Abstract
Objectives
To identify factors affecting functional hearing performance and quality of life (QoL) outcomes in paediatric cochlear implantation (CI) recipients at two University centres in mainland China.
Methods
Two university centres in mainland China, part of the prospective longitudinal Paediatric Implanted Recipient Observational Study (P-IROS), contributed participant data. Participants were aged under 10 years at time of CI. Functional hearing performance and QoL measures were collected prior to device activation, and at 6-monthly intervals for 2 years post-implantation. Functional hearing endpoints including Categories of Auditory Performance-II (CAP-II) and QoL were evaluated and analysed using ordinal mixed-effects regression models.
Results
Data were from 288 children with a mean age at implant of 2.74 years. Overall follow-up at 1 year was 59% and 51% at 2 years. Younger age at implantation (p<0.001) and hearing aid use preimplantation (p=0.026) were associated with significant benefit. Bilateral device users (both CI and bimodal) achieved significantly better functional hearing performance on the CAP-II than unilateral CI users (p<0.001). Slower functional hearing improvements were observed in those with lower parental expectations compared to higher expectations (p<0.001). QoL improved over time but followed a different initial trajectory between centres.
Conclusion
All participants demonstrated significant improvements in auditory performance and QoL over time. Younger age at CI, and bilateral/bimodal device fitting contributed to earlier improvements. Other potential factors that could help inform families, professionals, and health authorities about choice of hearing device and educational supports required included aetiology of hearing loss and level of maternal education.
Introduction
Hearing implants, including cochlear implants and bone conduction implants, have become the standard of care for children with hearing losses who do not benefit from conventional devices such as hearing aids (Sininger et al., Citation2010). Cochlear implantation (CI) is a well-established, safe, and effective way to provide access to sound in children with permanent moderate to severe-profound sensorineural hearing loss (Ching et al., Citation2017; Yoshinaga-Itano et al., Citation2018). Initially, research involving hearing implants in paediatric populations focused primarily on hearing performance for unilateral CI users and reporting of behavioural outcomes (Geers et al., Citation2002; Sarant et al., Citation2001). Over time the importance of bilateral device usage became evident (Sarant et al., Citation2014) and age of implantation decreased. With broadening criteria for implantation there was a simultaneous increase in the use of self-assessment scales to holistically reflect patient and family related benefits, some of which have become embedded in routine clinical practice (Davis et al., Citation2022; Galvin et al., Citation2014).
As access to CI across many healthcare systems increases globally, it is important to demonstrate to a range of stakeholders that interventions are clinically effective in a real-world environment (Saunders et al., Citation2020) including across countries and within different cohorts. Registries can play an important role in ensuring quality of clinical interventions, adherence to protocols and development of clinical guidelines and standards (Hoque et al., Citation2017). This in turn can have a positive impact on clinical outcomes.
The Paediatric Implanted Recipient Observational Study (P-IROS) registry was a timeline driven patient data registry that collected an agreed core set of data with the aim to provide generalizable outcome with a real-world perspective that could inform future clinical decision making across the globe (Mauch et al., Citation2021; Sanderson et al., Citation2014). In order to monitor development of speech and language skills during early childhood through to school age, the P-IROS aimed to collect self-assessed outcomes via parent and clinician proxy for up to 4 years after implantation using outcomes consistent with other longitudinal studies with children with hearing loss (Ching et al., Citation2017). In China, there has been a recent emergence of literature demonstrating the importance of longitudinal data of outcomes (Li et al., Citation2024; Yang et al., Citation2022).
This has served to enable parents and caregivers to develop realistic expectations when considering cochlear implantation for their children with bilateral moderate to profound sensorineural hearing loss. Whilst providing a significant contribution to the literature, these studies have been performed within China, whereas this current study was part of a broader global register.
This study had three aims (i) to determine which baseline characteristics differed between children from the two centres in mainland China and (ii) to determine whether there were differences in change in outcomes over time between the two centres in mainland China and (iii) to assess and identify key characteristics that may inform the functional hearing and humanistic benefits of CI outcomes among children in mainland China after adjusting for centre differences.
Materials and methods
Study design and setting
The two mainland China centres explored in this study are a subset of the P-IROS registry. P-IROS was a global, prospective, longitudinal registry study for children receiving CI in routine clinical practice with intra-subject control that collected patient-related data from standardized and non-standardized self-report measures accrued by parent or guardian proxy for children implanted before the age of 10 years.
The two sites both agreed to participate in the P-IROS study. A third site in mainland China was unable to recruit sufficient participants and withdrew from the study. There were geographic and demographic differences between the two study centres, with Centre 1 located in a larger city with a more populous and wealthier demographic than Centre 2. At the time of the registry, centres also differed in their funding options with self-funding enabling greater access to hearing technology (CI and / or hearing aids) for participants attending Centre 1.
