ABSTRACT
Introduction
Despite setbacks in clinical trials for systemic lupus erythematosus (SLE), three drugs have been approved for SLE and lupus nephritis (LN) treatment in the past decade. Several ongoing clinical trials, some viewed optimistically by the scientific community, underscore the evolving landscape. Emerging clinical data have established specific therapeutic targets in routine clinical practice for treating SLE, aiming to improve long-term outcomes.
Areas covered
Research related to treatment of SLE and LN is discussed, focusing on randomized clinical trials during the last 5 years and recommendations for the management of SLE published by the European Alliance of Associations for Rheumatology (EULAR), American College of Rheumatology (ACR), Asia Pacific League of Associations for Rheumatology (APLAR), and Pan-American League of Associations of Rheumatology (PANLAR).
Expert Opinion
The landscape of SLE and LN treatments is evolving, as new drugs and combination treatment approaches redefine the traditional concepts of induction and maintenance treatment phases. As the therapeutic armamentarium in SLE continues to expand, the research focus is shifting from the imperative for new therapies to advancing our understanding of optimal treatment selection for individual patients, steering toward precision medicine strategies.
1. Introduction
The unpredictable presentation, course, and prognosis of systemic lupus erythematosus (SLE) pose a significant challenge for treatment approaches. Since the initial publication of the European Alliance of Associations for Rheumatology (EULAR) recommendations in 2008, there have been significant advancements in our understanding of SLE pathogenesis, treatment strategies, and established treatment goals. This progress led to the development to specific recommendations concerning disease monitoring [Citation1,Citation2], as well as management of lupus nephritis (LN) [Citation3,Citation4], neuropsychiatric SLE (NPSLE) [Citation5], antiphospholipid syndrome (APS) [Citation6], and guidance on pregnancy and women’s health issues in individuals with SLE [Citation7].
During the last decade, new data have emerged, providing insights into alternative glucocorticoid (GC) regimens, the effectiveness of ‘multitargeted’ therapy involving calcineurin inhibitors (CNIs) in treating LN, and the approval of belimumab, the first biological therapy for SLE. Recently, a second biological drug, anifrolumab, received approval for the treatment of SLE. At the same time, significant advancements in the field of LN have occurred with the approval of belimumab and voclosporin, a novel CNI, for patients with active LN. The evolving landscape of treatments has prompted the need for updates to the management of SLE, highlighted in the 2019 [Citation8] and 2023 [Citation9] EULAR recommendation updates.
In this review, we delve into therapeutic options based on organ involvement, with a particular focus on the latest EULAR recommendations for managing SLE [Citation9]. Our goal is to present these current recommendations in a digestible format for clinicians, enhancing their practical use. To achieve this, we have placed significant emphasis on educational illustrations.
2. Pharmacological management of SLE
In recent years, the emerging concept of ‘treat-to-target’ i.e. letting the degree of disease activity drive medication adjustments, has gained recognition [Citation10]. The core idea is to intensify treatment to the point where the patient reaches remission, and in patients where remission is deemed unachievable, to a point of low disease activity [Citation11]. Although widely embraced in the researcher community upon demonstration of their associations with prevention of organ damage accrual [Citation12,Citation13], a better HRQoL experience [Citation14], and upregulation of inhibitory immune pathways in support of their biological relevance [Citation15], challenges remain for the implementation of these states in routine clinical practice, in particular due to the complexity of current definitions that incorporate multiple elements to be assessed and scored, making real-time calculations cumbersome [Citation16]. Thus, for the moment, while the importance of attaining disease control aiming for remission or low disease activity receives wide acceptance, these states are not applied in clinical practice, awaiting simplification of their assessment.
Although pharmacotherapy should always be tailored to the patient, hydroxychloroquine (HCQ) as a first-line treatment for all patients with SLE is universally recommended by the EULAR [Citation9], the Asia-Pacific League of Associations for Rheumatology (APLAR) [Citation11], and the Pan-American League of Associations of Rheumatology (PANLAR) [Citation17], unless contraindicated. The EULAR recommendations that were updated in 2023 suggest a ‘target dose’ of 5 mg/kg real body weight/day up to a maximum of 400 mg/day, whereas the APLAR states that the former dose should not be exceeded. Chloroquine may be a more cost-effective option in countries where availability of HCQ is limited, with the downside of increased risk for retinal toxicity [Citation18]. Weighing flare frequency and disease severity against retinal toxicity is central when titrating HCQ [Citation19].
In addition to HCQ, GC treatment is used in a large portion of patients with SLE to restrain disease activity, although their detrimental long-term effects have been well-known for a long time [Citation20]. Besides clinical experience, detrimental side-effects of GCs are concretely shown in cohort studies, and include but are not limited to an increased risk for severe infections, cardiac complications, premature death, osteoporosis and osteoporotic fractures, gastrointestinal bleeding, sleep disturbances, depressive symptoms, blood pressure increase, and glaucoma [Citation21,Citation22]. Despite the adverse effects, GCs are often deemed necessary to reach a target disease state and should preferably not exceed 5 mg prednisone equivalents (pEq)/day in the context of maintenance use [Citation9]. Pulse therapy with intravenous methylprednisolone (IV-MP), e.g. 125–1000 mg per day, for 1–3 days, can be used in acute and severe SLE flares, allowing quick tapering, and reducing GC-related harm [Citation23,Citation24]. Medications recommended for specific SLE manifestations are mentioned in the respective sections below. A graphical summary of the pharmacological management of SLE is provided in .
Figure 1. An illustrative figure of drugs commonly used in patients with systemic lupus erythematosus (SLE) with different organ manifestations including lupus nephritis (kidneys), neuropsychiatric SLE (brain), cutaneous SLE (skin), lupus arthritis (hand), and immune-mediated cytopenia (immune cells). HCQ is recommended in all patients unless contraindicated. Glucocorticoids are commonly used to control disease activity, but daily dose should be reduced to <5 mg/day prednisone equivalent within 3–6 months. Further choice of immunosuppressants should be made based on disease severity and prevailing manifestation(s). Colours represent the grade of recommendation according to 2023 EULAR recommendation of the management of SLE [Citation9], as classified by the oxford center for evidence-based medicine [Citation9] (green, level of evidence A; yellow, level of evidence B; orange, level of evidence C). HCQ: hydroxychloroquine; CYC: cyclophosphamide; MMF: mycophenolate mofetil; MTX: methotrexate; AZA: azathioprine, CNI: calcineurin inhibitor; IVIG: intravenous immunoglobulin.
![Figure 1. An illustrative figure of drugs commonly used in patients with systemic lupus erythematosus (SLE) with different organ manifestations including lupus nephritis (kidneys), neuropsychiatric SLE (brain), cutaneous SLE (skin), lupus arthritis (hand), and immune-mediated cytopenia (immune cells). HCQ is recommended in all patients unless contraindicated. Glucocorticoids are commonly used to control disease activity, but daily dose should be reduced to <5 mg/day prednisone equivalent within 3–6 months. Further choice of immunosuppressants should be made based on disease severity and prevailing manifestation(s). Colours represent the grade of recommendation according to 2023 EULAR recommendation of the management of SLE [Citation9], as classified by the oxford center for evidence-based medicine [Citation9] (green, level of evidence A; yellow, level of evidence B; orange, level of evidence C). HCQ: hydroxychloroquine; CYC: cyclophosphamide; MMF: mycophenolate mofetil; MTX: methotrexate; AZA: azathioprine, CNI: calcineurin inhibitor; IVIG: intravenous immunoglobulin.](/cms/asset/9cf71e82-e6ed-4c09-b7de-24aaff56dc01/ieop_a_2354457_f0001_oc.jpg)
Regarding new advances in pharmacological management, chimeric antigen receptor (CAR)-T cells targeting CD19+ B cells have shown potential in refractory SLE [Citation25], constituting a new hope. With a strong ability to deplete their target cells, anti-CD19 CAR-T cells demonstrate remarkable efficacy upon deep depletion of CD19+ B cells and plasmablasts in tissues, overcoming the limitations of existing therapies [Citation26]. Current evidence comes from eight patients with refractory SLE/LN who underwent treatment with anti-CD19 CAR-T cell therapy [Citation27]. Following the administration of anti-CD19 CAR-T cells, all patients achieved Definition of Remission In Systemic Lupus Erythematosus (DORIS) remission within 6 months (SLEDAI-2K = 0). Analysis of the British Isles Lupus Assessment Group (BILAG) status revealed resolution of SLE symptoms across all major organ system domains after 6 months of therapy. A long-term follow-up extending up to 29 months demonstrated sustained absence of SLE disease activity (SLEDAI-2K = 0). Additionally, anti-dsDNA antibody levels turned negative, C3 levels were normalized, and proteinuria in LN patients resolved throughout the observation period. Notably, one patient experienced a transient rebound of proteinuria at 4 months post anti-CD19 CAR-T cell therapy, but kidney biopsy revealed no signs of active LN [Citation27]. Overall, anti-CD19 CAR-T cells hold promise for treating severe SLE, although further studies are required to consolidate treatment efficacy along with a favorable safety profile.
