ABSTRACT
Reuse of devulcanisates in virgin rubber compounds is the shortest loop in cradle-to-cradle cycles for elastomers. Due to the enormous complexity of these loops, the technology still stands in its infancy. Two elastomer types, EPDM (Ethylene–Propylene-Diene Terpolymer) and SBR (Styrene–Butadiene Rubber), are compared as to their ease of devulcanisation with an optimized devulcanisation aid. EPDM is relatively easy to devulcanise into a quality which allows for reuse of large amounts till 50% in virgin compounds for applications like roofing sheets and others. This is already being done on industrial scale. SBR is more difficult to devulcanise due to re-crosslinking under the influence of oxygen. Furthermore, tyre scrap is a mix of compounds and elastomers, which are impossible to segregate before devulcanisation. A lot more research and development is needed to bring this to a success.
CLASSIFICATIONS:
Introduction
Devulcanisation of elastomers is the most effective way of recycling: it is the shortest loop and it allows to reuse the material in its original application. However, the technology for this is still in its infancy; the current devulcanisation processes described in the literature [Citation1–5] are a combination of crosslink scission and polymer breakdown. The challenge is to shift the balance towards crosslink scission and to avoid polymer breakdown as far as possible. General rules to achieve this are a low devulcanisation temperature, the absence of oxygen and the presence of a devulcanisation aid. The most common devulcanisation aid is DiPhenyl DiSulfide (DPDS); however, for Ethylene–Propylene-Diene Terpolymer (EPDM) its efficiency is low: a better choice for this polymer is an amine: HexaDecyl Amine (HDA) [Citation6].
Devulcanisation studies on model compounds were performed on tetramethylethylene and small-scale processes were developed for Natural Rubber [Citation7–10] besides EPDM and Styrene–Butadiene Rubber (SBR), which are discussed here.
Experimental
Materials
Within this study, different raw materials were used for the optimisation of the devulcanisation processes: the first part was done with conventionally sulphur cured and efficiently cured (EV) EPDM model compounds, ground to a granulate of a maximum particle size of 8 mm. The formulation of this model compound, which was also used for blending the devulcanisate with virgin rubber, is given in . In the second part, a SBR model compound according to was used for virgin/devulcanisate blends. This table also shows the formulations of the different tyre materials used for devulcanisation. All materials are specified in .
Table 1. EPDM masterbatch and curing formulations.
Table 2. SBR model compound according to ASTM D3185-99 and model compound for devulcanisation of tyre rubbers.
Table 3. Material specifications.
Mixing and vulcanisation
Compounds were made in a Brabender Plasticorder 350S mixer with a mixing chamber volume of 350 cm3, using a rotor speed of 60 rev min−1, a fill factor of 0.75 and an initial temperature setting of 50°C. Cure characteristics were tested using an RPA 2000 dynamic mechanical curemeter from Alpha Technologies at 170°C (EPDM) and at 160°C (SBR), 0.833 Hz and 0.2 degree strain, according to ISO 6502. The curing additives were mixed into the EPDM rubber masterbatch at room temperature on a laboratory size Schwabenthan two-roll mill (15 × 33 cm Polymix 80) with a friction ratio of 1:1.25; for the SBR compounds, the curatives were added in the mixer. Curing was done in a Wickert WLP1600 press at 170°C and 100 bar, time was taken as t90 + 130 seconds for 2-mm-thick sheets, and 1.5 * t90 to correct for the larger thickness when sheets of a thickness of 6 mm were cured from the EPDM rubber. The SBR tyre compounds were vulcanised for t90 + 5 minutes into 2-mm-thick sheets.
Cryogenic grinding
The cured materials were cryogenically ground in a Bauknecht grinder. The rubber samples were immersed in liquid nitrogen before grinding.
Devulcanisation
The ground rubber was devulcanised in a Brabender Plasticorder PL 2000 with a mixing chamber volume of 35 mL and a cam-type rotor. The fill factor was 0.7. The parameters temperature, time, rotor speed and amount of devulcanisation aid for the EPDM study were varied in a Design of Experiments setup within a parameter window as shown in , and a central composite face-centred model was used [Citation11]. A volume of 5 wt-% devulcanisation oil relative to the vulcanisate was added to each batch. EPDM was devulcanised with varying concentrations of HDA according to ; SBR was devulcanised with 5 wt-% DPDS.
Table 4. Devulcanisation conditions for EPDM devulcanisation.