Baseline data was gathered prior to first activation of the implant with subsequent longitudinal follow-up at consistently timed evaluation intervals. The study was administered through a secure, web-based, registry platform to collect outcome data from clinicians and patient proxy at consistent time intervals. Clinics enrolled patients into the study in order of implantation. Enrolment occurred immediately after implantation and needed to be prior to the first switch-on of the CI sound processor. This ensured the study was observational and mitigated recall bias of the pre-implant state. Evaluations with a 4-week recall were conducted at baseline 0.5, 1.0, 1.5-, and 2.0-years post-implant. A full description of the registry and overall data is published elsewhere (Cesur et al., Citation2023; Muller et al., Citation2023; Sanderson et al., Citation2014).
Participants
Participants were infants and children with significant hearing impairment, aged under 10 years at the time of first implantation. The study was open to any brand of cochlear implant. The study participants were from two centres in China with the first enrolment in May 2014 and the final data point being collected in May 2021. Participation was voluntary, and signed consent to participate in the study was required from the parent or guardian after the implantation procedure but before the device activation was completed. Children with prior experience of implantable hearing devices were excluded.
Evaluation questionnaires
All questionnaires and assessment tools were translated into simplified Chinese and either completed using paper (hard copy) or using a dedicated online portal or both. Completion of the assessment tools and questionnaires was either by parents / caregivers or clinicians or both.
Parent/ caregiver completed evaluations
Patient profile forms were completed at baseline and follow-up visits by the parent or caregiver. Information gathered included the implanted child’s hearing history, degree of residual hearing pre – and post-operatively, demographics and familial characteristics of the child. Additional questionnaires were completed to assess child and family quality of life, and functional listening.
The Children Using Hearing Implants Quality of Life (CuHIQoL) is a non-standardized 25-item questionnaire with a 5-point scale for each item (Looi et al., Citation2016). Raw ratings of 0 (strongly disagree) to 5 (strongly agree) were converted to a 0–100 scale for the analysis. The CuHIQoL was administered to examine three areas of quality of life: expectations for the child (7 questions. Example: I believe my child will easily make friends with other children), impact on the family (8 questions. Example: I always worry about whether the hearing device is working correctly) and quality of life (10 questions. Example: My child can communicate their needs using spoken language.) Negatively worded questions were scored in reverse so that higher scores on the scale of 0–100 indicate more positive outcomes. Evaluation of functional listening was provided via completion of the Speech Spatial Qualities of Hearing Scale, Parents’ Version (SSQ-P). The SSQ-P provides information across three auditory domains of speech recognition, spatial hearing and sound quality and was developed for children aged 4 years or over (Galvin and Noble, Citation2013). Completion of the SSQ-P was optional.
Clinician completed evaluations
The child’s clinician completed information pertaining to the technology (cochlear implant and sound processor) used by the child. Routine hearing threshold measures were performed at the clinic at baseline and follow-up and recorded via the online platform. The standardized questionnaire Categories of Auditory Performance-II (CAP-II) (Archbold et al., Citation1995; Illg et al., Citation2017; Nikolopoulos et al., Citation2005) was employed by the clinician in collaboration with the parent or guardian to evaluate the child’s auditory performance in everyday life. It comprises ten descriptions of auditory performance which are used to place participants into one of ten ordinal categories at the time of assessment. Scores range from 0 (no awareness of environmental sounds or voice) through to 9 (use of telephone with unknown speaker in unpredictable context) so that higher scores imply better performance. Changes in individual performance over time were assessed by repeating the CAP-II assessment at each evaluation time point. Refer to Evaluation Schedule in Supplementary Table 1.
Endpoints
In this post-hoc evaluation the primary endpoint was the change in CAP-II from pre-implantation baseline assessments through two years of six-monthly follow-up assessments.
Secondary endpoints were change in score for each question of the CuHIQoL from pre-implantation baseline assessments through two years of six-monthly follow-up assessments.
Statistical considerations
Data for children aged under 10 years of age with CI devices were extracted from the main study database for all participants from two centres in China. Participant demographics, pre-implantation clinical characteristics (including CAP-II, SSQ-P and QoL) and CI information were summarized descriptively for each site. Comparisons in baseline characteristics between centres were assessed using Wilcoxon rank-sum tests, or Fisher’s exact tests, as appropriate. Statistically significant baseline differences between centres would suggest the need to adjust for the effect of centre in analysis of change over time.