Immunometabolism has emerged as a key mechanism in the pathogenesis of SLE [Citation28]. Metformin has been repurposed for immune-mediated diseases due to its ability to reverse aberrant metabolic signaling in immune cells. Specifically, metformin suppresses oxidative phosphorylation by inhibiting mitochondrial electron transport-chain complex 1, and it acts through the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway or via AMPK-independent pathways [Citation28]. Indeed, previous evidence demonstrated that the addition of metformin to standard therapy for SLE was associated with reduced flares and reduced steroid doses [Citation29]; however, a randomized clinical trial was underpowered for drawing firm conclusions on the efficacy of metformin in SLE [Citation30]. Sirolimus (an mTOR inhibitor) has shown promise for treating SLE and is currently under investigation in clinical trial programs [Citation31]. Current evidence suggests that sirolimus is well-tolerated and effective in treating SLE patients, particularly those with arthritis and lupus nephritis; however, further research is required to consolidate its efficacy [Citation32,Citation33]. Moreover, sirolimus has been found to have comparable efficacy with tacrolimus, and it may even exhibit superior effects regarding serological improvements and tapering of GC doses [Citation34].
2.1. Lupus nephritis
According to data from the United States, renal involvement occurs in up to 40% of patients with SLE [Citation35]. It is imperative to treat active LN as early as possible, and to suppress disease activity as much as reasonably achievable [Citation9]. Before treatment initiation, it is desirable to obtain a kidney biopsy, as information gleaned therefrom is crucial when making therapeutical decisions [Citation36].
LN can be treated with low-dose intravenous cyclophosphamide (CYC) according to the Euro-Lupus-protocol or high-dose intravenous CYC according to the NIH regimen [Citation37]. The former is preferred as similar efficacy has been shown between the two regimens [Citation37], with the dosage being 500 mg on weeks 0, 2, 4, 6, 8, and 10 [Citation9]. Nevertheless, high-dose intravenous CYC still is preferred in several practices globally, especially for LN cases with rapidly progressive disease, e.g. rapid renal function loss, or features in the kidney biopsy that are coupled with poor long-term prognosis [Citation9,Citation38]. As an alternative to CYC, mycophenolate mofetil (MMF) or mycophenolic acid are increasingly used instead as the initial therapy. Given the critical and often irreversible organ damage caused by renal flares, LN is considered a severe manifestation of SLE no matter the degree of inflammation [Citation9], wherefore IV-MP also constitutes an option as a part of the initial treatment phase. This is emphasized in the EULAR recommendations stating that patients with histological presence of cellular crescents or fibrinoid necrosis, reduced glomerular filtration rate, or severe interstitial inflammation may be candidates for high-dose intravenous CYC and IV-MP in combination [Citation9]. In refractory cases, when intravenous CYC and mycophenolate regimens have failed or are contraindicated, off-label use of rituximab may be considered [Citation9], based on real-world evidence [Citation39], despite the failure of a clinical trial of rituximab in SLE [Citation40].
Two additional drugs, belimumab and voclosporin, have been approved after their respective successful clinical trials BLISS-LN [Citation41] (belimumab) and AURORA-1 [Citation42] (voclosporin). Both belimumab and voclosporin are approved as add-on medications for all patients with active LN. The 2023 update of the EULAR recommendations for the management of SLE state that add-on belimumab or add-on calcineurin inhibition should be considered on top of CYC or MMF (belimumab as add-on to CYC or MMF; voclosporin as add-on to MMF) [Citation9]. Once the renal flare has subsided, patients should remain on MMF, and add-on belimumab or voclosporin if this was a part of their initial therapy, for at least 3 years, and CYC administered as an initial treatment should be substituted with either MMF or azathioprine (AZA) for the same period [Citation9].
The 2023 update of the EULAR recommendations for the management of SLE have dedicated a section on LN [Citation9]. In those, addition of belimumab or addition of a calcineurin inhibitor should be considered as a part of the initial therapy. Along similar lines, the Kidney Disease Improving Global Outcomes (KDIGO) initiative released recently separate recommendations for the treatment of LN, separating the management by histopathological class and adding suggested schedules for the tapering of GCs [Citation38].
For SLE patients with renal involvement and histological evidence of thrombotic microangiopathy (TMA), anticoagulant medication is essential and has been shown protective in a multicentre cohort study [Citation43]. Eculizumab, a monoclonal antibody against complement protein C5 currently used to treat conditions such as atypical hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinuria, has shown efficacy in treating LN with TMA [Citation44].
In the setting of LN, adjunctive therapy with e.g. anti-hypertensive medications is central; blockade of the renin – angiotensin – aldosterone with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers is important for reducing proteinuria [Citation45]. Additionally, assessment of the cardiovascular risk is essential, and the threshold for lipid-lowering agents should in general be low [Citation46]. Non-pharmacological management is also important, including dietary interventions to restrict salt intake [Citation47].
2.2. Neuropsychiatric disease
To improve and standardize the care provided to SLE patients experiencing neuropsychiatric (NP) events, the EULAR endorsed eminence and evidence-based recommendations in 2010 [Citation5]. In the 2019 update of the recommendations for SLE, EULAR incorporated specific guidance for NPSLE emphasizing the attribution of NP symptoms to SLE-related and non-SLE-related causes as a part of the initial management [Citation8].
After evaluating and correcting any concurrent aggravating factors such as infections, drug adverse effects, or metabolic disturbances, the EULAR recommends the administration of symptomatic treatment including anticonvulsants, antidepressants, anxiolytics, and/or antipsychotics, tailored to the specific neuropsychiatric manifestations. Although symptomatic management represents an empiric strategy extrapolated from non-SLE cases, current evidence indicates comparable efficacy and safety with that seen in the general population [Citation48]. The subsequent therapeutic management in NPSLE is primarily guided by the presumed underlying mechanisms, whether inflammatory or thrombotic. However, these two entities are often challenging to distinguish in clinical practice and may even coexist. In particular, the administration of immunosuppressive agents should be considered in patients with i) NP manifestations traditionally classified as inflammatory, such as recurrent seizures, myelopathy, psychosis, optic neuritis, peripheral nervous system disease, and acute confusional state, ii) concurrent generalized disease activity, iii) severe, rapidly evolving, or deteriorating symptoms, iv) relapsing disease, and v) improvement observed after an initial trial with GCs [Citation49]. Patients presenting with a cerebrovascular event may be treated with immunosuppressants in addition to anticoagulants/antiplatelets, particularly in cases of recurrent strokes and absence of anti-phospholipid antibodies (aPL) and in cases with cardiovascular risk factors [Citation50]. Antiplatelet therapy should be individualized in patients with presumed ischemic-mediated manifestations and patients with medium to high aPL titers, while anticoagulation is not recommended in NPSLE patients without APS [Citation5].