Mooney viscosity
Mooney viscosities MLw (1 + 4)125°C were determined using a Mooney viscometer MV2000 VS from Alpha Technologies according to ISO R289 for EPDM, at 100°C for SBR.
Extractions
The soluble and insoluble fractions of the vulcanised compounds were determined by extraction in a Soxhlet apparatus: 24 hours in acetone, 48 hours in THF. Samples were dried under vacuum at 40°C until the weight was constant. The sol fraction was defined as the sum of the soluble fractions in acetone and THF.
Equilibrium swelling
The degree of swelling was measured with decahydronaphthalene for EPDM and toluene for SBR on the extracted samples. The crosslink density was calculated according to the Flory–Rehner equation [Citation12] with Kraus [Citation13] correction for filled compounds as given by Equations (1)–(3) [Citation14]:(1)
(2)
(3)
(4)
(5) where νapparent is the apparent measured chemical crosslink density from the swelling test, νactual is the actual chemical crosslink density corrected for the filler loading, K is a constant for a given filler, Φ is the volume fraction of filler in the specimen, Wb is the weight of specimen before extraction and Wa is the weight of specimen after extraction of all soluble material: polymer sol fraction, oil and soluble chemical residues. In the Flory–Rehner equation (Equation4
(4) ) for non-reinforced compounds, νe is the real crosslink density per unit volume, vr is the degree of swelling, with mr and ms the relative masses of rubber network and solvent at equilibrium swelling, and ρr and ρs the densities of the rubber and solvent. The Flory–Huggins polymer–solvent interaction parameter χ was taken as 0.37 for the system SBR/toluene [Citation15] and 0.121 + 0.278vr for the EPDM–decahydronaphthalene combination [Citation16].
Crosslink distribution
The specific crosslink distributions of the vulcanisates were studied using thiol/amine chemical probes [Citation17–20]. The thiol/amine probes were directly applied to the swollen samples. A solution of 0.4 M 2-propanethiol and 0.4 M piperidine in n-heptane was used for 2 hours at room temperature to selectively cleave polysulfidic crosslinks. 1 M 1-hexanethiol in piperidine was used for 48 hours at room temperature to break the poly- plus disulfidic crosslinks. The combination of the two treatments now allows for discrimination between the mono-, di- and polysulfidic crosslinks. The reactions were terminated by putting the samples in excess petroleum ether for 24 hours, followed by Soxhlet extraction in petroleum ether for another 24 hours and in THF again for 24 hours. The mono- and the sum of mono- and disulfidic crosslink densities of the samples after treatment were determined with equilibrium swelling for 72 hours in decahydronaphtalene according to Equations (4) and (5). A simple subtraction from the overall crosslink density before treatment results in the mono-, di-, and polysulfidic crosslink densities, separately.
Ratio random to main-chain scission
A useful tool to further understand the devulcanisation mechanism is the method developed by Horikx [Citation21]: he derived a theoretical relationship between the soluble fraction generated after degradation of a polymer network and the relative decrease in crosslink density, as a result of either main-chain scission or crosslink breakage. This treatment of polymer degradation can equally well be applied to rubber reclaiming, where also a mix of main-chain scission and crosslink breakage takes place. The details of this approach were developed by Verbruggen et al. [Citation22,Citation23].
Mixing and revulcanisation
After devulcanisation, the compounds were blended in varying proportions with the original virgin EPDM- or SBR masterbatches. The blends were cured again using the same formulation as applied for the original vulcanisates, see and . The devulcanisates and curing additives were blended into the virgin masterbatches either on a two-roll mill (for EPDM roof sheeting) or in a mixer (for SBR tyre compounds). The amount of curatives was based on the total polymer content, including the polymer content of the devulcanisates. No correction was made for residues of curatives in the devulcanisate.
Mechanical properties
Tensile and tear tests were carried out according to ISO 37 and ISO 34, respectively. Compression set tests were performed according to ISO 815. The hardness of the samples was measured with a Zwick hardness-meter Shore A type according to DIN 53503.
Results and discussion
Devulcanisation behaviour of sulphur cured EPDM
The crosslink densities, specific crosslink distributions and insoluble fractions of the vulcanised compounds A and B, before devulcanisation, are given in . Compound A, the conventionally sulphur cured material, is characterised by a relatively high overall crosslink density with a high percentage of poly- plus disulfidic crosslinks and only 15% of monosulfidic bonds. Compound B on basis of the EV cure system has 48% poly- plus disulfidic crosslinks, and a surplus of 52% monosulfidic, at an overall crosslink density less than half of compound A. The insoluble fraction of compound A is higher than that of compound B, corresponding to the higher overall crosslink density.
