177/1947601911423031
- Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26(33):5326–5334. doi: 10.1200/JCO.2008.16.3212
- Hurwitz HI, Bekaii-Saab, TS, Bendell, JC et al. Safety and effectiveness of bevacizumab treatment for metastatic colorectal cancer: final results from the Avastin® registry - investigation of effectiveness and safety (ARIES) observational cohort study. Clin Oncol. 2014;26(6):323–332. doi: 10.1016/j.clon.2014.03.001
- Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105(8):R15–R24. doi: 10.1172/JCI8829
- Melnyk O, Zimmerman M, Kim KJIN, et al. Neutralizing anti-vascular endothelial growth factor antibody inhibits further growth of established prostate cancer and metastases in a pre-clinical model. J Urol. 1999;161(3):960–963. doi: 10.1016/s0022-5347(01)61829-9
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1
- Masi G, Salvatore L, Boni L, et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol. 2015;26(4):724–730. doi: 10.1093/annonc/mdv012
- Bennouna J, Hiret S, Borg C et al. Bevacizumab (Bev) or cetuximab (Cet) plus chemotherapy after progression with bevacizumab plus chemotherapy in patients with wild-type (WT) KRAS metastatic colorectal cancer (mCRC): final analysis of a French randomized, multicenter, phase II study (PRODIGE 18). Ann Oncol. 2017;28:v159–v160. doi: 10.1093/annonc/mdx393.004
- Hecht JR, Cohn A, Dakhil S, et al. SPIRITT: a randomized, multicenter, phase II study of Panitumumab with FOLFIRI and Bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer. 2015;14(2):72–80. doi: 10.1016/j.clcc.2014.12.009
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer Previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. doi: 10.1200/JCO.2012.42.8201
- Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blin. Lancet Oncol. 2015;16(5):499–508. doi: 10.1016/S1470-2045(15)70127-0
- Grothey A, Cutsem EV, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (London, England). 2013 Jan;381(9863):303–312.
- Prager GW, Taieb J, Fakih M et al. Trifluridine-tipiracil and Bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. 2023 May;388(18):1657–1667. doi: 10.1056/NEJMoa2214963
- Sun Q, Zhou J, Zhang Z, et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther. 2014;15(12):1635–1645. doi: 10.4161/15384047.2014.964087
- Gu Y, Wang J, Li K, et al. Preclinical pharmacokinetics and disposition of a novel selective VEGFR inhibitor fruquintinib (HMPL-013) and the prediction of its human pharmacokinetics. Cancer Chemother Pharmacol. 2014 Jul;74(1):95–115.
- Fakih M, Raghav KPS, Chang DZ, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. 2023 Apr;58:101917. doi: 10.1016/j.eclinm.2023.101917
- Li Q, Cheng X, Zhou C, et al. Fruquintinib enhances the antitumor immune responses of anti-programmed death receptor-1 in colorectal cancer. Front Oncol. 2022;12:841977. doi: 10.3389/fonc.2022.841977
- Li J, Qin S, Xu R-H, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018 Jun;319(24):2486–2496. doi: 10.1001/jama.2018.7855
- Shirley M. Fruquintinib: first global approval. Drugs. 2018 Nov;78(16):1757–1761. doi: 10.1007/s40265-018-0998-z
- Zhang Q, Wang Q, Wang X, et al. Regorafenib, TAS-102, or fruquintinib for metastatic colorectal cancer: any difference in randomized trials? Int J Colorectal Dis. 2020 Feb;35(2):295–306. doi: 10.1007/s00384-019-03477-x
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015 Jun;16(6):619–629.
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015 May;372(20):1909–1919.
- Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of Trifluridine/Tipiracil (TAS-102) monotherapy in Asian patients with Previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol Off J Am Soc Clin Oncol. 2018 Feb;36(4):350–358.
- Moriwaki T, Fukuoka S, Taniguchi H, et al. Propensity Score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese society for cancer of the colon and rectum multicenter observational study. Oncology. 2018 Jan;23(1):7–15.
- Nevala-Plagemann C, Sama S, Ying J, et al. A real-world comparison of regorafenib and trifluridine/tipiracil in refractory metastatic colorectal cancer in the United States. J Natl Compr Canc Netw. 2023 Feb;21(3):257–264.
- Sueda T, Sakai D, Kudo T, et al. Efficacy and safety of regorafenib or TAS-102 in patients with metastatic colorectal cancer refractory to standard therapies. Anticancer Res. 2016 Aug;36(8):4299–4306.
- Masuishi T,Taniguchi H, Hamauchi S, et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: a retrospective comparison. Clin Colorectal Cancer. 2017 Jun;16(2):e15–e22.
- Dasari A, Lonardi S, Garcia-Carbonero R, et al. Subgroup analyses of safety and efficacy by number and types of prior lines of treatment in FRESCO-2, a global phase III study of fruquintinib in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2023 May;41(16_suppl):3604.