Concerning specific immunosuppressive treatment, the initial approach involves the administration of IV – MP pulses, followed by moderate-to-high daily oral doses. The choice of dosage depends on the severity of the presentation and the clinical response. Intravenous CYC, typically administered for 6 months, stands as the main therapeutic option for severe NPSLE [Citation51]. Rituximab (RTX) has also been used in severe NPSLE cases, showing encouraging response rates [Citation52]. AZA can be considered in cases of moderate NPSLE, mainly as a GC-sparing agent or for sustaining treatment response. Limited observational studies also suggest the potential use of MMF in managing NP manifestations, particularly for maintenance therapy [Citation53]. In case of peripheral neuropathy or other manifestations in the peripheral nervous system, intravenous immunoglobulin (IVIG) may be used as an alternative treatment, while plasma exchange should only be considered as a ‘rescue’ therapy for severe and refractory cases [Citation5]. The duration of treatment should be personalized for each patient through close monitoring, yet most patients will require extended periods of immunosuppression to consolidate and sustain a clinical response.
2.3. Hematological disease
Hematological complications of SLE include hemophagocytosis [Citation11], thrombocytopenia, anemia, lymphopenia, and pancytopenia. Furthermore, these hematological manifestations can occur as secondary to another disease, or as side-effects of medications used to treat SLE, particularly in the case of anemia [Citation54–58]. Revising the current medications received by the patient can therefore be of use when trying to discern whether a cell-line depletion is directly caused by SLE.
The EULAR recommendations address the treatment of severe autoimmune thrombocytopenia, typically defined as a platelet count below 20–30000/mm, and advocate high-dose GCs (including IV-MP) with or without IVIG, RTX, and high-dose intravenous CYC [Citation9] as an initial treatment, followed by maintenance therapy with either RTX, AZA, mycophenolate, or cyclosporine3. This recommendation remains virtually unchanged and largely in line with the statements from the PANLAR [Citation17].
For hemolytic anemia in SLE, the PANLAR recommends high-dose GCs as first-line and RTX as second-line treatment [Citation17]. Conversely, slightly newer recommendations from the APLAR advocate for CYC in addition to GCs for the treatment of alveolar hemorrhage, thrombotic thrombocytopenic purpura, myocarditis, shrinking lung syndrome, and hemophagocytosis [Citation11], although the primary research cited for this statement is either retrospective [Citation59] (29 patients) or on a case-report level [Citation60] (two patients). APLAR also recommends consideration of plasmapheresis in patients with SLE who have thrombotic thrombocytopenic purpura, diffuse alveolar hemorrhage, or hemophagocytosis [Citation11].
Among the novel treatment regimens whose development is underway, combination therapy with initial RTX infusion and subsequent belimumab injections show safety and efficacy in patients with persistent immune thrombocytopenia [Citation61].
2.4. Skin disease
Cutaneous manifestations in people with SLE are common and with a wide range of presentations. Lupus limited to the skin constitutes a separate spectrum of diseases [Citation62]. For treating active skin disease, topical agents such as GCs and/or calcineurin inhibitors (e.g. tacrolimus) are a first-line option and, depending on the severity of the skin involvement, they are usually preferred to systemic agents (with the exception of maintenance therapy with HCQ, which is broadly recommended to all individuals with SLE [Citation9]). For patients with active mucocutaneous SLE, quinacrine is an optional antimalarial in addition to HCQ, or as a substitute in cases where HCQ is not well tolerated [Citation63]. Oral GCs are also an alternative and can be used both short-term during flares and less preferably as maintenance therapy at low doses, as stated above.
If treatments with topical medications, antimalarials, and/or oral GCs do not suffice, which usually accounts for up to four out of ten patients [Citation63], other immunosuppressants to treat active skin disease in lupus are mycophenolate and methotrexate (MTX) [Citation64], as well as the biological agents belimumab [Citation65] and anifrolumab [Citation66], with anifrolumab showing slightly clearer results by measurements with a disease activity index specific for skin-disease (Cutaneous Lupus Erythematosus Disease Area and Severity Index, CLASI [Citation67]).
Thalidomide [Citation68] and lenalidomide [Citation69] have shown efficacy in the treatment of cutaneous lupus erythematosus, but should be prescribed with caution, especially to women of reproductive age (i.e. the majority of patients with new-onset SLE), due to their teratogenic effects. These pharmaceuticals should in principle not be considered unless the patient has proven treatment-refractory to several other alternatives. Vitamin A derivatives and dapsone are other non-anti-rheumatic drugs that can be tried in difficult-to-treat-cases of cutaneous lupus and should preferably be managed through or in close contact with a dermatology unit [Citation9].
2.5. Musculoskeletal disease
Musculoskeletal manifestations of SLE include arthritis and myositis [Citation70], and affect a majority of people with SLE [Citation71]. An arthritic component of SLE can vary in severity but is typically non-erosive. More severe cases may cause bone erosion [Citation72], and there can sometimes be a phenotypical overlap between SLE and rheumatoid arthritis, often referred to as rhupus [Citation73].
Concerning biologic agents, belimumab proved efficacious in managing arthritis in the clinical trials leading to its approval [Citation74,Citation75], as well as in real-world settings [Citation76,Citation77]. Anifrolumab showed a significant reduction in swollen joint count compared to placebo in one clinical trial [Citation78], but failed to show the same in another [Citation79]. RTX has been assessed for the treatment of musculoskeletal disease activity but failed to show efficacy compared to placebo [Citation80].
Concerning non-biologic agents, MTX has proven effective against musculoskeletal disease activity in general [Citation81], as well as patients’ joint-related complaints and subjective assessment of pain specifically [Citation82]. These results are in line with the APLAR recommendations that advocate for MTX therapy in articular manifestations [Citation11]. Mycophenolate sodium proved more effective for attaining remission as compared to AZA both at short-term (3-month) and long-term (24-month) follow-up [Citation83].
The Janus kinase inhibitor baricitinib has been tested for SLE in two clinical trials, BRAVE-1 [Citation84] and BRAVE-2 [Citation85], which both showed improvement of musculoskeletal involvement at doses of 4 mg/day, but not 2 mg/day.
In general, international recommendations for pharmacotherapy in musculoskeletal SLE are sparse; the EULAR recommendations include few specific recommendations on musculoskeletal involvement [Citation9], and the PANLAR grade the strength of their recommendations for both first-line (antimalarials and GCs) and second-line (MTX, leflunomide, belimumab or abatacept) therapy as low [Citation17,Citation76].
2.6. Antiphospholipid syndrome in SLE
Although often occurring secondary to SLE, APS is its own diagnostic entity with separate proposed classification criteria [Citation86]. The recommendations from EULAR for both APS [Citation6] and SLE [Citation9] underscore the significance of primary prevention in individuals carrying aPL. Importantly, only patients with persistently positive medium/high titers or multiple positivity for aPL should be considered high-risk and should receive primary prophylaxis with antiplatelets, especially in the presence of thrombotic risk factors. Low-dose aspirin (LDA) has been shown to reduce the likelihood of a first thrombosis by around 50% in asymptomatic aPL carriers [Citation87], while anticoagulation is not recommended for primary prevention. The therapeutic approach for patients with APS secondary to SLE should align with primary APS [Citation6]. Specifically, SLE patients with definite APS and a history of venous thrombosis should be treated with vitamin K antagonist (VKA) with a target international normalized ratio (INR) of 2–3; anticoagulation should be continued long term for unprovoked thrombotic events. Addition of LDA, increase of INR target to 3–4, or change to low-molecular weight heparin (LMWH) should be considered only for patients with good adherence to VKA and recurrent venous thrombosis. For the first arterial thrombotic event, VKA are recommended over treatment with LDA alone based on two observational studies which demonstrated decreased probability of recurrent thrombotic events among APS patients treated with VKA compared to patients treated with LDA [Citation88,Citation89]. For those patients, the VKA target (INR 2–3 or 3–4) should be individualized based on risk for bleeding and recurrent thrombosis, while a combination of LDA plus VKA with INR 2–3 represents an alternative therapeutic option [Citation6]. For individuals with APS exhibiting triple positivity, the use of direct oral anticoagulants (DOACs) is not recommended based on a prematurely terminated randomized controlled trial (RCT) [Citation90] that compared VKA with the DOAC rivaroxaban. The trial was terminated due to a greater occurrence of arterial events in the rivaroxaban arm, highlighting an elevated likelihood of recurrent thrombosis in APS patients with triple positivity. Accordingly, EULAR recommends DOACs only for APS patients not able to achieve the target INR despite good adherence to VKA or those with contraindications to VKA [Citation6]. A schematic summary of the pharmacological management of patients with APS in SLE is presented in .