Table 5. Crosslink densities and insoluble fractions of the original EPDM compounds.
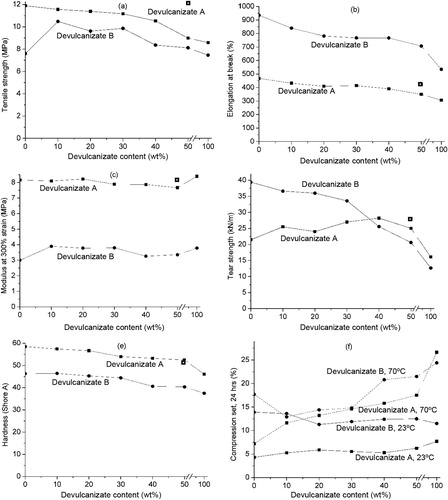
After grinding, both vulcanised compounds A and B were devulcanised under various conditions as given in . The influence of the variable factors of devulcanisate A is shown in –. When parameters needed to be set constant for creating the figures, they were fixed at devulcanisation temperature: 250°C; devulcanisation time: 7.5 minutes; rotor speed: 75 rev min−1 and amount of HDA devulcanisation aid: 5 wt-%.
Figure 1. Influence of (a) HDA concentration and temperature and (b) rotor speed and devulcanisation time on the Mooney viscosity of devulcanisate A.
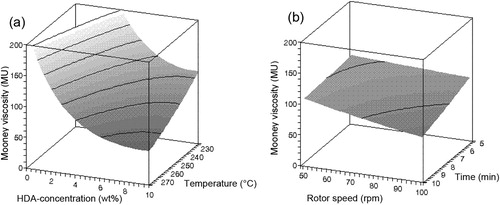
Figure 2. Influence of (a) HDA concentration and temperature and (b) rotor speed and devulcanisation time on the insoluble fraction of devulcanisate A.
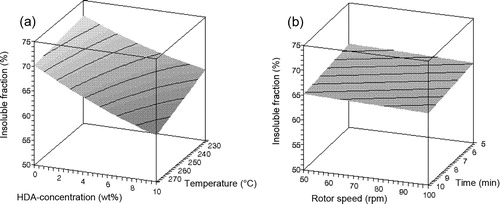
Figure 3. Influence of (a) HDA concentration and temperature and (b) rotor speed and devulcanisation time on the overall crosslink density of devulcanisate A.
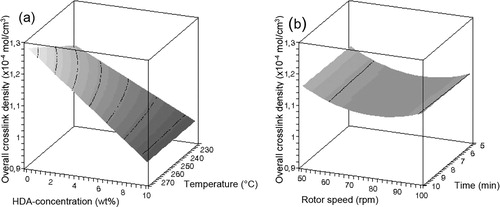
Figure 4. Influence of (a) HDA concentration and temperature and (b) rotor speed and devulcanisation time on the monosulfidic crosslink density of devulcanisate A.
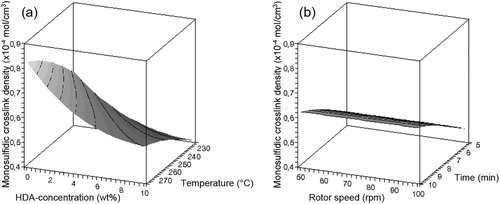
gives an overview of the influence of the process parameters on the properties of the devulcanisate.
Table 6. Overview of the influence of different parameters on devulcanisate properties for conventionally sulphur and EV cured EPDM rubber.
– show that there is only a minor influence of the factors rotor speed and devulcanisation time on the responses within the experimental window, compared to the influences of the factors devulcanisation temperature and HDA concentration. The influence of the amount of HDA on the Mooney viscosity is quite strong up to a concentration of approximately 5 wt-%, to level off at higher concentrations for all devulcanisation temperatures. Without the addition of HDA, the Mooney viscosity values of devulcanisate A are above 200 MU, outside the measuring range of the Mooney viscometer. The Mooney viscosity decreases significantly with increasing devulcanisation temperature. The insoluble fraction decreases with increasing concentration of HDA and higher devulcanisation temperature. At high concentrations of HDA, the influence of temperature is most pronounced. With increasing HDA concentration, a linear decrease in overall crosslink density is obtained for devulcanisate A. For devulcanisation with and without the addition of HDA, the final crosslink density is lower when devulcanisation is done at a lower temperature, except for 10 wt-% HDA. Without the addition of HDA, just by a thermo-mechanical treatment, the crosslink density already decreases from the original value of 2.39 × 10−4 to approximately 1.16–1.28 × 10−4 mol cm−3, depending on the temperature applied: the main decrease in crosslink density for devulcanisate A is therefore caused by the mere thermal process; addition of HDA has only little additional effect. HDA is able to break monosulfidic crosslinks at all temperatures, although the influence of HDA is not strong enough to compensate for the extra formed monosulfidic bonds by mere thermal devulcanisation.
shows the general trends found with variation of the process parameters for both rubber types.