Linear mixed-effects models were used to estimate the association between characteristics of interest and change in outcome measures, CAP-II or quality of life, over time. The mixed models included a random effect for participants nested within centre to control for the correlation induced by repeated measures on participants within centres over time. Characteristics of interest included age at implant, modality (unilateral, bilateral/bimodal), pre-implant hearing aid use, and maternal and paternal education.
All analyses were conducted using R Statistical Software version 4.2.2 (R Core Team, Citation2021).
Ethics statement
The Cochlear P-IROS was conducted according to the guidelines established in the Declaration of Helsinki (World Medical Association, Citation2013). Ethics approvals were obtained as required for the participating sites. The investigating clinician obtained written informed consent prior to enrolling each patient in the registry. This occurred after each patient was implanted when the decision for type and device configuration had already been made.
Results
Participants
Data from 288 patients from two sites in China were extracted from the study database: 172 patients from Centre 1 and 116 patients from Centre 2. The children recruited to the study were bilaterally profoundly deaf, and 53% of all participants (56% of participants at Centre 1 and 52% at Centre 2, respectively) were male.
Baseline characteristics
All children had baseline unaided hearing thresholds for both the implanted and contralateral ears that were severe-profound. As shown in , the median age of the participants at time of surgery was 2 years (range 0–9 years) with a mean (SD) of 2.46 (2.10) years for Centre 1 and 3.02 (2.46) years for Centre 2 (). A significantly higher percentage of Centre 1 participants had not yet entered school (92% vs 73%, p < 0.001). Aetiologies differed significantly between centres, with a higher proportion of children at Centre 1 having hereditary or large vestibular aqueduct syndrome hearing loss compared to Centre 2 (75% vs 45%) and a smaller proportion having other aetiologies (20% vs 53%, p < 0.001).
Table 1 Baseline characteristics by site
Devices
A significantly higher proportion of children at Centre 1 used hearing aids prior to receiving a CI (73% vs 60%, p = 0.035). Participants mainly received Cochlear devices (90%). There were no bone conduction device users. The most commonly implanted devices were Nucleus™ CI24(RE) ST or Nucleus™ CI24(RE)CA (192 patients, 67%) or CI422 (42 patients, 15%). Only 4.2% of participants received bilateral CI and of the 276 unilaterally implanted participants, 64.1% were bimodal device users (i.e. they used a hearing aid in their non-implanted ear). Modalities differed significantly between centres (p < 0.001, ) with a higher proportion of participants at Centre 1 (68% vs 51%) being bimodal device users.
Parental education
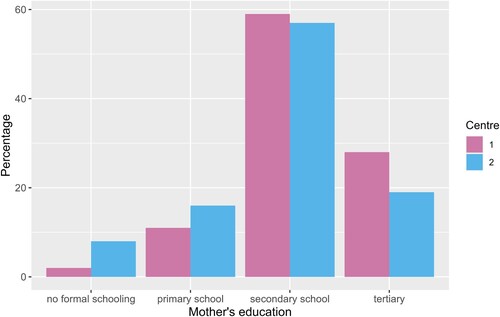
Maternal education, as shown in and , was significantly different between the two centres (p = 0.029), there was a higher proportion of mothers who had tertiary levels of education in centre 1 (27% vs 19%), with a higher proportion of primary school education or less for centre 2 (24% vs 13%). Paternal education was not significantly different between centres (p = 0.19).
Baseline auditory performance
Baseline CAP-II scores were significantly higher (better) at Centre 1 than Centre 2 (p < 0.001; ). Subscales of the CuHIQoL were compared. At baseline, parents of children at Centre 1 had significantly higher expectations for their child (p < 0.001) and were more positive about the impact of their child’s hearing impairment (p < 0.001) compared to parents at Centre 2 ().
Table 2 Baseline performance scores by centre
Follow-up
Study follow-up for this voluntary registry was expected for the first two years after CI, with an optional third – and fourth-year interval. In China, overall follow-up from both centres at 0.5 years was 71%, 59% at 1 year, 54% at 1.5 years and 51% at 2 years. Almost 30% of participants continued to year 3 of the registry. Centre 1 had 37/172 (22%) patients attend the year 2 follow-up visit, while Centre 2 had 111/116 (96%) patients attend. Because of attrition beyond 2 years, we analysed follow-up data to 2 years post-implant.
Change in auditory performance
CAP-II
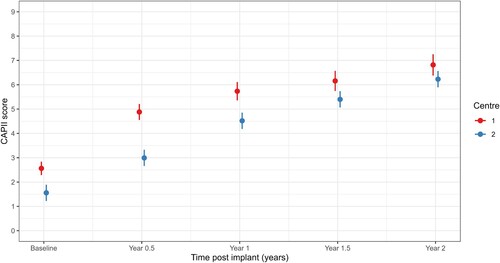
A linear mixed-effects model with centre as a fixed effect indicated differences in auditory performance over time between the two centres (interaction effect p < 0.001, , ); estimated marginal means indicate that CAP-II scores are higher at Centre 1 but Centre 2 approaches Centre 1 mean scores by 2 years. All scores significantly improved over time (visit p < 0.001, , ).