Figure 2. An illustrative figure of drugs commonly used in patients with systemic lupus erythematosus (SLE) with antiphospholipid syndrome (APS; right) and during pregnancy (left). HCQ is recommended in all patients unless contraindicated. Antiphospholipid antibody (aPL) profile should be considered for preventive strategies. Colours represent the safety of each medication during pregnancy (green, considered safe; yellow, insufficient data to evaluate the risk during pregnancy; orange, contraindicated). HCQ: hydroxychloroquine; CYC: cyclophosphamide; MMF: mycophenolate mofetil; AZA: azathioprine; CsA: cyclosporine A; TAC: tacrolimus; IVIG: intravenous immunoglobulin.
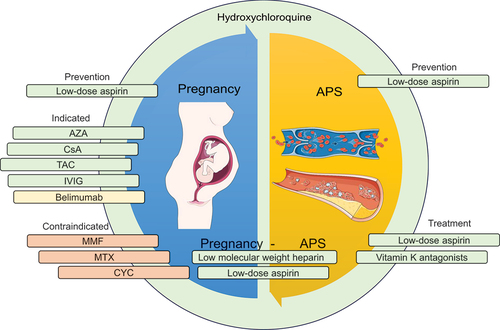
Therapeutic approaches with anti-inflammatory agents, such as GCs and anti-complement therapy, should be considered to treat certain APS-related phenotypes like refractory or catastrophic APS, which indicates the involvement of an inflammatory response mediated by aPL [Citation6]. Of note, the mTORC pathway is activated in arteries of patients with APS in a PI3K-AKT dependent manner suggesting that mTORC may represent a promising therapeutic target. From a clinical standpoint, kidney transplant recipients with a high-risk aPL profile who were treated with the mTORC inhibitor sirolimus exhibited a downregulation of the mTORC pathway, resulting in favorable kidney-related outcomes [Citation91]. However, the current management of severe APS cases with immunosuppressants lacks randomized evidence, underscoring the need for further research to support these interventions.
2.7. Pregnancy
Pregnancy is a topic of particular interest in SLE, as the disease mainly affects women of child-bearing age. EULAR has compiled separate European recommendations for family planning and pregnancy in people with SLE and/or APS [Citation7].
There is evidence supporting the use of HCQ as safe and effective not only before, but also during pregnancy for reducing disease activity and for flare-prevention [Citation92–95]. GCs can also be given during pregnancy, both orally [Citation96,Citation97] and intravenously [Citation98,Citation99], although the latter should be reserved for treating moderate-to-high disease activity in a pregnant patient. Plasmapheresis [Citation100,Citation101] and IVIG [Citation96,Citation102] are other intravenous therapies that could be considered in these cases. It is important to note that evidence for plasmapheresis is on a case report level.
CYC is contraindicated during pregnancy, especially during the first trimester, due to increased risk of intra-uterine fetal death [Citation102,Citation103]. Patients receiving CYC who wish to bear children in the future can be offered gonadotropin-releasing hormone analogues for the prevention of premature gonadal failure [Citation104]. Caution is mainly warranted for women older than 35 years of age and women receiving high-dose CYC; low-dose CYC-regimens have been reported to spare the ovarian reserve [Citation105]. MMF and MTX should be discontinued before and should be avoided during pregnancy due to their teratogenic effects [Citation99]. Substances on which there are insufficient data upon which grounded conclusions can be drawn, such as belimumab [Citation106] or leflunomide, should be avoided [Citation99]. AZA [Citation107], cyclosporine [Citation108] and tacrolimus [Citation109] are considered compatible with pregnancy. As biologic agents are immunoglobulins, they may not be expected to pass the placenta until the third trimester. Although data on voclosporine are lacking, it may be assumed to prove a safe drug when data become available, based on safety data inferred from studies on cyclosporine A and tacrolimus in patient populations including patients with SLE, given their similar mechanism of action.
Another consideration concerning reproductive health is the use of contraceptives. Intrauterine devices and contraceptives containing progesterone can be considered for all patients [Citation7,Citation110]. Combined oral contraceptives containing estrogen and progesterone might be considered for patients with stable disease who do not have aPL [Citation7,Citation110]. However, the use of combined oral contraceptives should be carefully weighed against the risk of flare in the individual patient. A schematic summary of the pharmacological management of patients with SLE during pregnancy is shown in .
3. Non-pharmacological management of SLE
Although this review pertains to the pharmacological management of SLE, it should be noted that non-pharmacological management, on which EULAR has developed separate recommendations [Citation47,Citation111], comprise an important part of SLE therapy. Efficacious interventions range from physical activity [Citation112] for the management of fatigue, to psychosocial interventions for the management of depressive symptoms [Citation113].
In contrast to active interventions, reduction of harmful exposures such as the sunlight [Citation111] also constitutes an important non-pharmacological strategy in the management of SLE. Furthermore, smoking is not only associated with increased disease severity [Citation114], but also to decreased efficacy of medications such as belimumab [Citation76,Citation115] and HCQ [Citation116]. Effective weight management strategies for patients who are over- or underweight may improve health-related quality of life (HRQoL) [Citation117]. Concerning food intake, cross-sectional data show associations between the Mediterranean diet and lower measures of disease activity as well as organ damage accrual [Citation118].
Social support and a sense of community can also help with managing SLE [Citation119], and attending support groups can be beneficial for patients’ HRQoL [Citation120]. Interhuman relations are also important within the healthcare setting, as it can enhance medication adherence [Citation121]. Navigating healthcare systems [Citation122] and misunderstandings in communication with healthcare providers have been described as obstacles between patients with SLE and adequate healthcare, stressing the need for soft skills among physicians and other healthcare professionals [Citation123,Citation124].
4. Expert opinion
The pharmacological management of SLE has long encountered formidable challenges but has also witnessed substantial progress during the last years, particularly concerning lupus nephritis. The authors advocate for holistic and individualized management approaches, centered around three interconnected principles: minimizing GC usage, targeting to the attainment of remission, or low disease activity when remission cannot be achieved, and ensuring early and assertive utilization of suitable management for SLE, which incorporates consideration of the patient’s disease experience, individual needs, and preferences. All the above are anticipated to mitigate organ damage accrual, contribute to improved HRQoL, and ultimately, prolong patients’ life-length.
For LN in particular, the authors advocate for early combination therapy with addition of belimumab on top of CYC or MMF or addition of a calcineurin inhibitor on top of MMF. While the current versions of the EULAR and KDIGO recommendations for the management of SLE and LN prompt consideration of early combination with these add-on options, we instead advocate that early combinatory regimens are desirable in all cases. With the current standard therapies, complete renal response is achieved in a disappointing 20–30% of the patients within 1–2 years of therapy, as evidenced in the placebo arms of the belimumab (BLISS-LN) and voclosporin (AURA-LV and AURORA 1) clinical trials [Citation41,Citation42,Citation125]. Thus, while combinatory regimens may overtreat a few patients in whom the CYC or MMF regimen alone might have been sufficient, they will certainly benefit a substantial number of patients with LN, mainly comprising young women during their fertile years of age. Aggressive regimens are therefore preferable during the initial phase of LN therapy, and the risk of overtreatment may better be mitigated by tapering the degree of immunosuppression upon successful induction of complete renal response [Citation126,Citation127]. In this regard, a repeat kidney biopsy may be essential for the early evaluation of the treatment outcome [Citation128,Citation129], at least until biomarkers that reliably reflect activity and chronicity features in the LN kidney tissue exist and are used for liquid biopsies that effectively substitute tissue-based assessments.
4.1. Tapering prednisone for prolonged well-being
A fundamental principle in the pharmacological management of SLE is the tapering of GCs to curtail prolonged exposure and alleviate the adverse effects linked to chronic GC use. The prevailing treatment paradigm underscores an initial phase aimed at inducing remission, recognizing the imperative for GCs in the acute setting. However, the goal is to expeditiously reduce GC use to the lowest effective dose, and withdrawal when possible. The rationale behind this approach stems from the deleterious impact of protracted corticosteroid use on various organ systems. Importantly, data from the voclosporin trials demonstrate the feasibility of a forced, quick tapering to low-dose GC in lupus nephritis [Citation42,Citation125,Citation126].