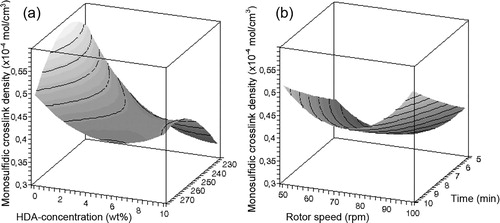
The devulcanisation behaviour of compound B is illustrated in –. Contrary to the results obtained for devulcanisate A, devulcanisate B shows a linear decrease in Mooney viscosity with increasing HDA concentration. The influence of temperature on the Mooney viscosity levels off with increasing temperature. The insoluble fraction decreases with rising temperature and higher HDA concentrations. The devulcanisate of compound B behaves quite differently from the devulcanisate from conventionally sulphur cured rubber; without the use of HDA, the crosslink density of the insoluble fraction remains more or less equal to the crosslink density of the original vulcanised compound B, see , for all devulcanisation temperatures; thermal treatment alone does not lead to a decrease of the crosslink density. When HDA is applied, a decrease in overall crosslink density of the insoluble fraction with increasing concentration of HDA is observed for devulcanisation temperatures of 200°C and 225°C, while the crosslink density at 250°C and 275°C rises with addition of HDA; a moderate devulcanisation temperature and the addition of HDA as devulcanisation aid are therefore required for a significant decrease in crosslink density, otherwise the opposite effect is obtained. After devulcanisation at 275°C, devulcanisate B has a much lower Mooney viscosity compared to devulcanisate A and demonstrates tacky behaviour, even though the crosslink density of the remaining insoluble fraction after extraction of this devulcanisate increases with increasing HDA concentration.
Figure 5. Influence of (a) HDA concentration and temperature and (b) rotor speed and devulcanisation time on the Mooney viscosity of devulcanisate B.
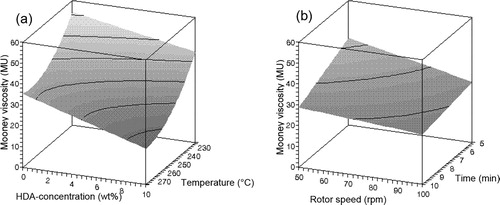
Figure 6. Influence of (a) HDA concentration and temperature and (b) rotor speed and devulcanisation time on the insoluble fraction of devulcanisate B.
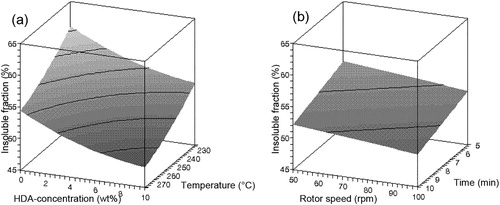
Figure 7. Influence of (a) HDA concentration and temperature and (b) rotor speed and devulcanisation time on the overall crosslink density of devulcanisate B.
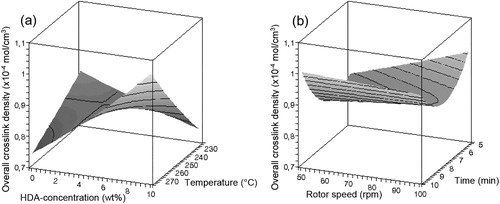
Figure 8. Influence of (a) HDA concentration and temperature and (b) rotor speed and devulcanisation time on the monosulfidic crosslink density of devulcanisate B.
It is well known that monosulfidic crosslinks have a higher dissociation energy than di- or polysulfidic bonds. Consequently, it was anticipated that vulcanisates with a high percentage of poly- and/or disulfidic crosslinks would be more prone to devulcanise than those with mainly monosulfidic bonds. This would favour compound A for easier devulcanisation than compound B. However, the outcome of this study shows a different picture.