Table 3 Estimated marginal means with standard error (SE) and p-values for the overall significance of the main effects and interactions from linear mixed-effects models for each characteristic of interest; each row shows the result of a separate model
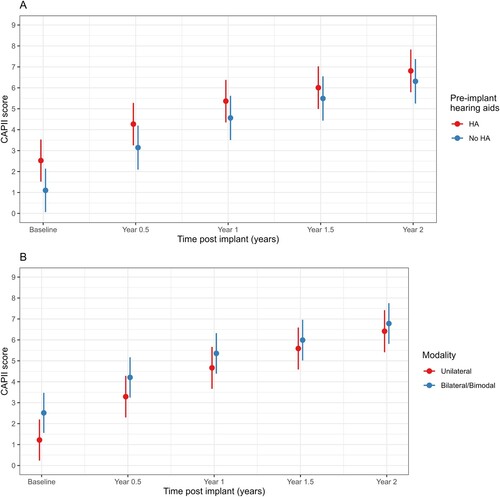
There was a significant interaction between age at implant and visit (p < 0.001, ). Results indicated that younger children had lower mean baseline CAP-II scores but increased by more than children who were older at implant. Pre-implantation hearing aid use was associated with higher CAP-II scores (p < 0.001, , A) but mean CAP-II scores for those who did not use hearing aids pre-implant approached the scores of hearing aid users by two years (interaction effect p < 0.001). There were few bilateral device users and so bilateral and bimodal device users were combined for analysis. There was a significant interaction between modality and time (p < 0.001, , B); bilateral/bimodal device use was associated with higher (better) auditory performance on the CAP-II at all time points with mean CAP-II scores for unilateral device users approaching that of bilateral/bimodal device users by 2 years (). There was no evidence of an association between mother’s education and CAP-II scores (p = 0.70) or change in CAP-II scores over time (p = 0.46). Aetiology was not analysed in the mixed-model analyses as the counts in some categories was too small.
SSQ-P
The SSQ-P questionnaire was poorly completed, with 75% of participants not completing these questions. Therefore, this questionnaire was not analysed further.
Quality of life (CuHIQoL)
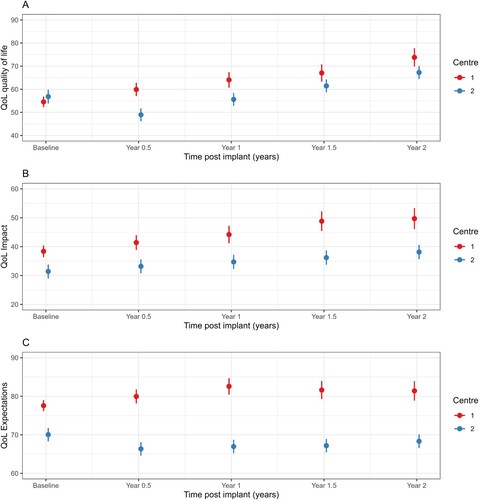
Overall QoL scores improved over time, however the trajectory of this improvement differed between sites (A; interaction p-value < 0.001, ) with mean scores at Centre 1 increasing at each follow-up time point, while mean scores initially decreased at Centre 2 but then increased at each time point thereafter. There was no evidence of a difference in trajectories over time for mean QoL impact scores (, interaction p-value = 0.06). Mean QoL impact scores increased similarly over time for both centres (B, main effect of visit p-value < 0.001) but Centre 1 had significantly higher scores than Centre 2 (main effect of centre p-value < 0.001). Mean QoL expectation scores differed between centres over time (C, interaction p-value < 0.001, ) whereby they initially increased for Centre 1 up to 1-year follow up and then remained steady but decreased for Centre 2 and then remained relatively stable.
Figure 4 CuHIQoL category results over time, by centre: A - Quality of Life, B - Impact on family, C - Expectations for child.
Age at implant was associated with increasing overall mean QoL and impact (, main effect of time p-value < 0.001, Supplementary Figure 1A, B) scores over time, but not parental expectation scores (, Supplementary Figure 1C) which showed no evidence of a change over time (main effect of time p-value = 0.25) or difference between age groups (main effect of age p-value = 0.85). Mean overall QoL scores were initially higher for children who were older at implant, but the scores were similar by 2 years follow-up regardless of age at implant (, interaction p-value < 0.001, Supplementary Figure 1A).