4.2. Striving for remission and minimal disease activity
An overarching goal in treating SLE is to attain remission, and in cases where this proves unachievable, to reach a state of minimal disease activity. The evolving treatment landscape introduces new-generation drugs such as belimumab, anifrolumab, voclosporin, and obinutuzumab. The decision-making process for clinicians becomes multifaceted, requiring consideration of drug availability, costs, and patient-specific factors. In the era of a growing array of options, precision medicine emerges as a critical tool in navigating this complexity [Citation130,Citation131].
4.3. Early intervention with cortisone-sparing agents
The imperative to treat rheumatic diseases, SLE included, early with cortisone-sparing agents is underscored by the acknowledgment of the long-term consequences of chronic steroid use, which are specified above. Lupus nephritis exemplifies the significance of this principle, as evidenced by trials incorporating initial combination treatments to circumvent prolonged corticosteroid exposure and mitigate organ damage accrual [Citation42,Citation125].
5. Conclusion
In summary, pharmacotherapy for SLE stands at a transformative juncture. While recent successes with novel drugs bring optimism, challenges persist in refining treatment strategies to achieve better outcomes. An emphasis on tapering GC dosages to mitigate long-term use, aspiring for remission and minimal disease activity, and early intervention with effective cortisone-sparing agents are central tenets. In LN in particular, a paradigm shift from sequential to combinatory regimens is being witnessed, which is anticipated to result in improved short-term outcomes and long-term prognosis for these patients. The change toward early multimodal treatment and the emphasis on personalized therapeutic approaches show promise for the treatment of patients with SLE. Navigating these changes, the integration of biomarkers and tissue-level mapping will play a pivotal role in refining treatment strategies and enhancing patient outcomes in the era of precision medicine.
Article highlights
Despite advances in pharmacotherapy and the introduction of biologics, antimalarial agents and glucocorticoids remain cornerstones in the medicinal management of SLE.
Although glucocorticoids remain an effective alternative for promptly and effectively suppressing disease activity, their use should be minimized and constantly reevaluated due to their long-term detrimental effects.
Pharmacotherapy in SLE should be tailored to the individual patient, based on disease activity, severity, serological profile, and organ involvement, among other factors.
Adjunctive therapy on top of immunosuppression is needed for treating or preventing acute and chronic complications, particularly for renal and neuropsychiatric involvement.
Targeted therapies from established biologics such as belimumab and anifrolumab to novel options such as anti-CD19 CAR-T cell therapy herald the transition from clinical to molecular phenotyping as the driving principle for treatment selection.
List of abbreviations
Declaration of interest
I Parodis has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia, Bristol-Myers Squibb, Elli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Otsuka, and Roche. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
Study conception and design: I Parodis, D Nikolopoulos. Literature search and data extraction: D Nikolopoulos, A Tsoi. Supervision: I Parodis. Manuscript writing – initial draft: D Nikolopoulos, A Tsoi. Manuscript writing – critical review and revisions: all authors. All authors have approved the final version of the manuscript and take full responsibility for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Mosca M, Tani C, Aringer M, et al. European league against rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis. 2010 Jul;69(7):1269–1274. doi: 10.1136/ard.2009.117200
- Chavatza K, Kostopoulou M, Nikolopoulos D, et al. Quality indicators for systemic lupus erythematosus based on the 2019 EULAR recommendations: development and initial validation in a cohort of 220 patients. Ann Rheum Dis. 2021 Sep;80(9):1175–1182. doi: 10.1136/annrheumdis-2021-220438
- Bertsias GK, Tektonidou M, Amoura Z, et al. Joint european league against rheumatism and european renal association-European dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012 Nov;71(11):1771–1782. doi: 10.1136/annrheumdis-2012-201940
- Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 update of the joint european league against rheumatism and european renal association-european dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020 Jun;79(6):713–723. doi: 10.1136/annrheumdis-2020-216924
- Bertsias GK, Ioannidis JP, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010 Dec;69(12):2074–2082. doi: 10.1136/ard.2010.130476
- Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019 Oct;78(10):1296–1304. doi: 10.1136/annrheumdis-2019-215213
- Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. 2017 Mar;76(3):476–485. doi: 10.1136/annrheumdis-2016-209770
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019 Jun;78(6):736–745. doi: 10.1136/annrheumdis-2019-215089
- Fanouriakis A, Kostopoulou M, Andersen J, et al. Response to: correspondence on ‘EULAR recommendations for the management of systemic lupus erythematosus: 2023 update’. Ann Rheum Dis. 2023 Oct 12:ard-2024–225617. doi: 10.1136/ard-2024-225617
- Zucchi D, Cardelli C, Elefante E, et al. Treat-to-target in systemic lupus erythematosus: reality or pipe dream. J Clin Med. 2023 May 8;12(9):3348. doi: 10.3390/jcm12093348
- Mok CC, Hamijoyo L, Kasitanon N, et al. The Asia-Pacific League of Associations for Rheumatology consensus statements on the management of systemic lupus erythematosus. Lancet Rheumatol. 2021 Sep 01;3(7):e517–e531. doi: 10.1016/S2665-9913(21)00009-6
- Kandane-Rathnayake R, Golder V, Louthrenoo W, et al. Lupus low disease activity state and remission and risk of mortality in patients with systemic lupus erythematosus: a prospective, multinational, longitudinal cohort study. Lancet Rheumatol. 2022 Dec;4(12):e822–e830. doi: 10.1016/S2665-9913(22)00304-6
- Gatto M, Zen M, Iaccarino L, et al. New therapeutic strategies in systemic lupus erythematosus management. Nat Rev Rheumatol. 2019 Jan;15(1):30–48. doi: 10.1038/s41584-018-0133-2
- Emamikia S, Oon S, Gomez A, et al. Impact of remission and low disease activity on health-related quality of life in patients with systemic lupus erythematosus. Rheumatology (Oxford). 2022 Nov 28;61(12):4752–4762. doi: 10.1093/rheumatology/keac185
- Parodis I, Lindblom J, Barturen G, et al. Molecular characterisation of lupus low disease activity state (LLDAS) and DORIS remission by whole-blood transcriptome-based pathways in a pan-European systemic lupus erythematosus cohort. Ann Rheum Dis. 2024 Feb 19:ard-2023–224795. doi: 10.1136/ard-2023-224795
- Felten R, Sagez F, Gavand PE, et al. 10 most important contemporary challenges in the management of SLE. Lupus Sci Med. 2019;6(1):e000303. doi: 10.1136/lupus-2018-000303
- Pons-Estel BA, Bonfa E, Soriano ER, et al. First Latin American clinical practice guidelines for the treatment of systemic lupus erythematosus: Latin American Group for the study of lupus (GLADEL, grupo latino americano de estudio del lupus)-Pan-American League of associations of rheumatology (PANLAR). Ann Rheum Dis. 2018 Nov;77(11):1549–1557. doi: 10.1136/annrheumdis-2018-213512