A conspicuous difference is observed in the devulcanisation behaviour of compounds A and B in and . For compound B, even by simple thermal treatment without the use of HDA, low Mooney values are obtained; whereas for compound A, thermal treatment alone is insufficient and addition of HDA is required. While the most obvious difference between compounds A and B was the more than double crosslink density of compound A before devulcanisation, a noticeable breakdown of the network and detachment of polymer chains are achieved only by a combined action of heat and devulcanisation agent.
Another difference is that the addition of increasing amounts of HDA enhances the temperature effect for compound A, whereas for material B the temperature sensitivity is not dependent on the HDA concentration. This again is an indication that HDA plays a more important role in the devulcanisation of compound A compared to material B, and that the reaction initiated by HDA is temperature dependent. Furthermore, compound B in general shows higher temperature sensitivity than material A.
For compound A, thermal treatment alone results only in a little decrease of the insoluble fraction, slightly dependent on the temperature applied. Or conversely, only 3–5% soluble devulcanised matter is generated in addition to the original vulcanised compound A, resulting in still unmeasurable high Mooney viscosity values. However, shows that a significant decrease in crosslink density is achieved relative to the initial value. For compound B, the opposite effect is seen: a significant amount of soluble material is generated, simply when the material is thermally treated. Dependent on the temperature applied, 6–18% sol fraction is generated in addition to the original vulcanised compound B, while the crosslink density within the non-soluble matter after devulcanisation is essentially still equal to that of the material before devulcanisation. Dependent on the devulcanisation temperature, the crosslink density de- or increases when HDA is added. Above a temperature of approximately 240°C, HDA reacts as crosslinking promoter rather than as devulcanisation aid.
The thus produced devulcanisates were analysed in a Horikx plot to evaluate the principal devulcanisation mechanism: polymer or crosslink scission. shows the position of the different devulcanisates in the Horikx plot. It is obvious that the EV cured rubber suffers more polymer scission: the sol content is rather high compared to the majority of the conventionally sulphur cured materials. This is due to the breakdown mechanism: when the polymer is broken, soluble fragments are formed in the early stages of devulcanisation. The reason for the predominance of polymer scission is the high content of monosulphidic bonds, which is 52% for the EV cured rubber. The conventionally sulphur cured rubber contains only 15% of these bonds. Besides, the position above both lines shows that there is another – mechanistic – difference: it is not a gradual devulcanisation to the core of the particles, but more a peeling off of the outer – devulcanised – layers with the remaining core still highly vulcanised. These two factors cause a high amount of soluble matter. The decrease in sol content above a certain threshold of devulcanisation indicates that recombination happens: the network is broken, but at the same time the reactive species, mainly radicals, react with each other and form new bonds reducing the sol content.
Figure 9. Fraction of sol generated during reclaiming versus the relative decrease in crosslink density. (—): only main-chain scission; (– –): only crosslink scission; (▴): reclaim A at 225°C; (▪): reclaim A at 250°C; (●): reclaim A at 275°C; (♦): reclaim B at 200°C and (×): reclaim B at 225°C.
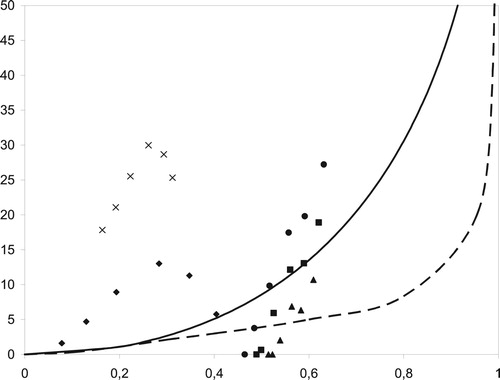
For the conventionally sulphur cured rubber, the situation is different: the degree of devulcanisation is higher, as the network has a higher content of longer sulphur bridges, which are easier to break. A higher devulcanisation temperature shifts the data points to a slightly lower devulcanisation level, for the same reason as mentioned above: recombination of active fragments. This chart shows that for conventionally sulphur cured rubber, under certain devulcanisation conditions crosslink scission is the predominant mechanism. However, it also shows that the devulcanisation mechanism is very sensitive to the process conditions. The samples having no sol content are partly devulcanised, but all polymer chains are still attached to the network and therefore no soluble matter is generated.
Revulcanisation behaviour of EPDM devulcanisates
A study of the rheograms showed that the maximum torque decreased with increasing content of devulcanisate. In the case of the conventionally sulphur cured EPDM, the maximum torque of the virgin compound was 4.0 dNm, while for a blend with 50% devulcanisate the maximum torque decreased to 2.5 dNm. In the case of the EV cured rubber, the starting values of the maximum torque were 9 dNm, and this was reduced to 5.9 dNm.