All QoL scores had significant interactions between time and pre-implant hearing aid use indicating that the hearing aid and non-hearing aid groups were different at two or more time points (, interaction p-value ≤ 0.033, Supplementary Figure 2). For overall QoL mean scores were similar at baseline between those who did and did not use hearing aids and both groups had higher scores by 2 years (main effect for visit p-value <0.001) however those without hearing aids had lower scores at each post-baseline time point (p = 0.004). For mean QoL impact scores, the mean scores for those with pre-implant hearing aids had slightly lower baseline scores and then slightly higher post baseline scores compared to those who did not use pre-implant hearing aids, though the groups were not significantly different (p = 0.56). Mean QoL parental expectations scores were similar but did not change substantially over time (p = 0.24). Similar results were found for device modality (, Supplementary Figure 3).
Change in mean QoL scores was not associated with mother’s education level (interaction p-value ≥0.260, ).
Discussion
This post-hoc subgroup analysis reports the outcomes of children from two distinct centres in China enrolled in the prospective P-IROS study and has highlighted characteristics that could impact the guidance and standards of care for paediatric cochlear implantation. Whilst the median age at implant at both centres was 2 years, there was a difference in the range and average age of implantation, with children from centre 1 receiving CI at a younger age. Centre 2 had higher retention of participants than Centre 1 at all points of the data collection. Children were primarily unilaterally implanted with CI24(RE) ST, CI24(RE) CA or CI422 devices. Approximately two thirds used bimodal hearing devices.
Overall, significant benefits were observed following implantation, both in terms of auditory performance which increased from either only awareness of environmental sounds and response to speech sounds, to the ability to use a telephone with known speaker or being able to follow group conversation in a reverberant room. The auditory performance scores achieved after two years of follow up (CAP-II level 6–7 on a scale of 0–9), were higher than the mean CAP-II score of 3.9 (scale of 0–8) at two years for unilateral CI in the French infant registry (Loundon et al., Citation2020) and the CAP-II level 5 reported in a long term outcomes study of CI users who were implanted in childhood (Illg et al., Citation2017). CAP-II scores were generally in line with those reported by others following CI in young children (Cesur et al., Citation2023; Guo et al., Citation2020; Kwak et al., Citation2020; Lyu et al., Citation2019).
The improvements in hearing were accompanied by an overall improvement in QoL and expectations for the child. Such improvements in QoL following paediatric CI have been observed elsewhere (Kumar et al., Citation2015; Warner-Czyz et al., Citation2022). Specifically, positive improvements in communication and social skills, as was shown in the current study, were associated with less positive improvement in the continued parental support required.
The CuHIQoL was only validated for a Mandarin speaking population in Singapore (Looi et al., Citation2016) and found to have consistency in self-report of children with normal hearing and their parents, whilst there was a difference between children with hearing loss and their parents. The CuHIQL had not been validated in the China mainland, however was used across the broader study population (Muller et al., Citation2023).
Warner-Czyz et al., (Citation2022) identified global differences when comparing QoL results across countries. They speculate cultural approaches may have an impact and identify China which has a more collectivist society as having fewer positive ratings compared to parents from more individualistic societies. In the current study, the overall QoL was not significantly different between sites, however scores for ‘impact’ and ‘expectation’ were numerically less positive for Centre 2. This may reflect the difference in the geographical and demographic areas and funding sources. Centre 1 was predominantly self-funded and had a more urban lifestyle where people may have leaned toward an individualistic approach. whilst Centre 2 had services provided by their local government area which could lead to a more collective approach.
A steeper initial trajectory for hearing improvements was observed for participants from Centre 1. These children were younger when implanted than those from centre 2, most had not yet commenced school, had worn a hearing aid prior to CI, used auditory-oral communication, had a higher proportion of residual hearing and progressive losses due to aetiology of Large Vestibular Aqueduct Syndrome (LVAS), and were more likely to have bimodal devices or bilateral hearing aids. There was a significantly higher proportion of mothers with tertiary education from Centre 1 compared to Centre 2 where there was a greater proportion of mothers with no formal schooling. Higher maternal education has been cited as one of the many factors that can enhance outcomes, in particular language development, for children following CI (Ching and Dillon, Citation2013). We did not find an association between maternal education and change in outcomes in this study. Significant benefits have also been reported for bimodal device users, supporting the current clinical recommendations to encourage the use of a contralateral hearing aid, even though there was little residual hearing in the sample (Cheng et al., Citation2018). Our study supports the use of CI in children of a younger age, in line with other studies and systematic reviews (Ching et al., Citation2017; Kwak et al., Citation2020; Wu et al., Citation2023; Yang et al., Citation2022). The higher proportion of hearing aid usage prior to CI, and retention of hearing aid use by some participants may have potentially been linked to aetiological factors. Twenty percent of the Centre 1 population had LVAS which is associated with lesser degrees of hearing loss and deterioration of hearing over time, which lends itself to benefiting from the acoustic signal of a hearing aid in addition to a cochlear implant (Hall et al., Citation2019). Regardless, unilateral CI has been found a cost-effective hearing solution for children with severe to profound sensorineural hearing loss (SNHL) in rural China (Qiu et al., Citation2017).