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmol. 2016 Jun;123(6):1386–1394. doi: 10.1016/j.ophtha.2016.01.058
- Jorge AM, Mancini C, Zhou B, et al. Hydroxychloroquine dose per ophthalmology guidelines and the risk of systemic lupus erythematosus flares. JAMA. 2022 Oct 11;328(14):1458–1460. doi: 10.1001/jama.2022.13591
- Northcott M, Gearing LJ, Nim HT, et al. Glucocorticoid gene signatures in systemic lupus erythematosus and the effects of type I interferon: a cross-sectional and in-vitro study. Lancet Rheumatol. 2021 May 01;3(5):e357–e370. doi: 10.1016/S2665-9913(21)00006-0
- Saag KG, Koehnke R, Caldwell JR, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994 Feb;96(2):115–123. doi: 10.1016/0002-9343(94)90131-7
- Huscher D, Thiele K, Gromnica-Ihle E, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009 Jul;68(7):1119–1124. doi: 10.1136/ard.2008.092163
- Kallas R, Li J, Petri M. Predictors of Osteonecrosis in systemic lupus erythematosus: a prospective cohort study. Arthritis Care Res (Hoboken). 2022 Jul;74(7):1122–1132. doi: 10.1002/acr.24541
- Tselios K, Gladman DD, Al-Sheikh H, et al. Medium versus high initial prednisone dose for remission induction in lupus nephritis: a propensity score-matched analysis. Arthritis Care Res (Hoboken). 2022 Sep;74(9):1451–1458. doi: 10.1002/acr.24592
- Nunez D, Patel D, Volkov J, et al. Cytokine and reactivity profiles in SLE patients following anti-CD19 CART therapy. Mol Ther Methods Clin Dev. 2023 Dec 14;31:101104. doi: 10.1016/j.omtm.2023.08.023
- Mackensen A, Müller F, Mougiakakos D, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022 Oct;28(10):2124–2132. doi: 10.1038/s41591-022-02017-5
- Müller F, Taubmann J, Bucci L, et al. CD19 CAR T-Cell therapy in autoimmune disease - a case series with follow-up. N Engl J Med. 2024 Feb 22;390(8):687–700. doi: 10.1056/NEJMoa2308917
- Morel L. Immunometabolism in systemic lupus erythematosus. Nat Rev Rheumatol. 2017 May;13(5):280–290. doi: 10.1038/nrrheum.2017.43
- Wang H, Li T, Chen S, et al. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in Systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 2015 Dec;67(12):3190–3200. doi: 10.1002/art.39296
- Sun F, Wang HJ, Liu Z, et al. Safety and efficacy of metformin in systemic lupus erythematosus: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Rheumatol. 2020 Apr;2(4):e210–e216. doi: 10.1016/S2665-9913(20)30004-7
- Lai ZW, Kelly R, Winans T, et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet. 2018 Mar 24;391(10126):1186–1196. doi: 10.1016/S0140-6736(18)30485-9
- Eriksson P, Wallin P, Sjöwall C. Clinical experience of sirolimus regarding efficacy and safety in systemic lupus erythematosus. Front Pharmacol. 2019;10:82. doi: 10.3389/fphar.2019.00082
- Yap DYH, Tang C, Chan GCW, et al. Longterm data on sirolimus treatment in patients with lupus nephritis. J Rheumatol. 2018 Dec;45(12):1663–1670. doi: 10.3899/jrheum.180507
- Jiang N, Li M, Zhang H, et al. Sirolimus versus tacrolimus for systemic lupus erythematosus treatment: results from a real-world CSTAR cohort study. Lupus Sci Med. 2022 Jan;9(1):e000617. doi: 10.1136/lupus-2021-000617
- Hoover PJ, Costenbader KH. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney Int. 2016 Sep;90(3):487–492. doi: 10.1016/j.kint.2016.03.042
- Moroni G, Depetri F, Ponticelli C. Lupus nephritis: when and how often to biopsy and what does it mean? J Autoimmun. 2016 Nov;74:27–40. doi: 10.1016/j.jaut.2016.06.006
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-lupus nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002 Aug;46(8):2121–2131. doi: 10.1002/art.10461
- Yu C, Li P, Dang X, et al. Lupus nephritis: new progress in diagnosis and treatment. J Autoimmun. 2022 Oct;132:102871. doi: 10.1016/j.jaut.2022.102871
- Suh CH. Rituximab can decrease proteinuria in refractory lupus nephritis. J Rheum Dis. 2022;29:59–60. Korea (South). doi: 10.4078/jrd.2022.29.2.59
- Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. 2012 Apr;64(4):1215–1226. doi: 10.1002/art.34359
- Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of Belimumab in lupus nephritis. N Engl J Med. 2020 Sep 17;383(12):1117–1128. doi: 10.1056/NEJMoa2001180
- Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021 May 29;397(10289):2070–2080. doi: 10.1016/S0140-6736(21)00578-X
- Sciascia S, Yazdany J, Dall’era M, et al. Anticoagulation in patients with concomitant lupus nephritis and thrombotic microangiopathy: a multicentre cohort study. Ann Rheum Dis. 2019;78: 1004–1006. England. doi: 10.1136/annrheumdis-2018-214559
- Wright RD, Bannerman F, Beresford MW, et al. A systematic review of the role of eculizumab in systemic lupus erythematosus-associated thrombotic microangiopathy. BMC Nephrol. 2020 Jun 30;21(1):245. doi: 10.1186/s12882-020-01888-5
- Tselios K, Gladman DD, Su J, et al. Impact of the new American college of cardiology/American heart association definition of hypertension on atherosclerotic vascular events in systemic lupus erythematosus. Ann Rheum Dis. 2020 May;79(5):612–617. doi: 10.1136/annrheumdis-2019-216764
- Kostopoulou M, Nikolopoulos D, Parodis I, et al. Cardiovascular disease in systemic lupus erythematosus: recent data on epidemiology, risk factors and prevention. Curr Vasc Pharmacol. 2020;18(6):549–565. doi: 10.2174/1570161118666191227101636
- Parodis I, Gomez A, Tsoi A, et al. Systematic literature review informing the EULAR recommendations for the non-pharmacological management of systemic lupus erythematosus and systemic sclerosis. RMD Open. 2023 Aug;9(3):e003297. doi: 10.1136/rmdopen-2023-003297
- Hermosillo-Romo D, Brey RL. Diagnosis and management of patients with neuropsychiatric systemic lupus erythematosus (NPSLE). Best Pract Res Clin Rheumatol. 2002 Apr;16(2):229–244. doi: 10.1053/berh.2001.0223
- Nikolopoulos D, Fanouriakis A, Bertsias G. Treatment of neuropsychiatric systemic lupus erythematosus: clinical challenges and future perspectives. Expert Rev Clin Immunol. 2021 Apr;17(4):317–330. doi: 10.1080/1744666X.2021.1899810
- Nikolopoulos D, Fanouriakis A, Boumpas DT. Cerebrovascular events in systemic lupus erythematosus: diagnosis and management. Mediterr J Rheumatol. 2019 Mar;30(1):7–15. doi: 10.31138/mjr.30.1.7
- Fanouriakis A, Pamfil C, Sidiropoulos P, et al. Cyclophosphamide in combination with glucocorticoids for severe neuropsychiatric systemic lupus erythematosus: a retrospective, observational two-centre study. Lupus. 2016 May;25(6):627–636. doi: 10.1177/0961203315622821
- Tokunaga M, Saito K, Kawabata D, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007 Apr;66(4):470–475. doi: 10.1136/ard.2006.057885
- Gupta N, Ganpati A, Mandal S, et al. Mycophenolate mofetil and deflazacort combination in neuropsychiatric lupus: a decade of experience from a tertiary care teaching hospital in southern India. Clin Rheumatol. 2017 Oct;36(10):2273–2279. doi: 10.1007/s10067-017-3775-6
- Hall AG, Tilby MJ. Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992 Sep;6(3):163–173. doi: 10.1016/0268-960X(92)90028-O
- Kuraitis D, Murina A. Facts, not fear: safety of Hydroxychloroquine. Am J Med Sci. 2020 Aug;360(2):199–200. doi: 10.1016/j.amjms.2020.05.003
- Kiang TKL, Ensom MHH. Exposure-toxicity relationships of mycophenolic acid in adult kidney transplant patients. Clin Pharmacokinet. 2019 Dec;58(12):1533–1552. doi: 10.1007/s40262-019-00802-z
- Formea CM, Myers-Huentelman H, Wu R, et al. Thiopurine S-methyltransferase genotype predicts azathioprine-induced myelotoxicity in kidney transplant recipients. Am J Transplant. 2004 Nov;4(11):1810–1817. doi: 10.1111/j.1600-6143.2004.00575.x