The mechanical properties of the revulcanised blends containing devulcanisates A and B in quantities up to 50 wt-% respectively of the pure revulcanised devulcanisates are shown in . Tensile strength, elongation at break and hardness decrease with increasing contents of devulcanisate A. This is caused by the addition of the devulcanisate with lower properties due to ageing and the breakdown process, as well as the reduction in crosslinking reactivity for revulcanisation. The compression set at 70°C shows a strong rise, while compression set at 23°C and modulus at 300% strain remain constant and tear strength shows a maximum with increasing amounts of devulcanisate A. The 50/50 blend with devulcanisate A of initial particle size smaller than 3.35 mm shows somewhat better properties compared to the same compound with devulcanisate of an initial particle size smaller than 8 mm, with hardness being independent of initial particle size. The revulcanisates containing devulcanisate B show a maximum in tensile strength and a decrease in tear strength with increasing concentration of devulcanisate. For all other properties, the same trends as for the blends with devulcanisate A are found.
Devulcanisation of SBR compounds
The second part of this study is focused on the devulcanisation of SBR as the most challenging polymer in whole passenger car rubber, with the final aim to develop a process mainly based on the devulcanisation of the sulphur bridges. For this purpose, the ground rubber with a particle size between 0.8 and 2.0 mm was in a first step devulcanised under different conditions: thermally (TT), thermally with quenching in liquid nitrogen (TL) in order to minimise contact with oxygen from the air, and devulcanised in absence of air (TN). shows the experimentally determined sol fractions of devulcanised SBR at various devulcanisation temperatures as a function of the relative decrease in crosslink density. For the thermally treated material, TT in (a), an increase of the devulcanisation temperature to 220°C results in a shift of the data point to the upper right-hand side of the graph, which indicates an increase of sol fraction and decrease of crosslink density. Nevertheless, a further increase of devulcanisation temperature to 260°C results in a back-turn of the experimental data points to the left, which is the reverse of the expected decrease of crosslink density. This reversion phenomenon is even more pronounced for devulcanisation up to 300°C; for this temperature the data point is even found at the left-hand side of the value for untreated SBR. The detrimental effect of the presence of oxygen in the devulcanisation process causes inefficient devulcanisation, in which the eventual crosslink density of the devulcanised rubber is even higher than that of the original untreated vulcanisate.
Figure 11. Sol fraction generated during devulcanisation versus the relative decrease in crosslink density of devulcanised SBR: (a) TT; (b) TL; (c) TN. [Reproduced from Ref. with permission from the Royal Society of Chemistry.]
![Figure 11. Sol fraction generated during devulcanisation versus the relative decrease in crosslink density of devulcanised SBR: (a) TT; (b) TL; (c) TN. [Reproduced from Ref. with permission from the Royal Society of Chemistry.]](/cms/asset/b4e01f1c-e857-4b0b-afae-99af4ce306e5/yprc_a_1464781_f0011_ob.jpg)
An improvement in the efficiency of the devulcanisation is observed when oxygen is excluded; the results of the TL and TN samples are shown in (b and c), respectively. They illustrate that the experimental data for treatment at 180°C are situated at more or less the same position as the data for TT. An increase of the devulcanisation temperature to 220°C exhibits the same trend as found for TT; the percentage of soluble polymer is increased and the crosslink density is decreased. For TL, the values after treatment at 260°C are more or less at the same position as the values for the materials devulcanised at 220°C; however, the reversion phenomenon still occurs when the devulcanisation temperature is further raised above 260°C. For TN, the data points first move to the right-hand side for a treatment temperature of 220°C, but then turn back to the left-hand side for devulcanisation temperatures of 260°C and 300°C.
The detrimental effect of the presence of oxygen in the devulcanisation is smallest with exclusion of oxygen as well as possibly by nitrogen present during devulcanisation and quenching in liquid nitrogen. The reversion phenomenon is less progressive in this case as the crosslink densities observed are still lower than the crosslink densities of devulcanised rubber treated at 180°C and untreated rubber. Thus it must be concluded that working under exclusion of oxygen during and after devulcanisation is a requirement for an efficient devulcanisation process of SBR, and the temperature of 220°C is the optimum for devulcanisation. Above 220°C reversion phenomena appear, whatever conditions are used. Based on the results, it is to be concluded that the devulcanisation as obtained under the present conditions is primarily via the mechanism of main-chain scission. Theoretically, the scission of C–S bonds and S–S bonds which have lower bond strength than C–C bonds should be the priority mechanism in network scission. However, practically, the data showed that main-chain scission mainly occurs. This is due to uncontrolled scission of the rubber network in a thermal process. As there is a majority of C–C bonds compared to C–S and S–S bonds, the probability of cleavage of the polymer backbone is higher.