- Garcia-Carbonero R, Van Cutsem E, Ciardiello F et al. Subgroup analysis of patients with metastatic colorectal cancer (mCRC) treated with regorafenib (REG) in the phase 3b CONSIGN trial who had progression-free survival (PFS) >4 months (m). Ann Oncol. 2016;27(Supplement 6):vi167. doi: 10.1093/annonc/mdw370.54
- Fukuoka S, Moriwaki T, Taniguchi H, et al. Regorafenib (REG) versus trifluridine/tipiracil (TAS-102) as salvage-line in patients with metastatic colorectal cancer refractory to standard chemotherapies (REGOTAS): a propensity score analysis from a JSCCR multicenter observational study. J Clin Oncol. 2017 May;35(15_suppl):3540.
- Schulz H, Janssen J, Strauss UP, et al. Clinical efficacy and safety of regorafenib (REG) in the treatment of metastatic colorectal cancer (mCRC) in daily practice in Germany: final results of the prospective multicentre non-interventional RECORA study. J Clin Oncol. 2018 Feb;36(4_suppl):748.
- Adenis A, de la Fouchardiere C, Paule B, et al. Erratum to: survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer. 2016 Jul;16(1):518.
- Grothey A, Huang L, Wagner A, et al. Hand-foot skin reaction (HFSR) and outcomes in the phase 3 CORRECT trial of regorafenib for metastatic colorectal cancer (mCRC). J Clin Oncol. 2017 May;35(15_suppl):3551. doi: 10.1200/JCO.2017.35.15_suppl.3551
- Li J,Qin S, Bai Y et al. Association between hand-foot skin reaction (HFSR) and survival benefit of fruquintinib in FRESCO trial. J Clin Oncol. 2019;37(15_suppl):e15012–15012. doi: 10.1200/jco.2019.37.15_suppl.e15012
- Antoniotti C, Marmorino F, Boccaccino A, et al. Early modulation of angiopoietin-2 plasma levels predicts benefit from regorafenib in patients with metastatic colorectal cancer. Eur J Cancer. 2022 Apr;165:116–124. doi: 10.1016/j.ejca.2022.01.025
- Cosso F, Lavacchi D, Fancelli S, et al. Long-term response of more than 9 years to regorafenib in a heavily pretreated patient with metastatic colorectal cancer. Anticancer Drugs. 2023 Mar;34(3):451–454.
- Marmorino F, Salvatore L, Barbara C, et al. Serum LDH predicts benefit from bevacizumab beyond progression in metastatic colorectal cancer. Br J Cancer. 2017 Jan;116(3):318–323.
- Iorio J, Lastraioli E, Tofani L, et al. hERG1 and HIF-2α behave as biomarkers of positive response to Bevacizumab in metastatic colorectal cancer patients. Transl Oncol. 2020 Mar;13(3):100740.
- van de Haar J, Ma X, Ooft SN, et al. Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer. Nat Med. 2023 Mar;29(3):605–614.
- Strickler JH, Cercek A, Siena S, et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol. 2023 May;24(5):496–508.
- Yoshino T, Di Bartolomeo M, Raghav K, et al. Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat Commun. 2023 Jun;14(1):3332.
- Cremolini C, Montagut C, Ronga P, et al. Rechallenge with anti-EGFR therapy to extend the continuum of care in patients with metastatic colorectal cancer. Front Oncol. 2022;12:946850. doi: 10.3389/fonc.2022.946850
- Henry JT. Comprehensive clinical and molecular characterization of KRAS G12C -mutant colorectal cancer. JCO Precis Oncol. 2021;5(5):613–621.
- Siena S, Sartore-Bianchi A, Marsoni S, et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2018 May;29(5):1108–1119.
- Tabernero J,Grothey A, Van Cutsem E et al. Encorafenib plus Cetuximab as a new standard of care for Previously treated BRAF V600E-Mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol Off J Am Soc Clin Oncol. 2021 Feb;39(4):273–284.
- Fakih MG, Salvatore L, Esaki T, et al. Sotorasib plus Panitumumab in refractory colorectal cancer with mutated KRAS G12C. N Engl J Med. 2023 Dec;389(23):2125–2139.
- Lonardi S, Pietrantonio F. New options for late-line treatment of metastatic colorectal cancer. Nat Rev Gastroenterol Hepatol. 2023 Dec;21(2):76–77. doi: 10.1038/s41575-023-00881-1
- Sartore-Bianchi A, Pietrantonio F, Lonardi S, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med. 2022 Aug;28(8):1612–1618.
- Antoniotti C, Rossini D, Pietrantonio F, et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022 Jul;23(7):876–887.
- Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol Off J Am Soc Clin Oncol. 2020 Jun;38(18):2053–2061.
- Zhang W, Zhang Z, Lou S, et al. Efficacy, safety and predictors of combined fruquintinib with programmed death-1 inhibitors for advanced microsatellite-stable colorectal cancer: a retrospective study. Front Oncol. 2022;12:929342. doi: 10.3389/fonc.2022.929342
- Zeng J, Baik C, Bhatia S, et al. Combination of stereotactic ablative body radiation with targeted therapies. Lancet Oncol. 2014 Sep;15(10):426–434.
- Wang K, Chen Y, Zhang Z et al. RIFLE: a phase II trial of stereotactic ablative radiotherapy combined with fruquintinib and tislelizumab in metastatic colorectal cancer. Gastroenterol Rep. 2023;11. doi: 10.1093/gastro/goad063