- Upadhyay R, Torley HI, McKinlay AW, et al. Iron deficiency anaemia in patients with rheumatic disease receiving non-steroidal anti-inflammatory drugs: the role of upper gastrointestinal lesions. Ann Rheum Dis. 1990 Jun;49(6):359–362. doi: 10.1136/ard.49.6.359
- Shen M, Zeng X, Tian X, et al. Diffuse alveolar hemorrhage in systemic lupus erythematosus: a retrospective study in China. Lupus. 2010 Oct;19(11):1326–1330. doi: 10.1177/0961203310373106
- Pérez-Sánchez I, Anguita J, Pintado T. Use of cyclophosphamide in the treatment of thrombotic thrombocytopenic purpura complicating systemic lupus erythematosus: report of two cases. Ann Hematol. 1999 Jun;78(6):285–287. doi: 10.1007/s002770050516
- Mahévas M, Azzaoui I, Crickx E, et al. Efficacy, safety and immunological profile of combining rituximab with belimumab for adults with persistent or chronic immune thrombocytopenia: results from a prospective phase 2b trial. Haematologica. 2021 Sep 1;106(9):2449–2457. doi: 10.3324/haematol.2020.259481
- Lu Q, Long H, Chow S, et al. Guideline for the diagnosis, treatment and long-term management of cutaneous lupus erythematosus. J Autoimmun. 2021 Sep;123:102707. doi: 10.1016/j.jaut.2021.102707
- Chasset F, Bouaziz JD, Costedoat-Chalumeau N, et al. Efficacy and comparison of antimalarials in cutaneous lupus erythematosus subtypes: a systematic review and meta-analysis. Br J Dermatol. 2017 Jul;177(1):188–196. doi: 10.1111/bjd.15312
- Keyes E, Jobanputra A, Feng R, et al. Comparative responsiveness of cutaneous lupus erythematosus patients to methotrexate and mycophenolate mofetil: a cohort study. J Am Acad Dermatol. 2022 Aug;87(2):447–448. doi: 10.1016/j.jaad.2021.09.017
- Kneeland R, Montes D, Endo J, et al. Improvement in cutaneous lupus erythematosus after twenty weeks of belimumab use: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2023 Aug;75(8):1838–1848. doi: 10.1002/acr.25058
- Morand EF, Furie RA, Bruce IN, et al. Efficacy of anifrolumab across organ domains in patients with moderate-to-severe systemic lupus erythematosus: a post-hoc analysis of pooled data from the TULIP-1 and TULIP-2 trials. Lancet Rheumatol. 2022 Apr 1;4(4):e282–e292. doi: 10.1016/S2665-9913(21)00317-9
- Albrecht J, Taylor L, Berlin JA, et al. The CLASI (cutaneous lupus erythematosus disease area and severity index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005 Nov;125(5):889–894. doi: 10.1111/j.0022-202X.2005.23889.x
- Chasset F, Tounsi T, Cesbron E, et al. Efficacy and tolerance profile of thalidomide in cutaneous lupus erythematosus: a systematic review and meta-analysis. J Am Acad Dermatol. 2018 Feb;78(2):342–350.e4. doi: 10.1016/j.jaad.2017.09.059
- Aitmehdi R, Arnaud L, Francès C, et al. Long-term efficacy and safety outcomes of lenalidomide for cutaneous lupus erythematosus: a multicenter retrospective observational study of 40 patients. J Am Acad Dermatol. 2021 Apr;84(4):1171–1174. doi: 10.1016/j.jaad.2020.11.014
- Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002 Feb;29(2):288–291.
- Vitali C, Bencivelli W, Isenberg DA, et al.;Disease activity in systemic lupus erythematosus: report of the Consensus Study Group of the European Workshop for Rheumatology Research. I. A descriptive analysis of 704 European lupus patients. European consensus study group for disease activity in SLE. Clin Exp Rheumatol. 1992 Sep;10(5):527–539.
- Ceccarelli F, Natalucci F, Pirone C, et al. Erosive arthritis in systemic lupus erythematosus: application of cluster analysis. Clin Exp Rheumatol. 2022 Nov;40(11):2175–2178. doi: 10.55563/clinexprheumatol/b8gaty
- Shovman O, Langevitz P, Shoenfeld Y. Rhupus; unusual presentations. Clin Rheumatol. 2015 Dec;34(12):2041–2046. doi: 10.1007/s10067-015-2978-y
- Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011 Dec;63(12):3918–3930. doi: 10.1002/art.30613
- Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011 Feb 26;377(9767):721–731. doi: 10.1016/S0140-6736(10)61354-2
- Parodis I, Gomez A, Frodlund M, et al. Smoking reduces the efficacy of belimumab in mucocutaneous lupus. Expert Opin Biol Ther. 2018 Aug;18(8):911–920. doi: 10.1080/14712598.2018.1494719
- Schwarting A, Schroeder JO, Alexander T, et al. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: results from the OBSErve Germany study. Rheumatol Ther. 2016 Dec;3(2):271–290. doi: 10.1007/s40744-016-0047-x
- Furie RA, Morand FE, Bruce I, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumat. 2019;1(4):e208–e219. doi: 10.1016/S2665-9913(19)30076-1
- Morand EF, Furie R, Tanaka Y, et al. Trial of Anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020 Jan 16;382(3):211–221. doi: 10.1056/NEJMoa1912196
- Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010 Jan;62(1):222–233. doi: 10.1002/art.27233
- Fortin PR, Abrahamowicz M, Ferland D, et al. Steroid-sparing effects of methotrexate in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2008 Dec 15;59(12):1796–1804. doi: 10.1002/art.24068
- Carneiro JR, Sato EI. Double blind, randomized, placebo controlled clinical trial of methotrexate in systemic lupus erythematosus. J Rheumatol. 1999 Jun;26(6):1275–1279.
- Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Ann Rheum Dis. 2017 Sep;76(9):1575–1582. doi: 10.1136/annrheumdis-2016-210882
- Morand EF, Vital EM, Petri M, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I). Lancet. 2023 Mar 25;401(10381):1001–1010. doi: 10.1016/S0140-6736(22)02607-1
- Petri M, Bruce IN, Dörner T, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet. 2023 Mar 25;401(10381):1011–1019. doi: 10.1016/S0140-6736(22)02546-6
- Barbhaiya M, Zuily S, Naden R, et al. The 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Arthritis Rheumatol. 2023 Oct;75(10):1687–1702. doi: 10.1002/art.42624
- Arnaud L, Mathian A, Ruffatti A, et al. Efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies: an international and collaborative meta-analysis. Autoimmun Rev. 2014 Mar;13(3):281–291. doi: 10.1016/j.autrev.2013.10.014
- Verro P, Levine SR, Tietjen GE. Cerebrovascular ischemic events with high positive anticardiolipin antibodies. Stroke. 1998 Nov;29(11):2245–2253. doi: 10.1161/01.STR.29.11.2245
- Wang CR, Liu MF. Rituximab usage in systemic lupus erythematosus-associated antiphospholipid syndrome: a single-center experience. Semin Arthritis Rheum. 2016 Aug;46(1):102–108. doi: 10.1016/j.semarthrit.2016.02.002
- Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018 Sep 27;132(13):1365–1371. doi: 10.1182/blood-2018-04-848333
- Canaud G, Bienaimé F, Tabarin F, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014 Jul 24;371(4):303–312. doi: 10.1056/NEJMoa1312890
- Levy RA, Vilela VS, Cataldo MJ, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10(6):401–404. doi: 10.1191/096120301678646137
- Al Arfaj AS, Khalil N. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus. 2010 Dec;19(14):1665–1673. doi: 10.1177/0961203310378669
- Clowse ME, Magder L, Witter F, et al. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006 Nov;54(11):3640–3647. doi: 10.1002/art.22159
- Koh JH, Ko HS, Kwok SK, et al. Hydroxychloroquine and pregnancy on lupus flares in Korean patients with systemic lupus erythematosus. Lupus. 2015 Feb;24(2):210–217. doi: 10.1177/0961203314555352
- Chakravarty EF, Colón I, Langen ES, et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol. 2005 Jun;192(6):1897–1904. doi: 10.1016/j.ajog.2005.02.063