Revulcanisation of SBR devulcanisates
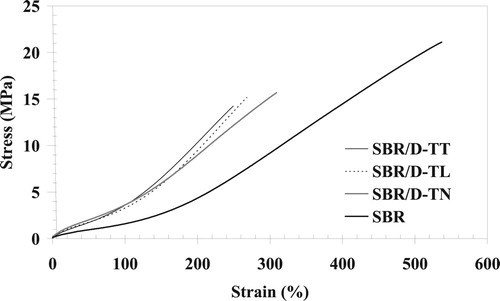
Stress–strain curves of vulcanised compounds of the original SBR and of 50/50 blends of SBR devulcanisates with the original SBR are shown in .
The tensile strength of the blends is lower than the strength of the original vulcanised SBR. Such a decrease in tensile strength with the addition of devulcanised SBR is reported by many researchers [Citation8,Citation24–27]; explanations given for the decrease in strength are as follows:
flaws in the structure of the blend interface between original and reclaimed materials, as co-vulcanisation between the two phases in general is poor;
an abrupt modulus change from the original compound, the continuous phase, to the reclaim particles, the discontinuous phase, resulting in inhomogeneities in stress distribution.
The reason for it is a mismatch in modulus, which can significantly reduce the strength of the material. The stress accumulates on the interface between the devulcanised particles and the matrix and fracture starts from this point. Elongation at break values of the blends is also lower than the values of the original rubber and they follow the same trend as the tensile strength values.
Typically, the mechanical properties of a blend of virgin and devulcanised rubber are affected by many factors such as follows:
Presence of gel in the reclaim
Bonding between reclaim and matrix
Particle size of the reclaim
Sulphur distribution between the matrix and reclaim
Crosslink density and distribution.
The most probable cause is a higher crosslink density in the blends, as the devulcanised rubber is only partially devulcanised and still contains reactive curatives. Under normal industrial compounding operations, the increase in hardness and moduli would have been corrected by adjustment of the compound formulation. The use of less vulcanisation ingredients would most probably have compensated for the hardness increase and brought the tensile properties back, close to those of the virgin SBR vulcanisates.
Conclusion
The results of the devulcanisation experiments on two different rubbers, as presented in this chapter, demonstrate the complexity of this technology. EPDM is commonly used in a more or less pure, unblended form in articles for roof sheeting. For such elastomer devulcanisation is fairly straightforward; the elastomer backbone is relatively stable due to its saturated character. With an amine-type devulcanisation aid like HDA a material is obtained, which can be blended with virgin rubber compounds in relatively high quantities till 50% and still satisfy practical requirements for roof sheeting applications. The devulcanisation of EPDM is indeed practised on industrial scale as of the time of writing this article.
For SBR as used in tyres, the situation is much more complicated. First the SBR is practically never used in pure form in tyres, but in blends with Natural Rubber, Butadiene Rubber and IIR (Butyl) innerliners: each of these elastomers showing their own specific performance as to devulcanisation and together ending in a blend of these different elastomers, which makes reuse in tyres most complicated. Second, the SBR by itself (and BR) is probably the most difficult of all these different elastomers to devulcanise due to its tendency to crosslink under the influence of environmental oxygen, which dictates extremely severe control of the oxygen levels in the devulcanisation process [Citation28].
The results presented in this article have shown an example where devulcanisation as the shortest cradle-to-cradle loop of recycling is a success: EPDM, but also where the technology as of the day if writing this contribution is still in its infancy; SBR, as representative of tyre rubbers. A lot more research and development is needed to bring this to a success.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jalilvand AR, Ghasemi I, Karrabi M, et al. Prog Rubber Plast Recycling Technol. 2008;24:33. doi: 10.1177/147776060802400103
- Myers RD, Nicholson P, MacLeod JB, et al. U.S. Patent 5,602,186 (to Exxon Research and Engineering Company). February 11, 1997.