Sociodemographic disparities in access to CI in 14 countries have recently been reviewed, with some measures such as age at CI and rates of CI consistently reported, while other measures, such as access to rehabilitation services, willingness to undergo CI, and daily CI use are rarely measured (Omar et al., Citation2022). There remains an unmet need for hearing rehabilitation in China (van Hasselt et al., Citation2019), particularly for those living in a rural community (Li et al., Citation2016).
Consistent with the literature, this study highlights the importance of early CI, and bilateral device fitting (Leigh et al., Citation2013). Due to the lower age of implantation, a higher proportion (92%) of the Centre 1 participants had not entered school compared to Centre 2 (73%), and a larger proportion of children from Centre 2 attended either a school for the deaf or mainstream school, compared to Centre 1 which was more than likely due to the child’s age. Overall, there was a significant difference in educational setting attendance between the two groups (p < 0.001).
The schooling and education of children who have undergone CI has been the subject of monitoring in many settings. Choo et al. (Citation2021) found that early CI and the absence of other disabilities were the two main factors that increased the likelihood of full-time enrolment in mainstream classes at regular schools. However difficulties in peer relationships sometimes remained (Choo et al., Citation2021). In contrast, Li et al. (Citation2023) in their study of 216 Mandarin-speaking children, found no differences in the trajectories of receptive and expressive vocabulary whether they received their first CI at 1, 2, or 3 years of age (Li et al., Citation2023).
Strengths and limitations
Strengths of this study included the registry-based approach, which allows assessment of the impact of a treatment through inclusion of chronologically treated individuals in an unbiased manner, (Muller et al., Citation2023; Sanderson et al., Citation2014) with repeated assessment intervals. The registry provided an easy-to-access, secure web platform with relevant language options and automated reports (Ahern et al., Citation2022; Sanderson et al., Citation2014).
This study had some limitations. As is the nature of a voluntary registry, compliance can be low. In the case of this study, only two centres in China had sufficient data to enable analysis. While the statistical methods used appropriately accounted for dropout, the attrition increased the uncertainty in estimated outcomes and there is the possibility that those who did not attend the follow-up visits had poor outcomes and this could potentially impact findings.
Different clinical approaches could also explain the superior retention of participants for Centre 2. The model of care for Centre 1 incurred costs on families for each of their visits, whereas Centre 2 had all services sponsored by the local government provider. Further, Centre 2 allocated a specific study coordinator to remind participants of study requirements.
QoL measures were completed by proxy by parents and caregivers. Most studies suggest disparity between ratings made by children with a CI who rate their experience more positively than their parents (Huber, Citation2005; Warner-Czyz et al., Citation2022). The current protocol did not include self-report measures for children, primarily due to relevant tools being unavailable in different languages at the time the register was developed (Muller et al., Citation2023).
Conclusions
This study found evidence of between site cohort differences between two centres in China. Factors, including age at implant, contributed to a steeper trajectory for improved audition and QoL outcomes in children following CI. While our study suggests that improved QoL and auditory benefits can still be obtained with later implant, the benefits will take longer to obtain. Increasing access to enable a younger age of cochlear implantation, bilateral device fitting, and parental awareness of factors that can impact their child’s cochlear implant journey, could lead to optimizing both expectations and the impact of the CI in the child and on their family’s quality of life.
Author contribution
CY, JL, MZ, RW, XC, JQ, LX contributed to the work equally and were study investigators. CP and PLG wrote the initial draft of the manuscript and analysed data. All authors had substantial contribution to conception and design, acquisition of data, or analysis and interpretation of data, and reviewed the draft article critically for important intellectual content and approved the final version of the article. All authors agree to be accountable for all aspects of the work.
Supplementary information V3.docx
Download MS Word (966 KB)Acknowledgments
The authors thank all the patients, parents, carers and clinic staff who participated across the sites. The authors thank Sally Costello of WriteSource Medical Pty Ltd, Sydney, Australia, for providing medical writing support funded by Cochlear Ltd in accordance with Good Publication Practice (GPP2022) guidelines (https://www.ismpp.org/gpp-2022)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, LX, upon reasonable request.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14670100.2024.2382579.