- Derksen RH, Bruinse HW, de Groot PG, et al. Pregnancy in systemic lupus erythematosus: a prospective study. Lupus. 1994 Jun;3(3):149–155. doi: 10.1177/096120339400300304
- Moroni G, Quaglini S, Banfi G, et al. Pregnancy in lupus nephritis. Am J Kidney Dis. 2002 Oct;40(4):713–720. doi: 10.1053/ajkd.2002.35678
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016 May;75(5):795–810. doi: 10.1136/annrheumdis-2015-208840
- Takeshita Y, Turumi Y, Touma S, et al. Successful delivery in a pregnant woman with lupus anticoagulant positive systemic lupus erythematosus treated with double filtration plasmapheresis. Ther Apher. 2001 Feb;5(1):22–24. doi: 10.1046/j.1526-0968.2001.005001022.x
- Abou-Nassar K, Karsh J, Giulivi A, et al. Successful prevention of thrombotic thrombocytopenic purpura (TTP) relapse using monthly prophylactic plasma exchanges throughout pregnancy in a patient with systemic lupus erythematosus and a prior history of refractory TTP and recurrent fetal loss. Transfus Apher Sci. 2010 Aug;43(1):29–31. doi: 10.1016/j.transci.2010.05.002
- Clowse ME, Magder LS, Witter F, et al. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum. 2005 Feb;52(2):514–521. doi: 10.1002/art.20864
- Cavallasca JA, Laborde HA, Ruda-Vega H, et al. Maternal and fetal outcomes of 72 pregnancies in Argentine patients with systemic lupus erythematosus (SLE). Clin Rheumatol. 2008 Jan;27(1):41–46. doi: 10.1007/s10067-007-0649-3
- Ben-Aharon I, Gafter-Gvili A, Leibovici L, et al. Pharmacological interventions for fertility preservation during chemotherapy: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010 Aug;122(3):803–811. doi: 10.1007/s10549-010-0996-7
- Tamirou F, Husson SN, Gruson D, et al. Brief report: the euro-lupus low-dose intravenous cyclophosphamide regimen does not impact the ovarian reserve, as measured by serum levels of anti-müllerian hormone. Arthritis Rheumatol. 2017 Jun;69(6):1267–1271. doi: 10.1002/art.40079
- Petri M, Landy H, Clowse MEB, et al. Belimumab use during pregnancy: a summary of birth defects and pregnancy loss from belimumab clinical trials, a pregnancy registry and postmarketing reports. Ann Rheum Dis. 2023 Feb;82(2):217–225. doi: 10.1136/ard-2022-222505
- Fischer-Betz R, Specker C, Brinks R, et al. Low risk of renal flares and negative outcomes in women with lupus nephritis conceiving after switching from mycophenolate mofetil to azathioprine. Rheumatology (Oxford). 2013 Jun;52(6):1070–1076. doi: 10.1093/rheumatology/kes425
- Hussein MM, Mooij JM, Roujouleh H. Cyclosporine in the treatment of lupus nephritis including two patients treated during pregnancy. Clin Nephrol. 1993 Sep;40(3):160–163.
- Webster P, Wardle A, Bramham K, et al. Tacrolimus is an effective treatment for lupus nephritis in pregnancy. Lupus. 2014 Oct;23(11):1192–1196. doi: 10.1177/0961203314540353
- Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken). 2020 Apr;72(4):461–488. doi: 10.1002/acr.24130
- Parodis I, Girard-Guyonvarc’h C, Arnaud L, et al. EULAR recommendations for the non-pharmacological management of systemic lupus erythematosus and systemic sclerosis. Ann Rheum Dis. 2023 Jul 10:ard-2023–224416. doi: 10.1136/ard-2023-224416
- Bostrom C, Elfving B, Dupre B, et al. Effects of a one-year physical activity programme for women with systemic lupus erythematosus - a randomized controlled study. Lupus. 2016;25(6):602–616. doi: 10.1177/0961203315622817
- Zhang J, Wei W, Wang CM. Effects of psychological interventions for patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus. 2012;21(10):1077–1087. doi: 10.1177/0961203312447667
- Kallas R, Li J, Petri M. Association of African-American ethnicity and smoking status with total and individual damage index in systemic lupus erythematosus. Clin Rheumatol. 2020 Feb;39(2):365–373. doi: 10.1007/s10067-019-04800-1
- Parodis I, Sjöwall C, Jönsen A, et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev. 2017 Apr;16(4):343–351. doi: 10.1016/j.autrev.2017.02.005
- Chasset F, Francès C, Barete S, et al. Influence of smoking on the efficacy of antimalarials in cutaneous lupus: a meta-analysis of the literature. J Am Acad Dermatol. 2015 Apr;72(4):634–639. doi: 10.1016/j.jaad.2014.12.025
- Borg A, Gomez A, Cederlund A, et al. Contribution of abnormal BMI to adverse health-related quality of life outcomes after a 52-week therapy in patients with SLE. Rheumatology (Oxford). 2021 Sep 1;60(9):4205–4217. doi: 10.1093/rheumatology/keaa909
- Pocovi-Gerardino G, Correa-Rodriguez M, Callejas-Rubio JL, et al. Beneficial effect of mediterranean diet on disease activity and cardiovascular risk in systemic lupus erythematosus patients: a cross-sectional study. Rheumatology. 2021;60(1):160–169. doi: 10.1093/rheumatology/keaa210
- Li X, He L, Wang J, et al. Illness uncertainty, social support, and coping mode in hospitalized patients with systemic lupus erythematosus in a hospital in Shaanxi, China. PLOS ONE [Electronic Resource]. 2019;14(2):e0211313. doi: 10.1371/journal.pone.0211313
- Dorsey RR, Andresen EM, Moore TL. Health-related quality of life and support group attendance for patients with systemic lupus erythematosus. JCR: J Clinic Rheumatol. 2004;10(1):6–9. doi: 10.1097/01.rhu.0000111311.38407.15
- Ganachari MS, Almas SA. Evaluation of clinical pharmacist mediated education and counselling of systemic lupus erythematosus patients in tertiary care hospital. Indian J Rheumatol. 2012;7(1):7–12. doi: 10.1016/S0973-3698(12)60003-X
- Feldman CH, Bermas BL, Zibit M, et al. Designing an intervention for women with systemic lupus erythematosus from medically underserved areas to improve care: a qualitative study. Lupus. 2013 Jan;22(1):52–62. doi: 10.1177/0961203312463979
- Sloan M, Bosley M, Blane M, et al. ‘But you don’t look sick’: a qualitative analysis of the LUPUS UK online forum. Rheumatol Int. 2021 Apr;41(4):721–732. doi: 10.1007/s00296-020-04726-x
- Emamikia S, Gentline C, Enman Y, et al. How can we enhance adherence to medications in patients with systemic lupus erythematosus? Results from a qualitative study. J Clin Med. 2022 Mar 27;11(7):1857. doi: 10.3390/jcm11071857
- Rovin BH, Solomons N, Pendergraft WF 3rd, et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019 Jan;95(1):219–231. doi: 10.1016/j.kint.2018.08.025
- Parodis I, Houssiau FA. From sequential to combination and personalised therapy in lupus nephritis: moving towards a paradigm shift? Ann Rheum Dis. 2022 Jan;81(1):15–19. doi: 10.1136/annrheumdis-2021-221270
- Parodis I, Depascale R, Doria A, et al. When should targeted therapies be used in the treatment of lupus nephritis: early in the disease course or in refractory patients? Autoimmun Rev. 2023 Aug 23;23(1):103418. doi: 10.1016/j.autrev.2023.103418
- Parodis I, Tamirou F, Houssiau FA. Treat-to-target in lupus nephritis. What is the role of the repeat kidney biopsy? Arch Immunol Ther Exp (Warsz). 2022 Feb 11;70(1):8. doi: 10.1007/s00005-022-00646-9
- Parodis I, Moroni G, Calatroni M, et al. Is per-protocol kidney biopsy required in lupus nephritis? Autoimmun Rev. 2023 Aug 24;23(1):103422. doi: 10.1016/j.autrev.2023.103422
- Parodis I. Molecular signature-based decision making in the era of targeted therapies for systemic lupus erythematosus. Lancet Rheumatol. 2023 Jan;5(1):e4–e6. doi: 10.1016/S2665-9913(22)00358-7
- Lindblom J, Toro-Domínguez D, Carnero-Montoro E, et al. Distinct gene dysregulation patterns herald precision medicine potentiality in systemic lupus erythematosus. J Autoimmun. 2023 Apr;136:103025. doi: 10.1016/j.jaut.2023.103025