- Kojima M, Ogawa K, Mizoshima H, et al. Devulcanization of sulfur-cured isoprene rubber in supercritical carbon dioxide. Rubber Chem Technol. 2003;76:957–968. doi: 10.5254/1.3547784
- De D, et al. Raw Mater Appl. 2000;6:346.
- Zanchet A, et al. Use of styrene butadiene rubber industrial waste devulcanized by microwave in rubber composites for automotive application. Mater Des. 2012;39:437–443. doi: 10.1016/j.matdes.2012.03.014
- Dijkhuis KAJ, Babu I, Lopullissa JS, et al. A mechanistic approach to EPDM devulcanization. Rubber Chem Technol 2008;81:190–208. doi: 10.5254/1.3548204
- Rajan VV, Dierkes WK, Joseph R, et al. Effect of diphenyldisulfides with different substituents on the reclamation of NR based latex products. J Appl Polym Sci. 2007;104:3562–3580. doi: 10.1002/app.25925
- Rajan VV, Dierkes WK, Joseph R, et al. Recycling of NR based cured latex material reclaimed with 2,2′-dibenzamidodiphenyldisulphide in a truck tire tread compound. J Appl Polym Sci. 2006;102:4194–4206. doi: 10.1002/app.24563
- Rajan VV, Dierkes WK, Noordermeer JWM, et al. Comparative investigation on the reclamation of NR based latex products with amines and disulfides. Rubber Chem Technol. 2005;78:855–867. doi: 10.5254/1.3547918
- Dierkes W, Rajan V, Noordermeer JWM. Kautsch Gummi Kunstst. 2005;58:312.
- Eriksson L, Johanssen E, Kettaneh-Wold N, et al. Design of experiments. principles and applications. Umea: Umetrics Academy; 2000.
- Flory PJ, Rehner J. Statistical mechanics of cross-linked polymer networks. II. Swelling. J Chem Phys. 1943;11(11):521–526. doi: 10.1063/1.1723792
- Kraus G. Swelling of filler-reinforced vulcanizates. Appl Polym Sci. 1963;7:861–8871. doi: 10.1002/app.1963.070070306
- Porter M. Structural characterization of filled vulcanizates. Part 1. Determination of the concentration of chemical crosslinks in natural rubber vulcanizates containing high abrasion furnace black. Rubber Chem Technol. 1967;40:866–882. doi: 10.5254/1.3539101
- George SC, Kilian KN, Thomas S. Polym Compos. 1999;7:343.
- Dikland HG. Coagents in peroxide vulcanisations of EPDM rubber [PhD thesis]. University of Twente, Enschede, the Netherlands;1992.
- Campbell DS. Structural characterization of vulcanizates. Part X. Thiol-disulfide interchange for cleaving disulfide crosslinks in natural rubber vulcanizates. J Appl Polym Sci. 1969;13(6):1201–1214. doi: 10.1002/app.1969.070130609
- Campbell DSSaville B. Proceedings of the First International Rubber Conference, Brighton, UK; 1967.
- Kiroski D, Sims J, Packham DE, et al. Kautsch Gummi Kunstst. 1997;50:716.
- Saville B, Watson AA. Structural characterization of sulfur-vulcanized rubber networks. Rubber Chem Technol. 1967;40:100–148. doi: 10.5254/1.3539039
- Horikx MM. Chain scissions in a polymer network. J Polym Sci. 1956;19:445–454. doi: 10.1002/pol.1956.120199305
- Verbruggen MAL. Devulcanization of EPDM rubber [Ph.D. thesis]. University Twente, Enschede, the Netherlands;2007.
- Verbruggen MAL, van der Does L, Dierkes WK, et al. Functionalized SBRs in silica-reinforced tire tread compounds: evidence for interactions between silica filler and zinc oxide. Rubber Chem Technol 2016;89:559–572. doi: 10.5254/rct.16.83776
- Fujumoto K, Nishi T, Okamoto T. Int Polym Sci Technol. 1981;8(8):T/30.
- J. K. Kim, P. Saha, S. Thomas, J. T. Haponiuk (eds.), “Rubber Recycling: Challenges and New Developments”, chapter 8, Royal Society of Chemistry, London, Great Britain (2018).
- Burgoyne M, Leaker G, Krekic Z. The effect of reusing ground flash and scrap rubber in parent compound. Rubber Chem Technol. 1976;49:375–378. doi: 10.5254/1.3534972
- Fesus EM, Eggleton RW. Rubber World. 1991;203(6):23.
- Knorr K. Kautsch. Gummi Kunstst. 1994;47:54.