Additional information
Funding
Notes on contributors
Chongxian Yu
Chongxian Yu is a Medical Master, Doctor of the Department of Otolaryngology Head and Neck Surgery, the First Affiliated Hospital of Anhui Medical University. Regular Member of the International Association of Physicians in Audiology.
Jianfen Luo
Jianfen Luo is the director and Deputy chief physician of Auditory Implant Department, Shandong Otolaryngology Hospital, Associate professor and master tutor of Shandong University, and co-trained doctor of University of Pennsylvania. Member of the Professional Committee for the Prevention and Treatment of otolaryngology diseases of China Maternal and Child Health Association, member of the Hearing and Balance Medicine Branch of Shandong Province, senior consultant for the prevention and control of birth defects.
Mei Zhong
Mei Zhong is a graduated from Wannan Medical College Clinical medical school and completed a Masters at Anhui Medical University Otolaryngology. Currently is completing a PhD student: School of Education, Anhui Medical University working in the First Affiliated Hospital of Anhui Medical University Professional Title as an Attending physician. Has a strong research focus on diagnostics in otolaryngology and audiology with 3 published articles on postoperative effects of cochlear implants.
Ruijie Wang
Ruijie Wang has a PhD of Otorhinolaryngology, and the Deputy director of Auditory Electrophysiology Laboratory Department. Mainly work on pediatric behavioral audiometry and speech assessment, cochlear implant mapping, bimodal mapping, bone bridge mapping and the auditory nerve function evaluation. Acquire the senior scholar of Salus University Cochlear Implant Program and senior doctor for prevention and control consultant of hearing birth defects. Participated in two National Natural Science Foundation Projects and two Natural Foundation Projects of Shandong Province, and have published four SCI papers as the first author, with a total of more than 10 SCI papers.
Xiuhua Chao
Xiuhua Chao has a medical doctor and masters degree. She is the Deputy Director of the Laboratory of Auditory Electrophysiology. She is mainly engaged in the evaluation of auditory nerve function after cochlear implantation and is skilled in cochlear implant programming for patients with various severe cochlear deformities.
Jianxin Qiu
Jianxin Qiu is a Doctor, Professor, Chief physician, chief director of Department of otolaryngology ward two. Specializing in clinical work of Otolaryngology for 30 years. She has excelled in her cochlear implant experience at the Swedish Royal Karolinska Institute, engaged in the study of inner ear diseases and clinical research of Electronic cochlear and the Otology Center of University of Würzburg in Germany. She has performed more than 3000 cochlear implant operations 3000 cases in Anhui province. She is frequently invited to international meetings on cochlear implant and has published more than 70 articles. She has served on the committee of WHO Fangrong Cooperation Center, vice chairman of Anhui Province Academy Committee of Otorhinolaryngology, vice chairman of Anhui Province Allergy Committee.
Lei Xu
Lei Xu is a Medical Doctor, Chief Physician, Professor, Master Supervisor, Young Expert of Taishan Scholars in Shandong Province, and Vice-president of Shandong Second Provincial General Hospital (Shandong Provincial ENT Hospital), Deputy Director of Otology Center, Chief Expert in Auditory Implantation Department, Visiting Scholar in University of Pennsylvania. He is a standing Committee Member and Secretary General of the Otolaryngology Head and Neck Surgery Branch of China International Exchange and Promotive Association for Medical and Health care, Secretary General of China Otorhinolaryngology Collaborative Innovation Platform , Appointed Chairman of the Hearing and Balance Medicine Branch of Shandong Medical Association, Committee Member of the Otolaryngology Head and Neck Surgery Branch of Shandong Medical Association, Director of the Office of the Technical Guidance Group for Preventing and Treating Deafness in Shandong Province.
Petra L. Graham
Petra L. Graham is Associate Professor and biostatistician in the School of Mathematical and Physical Sciences at Macquarie University. Petra enjoys cross-disciplinary research, applying often complex statistical techniques to problems in health. Her research work aims to inform policy and change clinical practice.
Colleen Psarros
Colleen Psarros has a PhD in Audiology and has recently completed her doctoral thesis examining the Clinical Models of Service Delivery for Cochlear Implants and the Role of Telepractice. Her clinical expertise in the field of CI has spanned over 35 years within clinical and research. She is an Honorary Senior Research Fellow at Macquarie University and has coauthored numerous publications on clinical practice and electrophysiology.
References
- Ahern, S., Gabbe, B.J., Green, S., Hodgson, C.L., Wood, E.M., Zalcberg Oam, J.R., Zazryn, T. 2022. Realising the potential: Leveraging clinical quality registries for real world clinical research. Medical Journal of Australia, 216(6): 273–277. doi:10.5694/mja2.51443.
- Archbold, S., Lutman, M.E., Marshall, D.H. 1995. Categories of auditory performance. The Annals of Otology, Rhinology & Laryngology. Supplement, 166: 312–314.
- Cesur, S., Ciprut, A., Terlemez, S. 2023. Observational study of pediatric cochlear implant recipients: Two-year follow-up outcomes. Medeniyet Medical Journal, 38(1): 78–87. doi:10.4274/MMJ.galenos.2023.35305.
- Cheng, X., Liu, Y., Wang, B., Yuan, Y., Galvin, J.J., Fu, Q.-J., et al. 2018. The benefits of residual hair cell function for speech and music perception in pediatric bimodal cochlear implant listeners. Neural Plasticity, 4610592. doi:10.1155/2018/4610592.
- Ching, T., Dillon, H. 2013. Major findings of the LOCHI study on children at 3 years of age and implications for audiological management. International Journal of Audiology, 52(sup2): S65–S68. doi:10.3109/14992027.2013.866339.
- Ching, T.Y., Dillon, H., Button, L., Seeto, M., Van Buynder, P., Marnane, V., et al. 2017. Age at intervention for permanent hearing loss and 5-year language outcomes. Pediatrics, 140(3), doi:10.1542/peds.2016-4274.
- Choo, O.S., Kim, H., Kim, Y.J., Roh, J., Jang, J.H., Park, H.Y., Choung, Y.H. 2021. Effect of age at cochlear implantation in educational placement and peer relationships. Ear & Hearing, 42(4): 1054–1061. doi:10.1097/aud.0000000000001000
- Davis, A., Harrison, E., Cowan, R. 2022. The feasibility of the functional listening index–paediatric (FLI-P®) for young children with hearing loss. Journal of Clinical Medicine, 11(10): 2764. https://www.mdpi.com/2077-0383/11/10/2764.
- Galvin, K.L., Holland, J.F., Hughes, K.C. 2014. Longer-term functional outcomes and everyday listening performance for young children through to young adults using bilateral implants. Ear & Hearing, 35(2): 171–182. doi:10.1097/01.aud.0000436923.96492.3a.
- Galvin, K.L., Noble, W. 2013. Adaptation of the speech, spatial, and qualities of hearing scale for use with children, parents, and teachers. Cochlear Implants International, 14(3): 135–141. doi:10.1179/1754762812Y.0000000014.
- Geers, A., Uchanski, R., Brenner, C., Tye-Murray, N., Nicholas, J., Tobey, E. 2002. Rehabilitation factors contributing to implant benefit in children. Annals of Otology, Rhinology & Laryngology, 111(5_suppl): 127–130. doi:10.1177/00034894021110S525.
- Guo, Q., Lyu, J., Kong, Y., Xu, T., Dong, R., Qi, B., et al. 2020. The development of auditory performance and speech perception in CI children after long-period follow up. American Journal of Otolaryngology, 41(4): 102466. doi:10.1016/j.amjoto.2020.102466.
- Hall, A.C., Kenway, B., Sanli, H., Birman, C.S. 2019. Cochlear implant outcomes in large vestibular aqueduct syndrome–should We provide cochlear implants earlier? Otology & Neurotology, 40(8): e769–e773. doi:10.1097/MAO.0000000000002314.
- Hoque, D.M.E., Kumari, V., Hoque, M., Ruseckaite, R., Romero, L., Evans, S.M. 2017. Impact of clinical registries on quality of patient care and clinical outcomes: A systematic review. PLoS One, 12(9): e0183667. doi:10.1371/journal.pone.0183667.
- Huber, M. 2005. Health-related quality of life of Austrian children and adolescents with cochlear implants. International Journal of Pediatric Otorhinolaryngology, 69(8): 1089–1101. doi:10.1016/j.ijporl.2005.02.018.
- Illg, A., Haack, M., Lesinski-Schiedat, A., Büchner, A., Lenarz, T. 2017. Long-term outcomes, education, and occupational level in cochlear implant recipients who were implanted in childhood. Ear & Hearing, 38(5): 577–587. doi:10.1097/AUD.0000000000000423.
- Kumar, R., Warner-Czyz, A., Silver, C.H., Loy, B., Tobey, E. 2015. American parent perspectives on quality of life in pediatric cochlear implant recipients. Ear & Hearing, 36(2): 269–278. doi:10.1097/AUD.0000000000000108.
- Kwak, M.Y., Lee, J.Y., Kim, Y., Seo, J.W., Lee, J.Y., Kang, W.S., et al. 2020. Long-term change in the speech perception ability in pediatric cochlear implants and the effect of the age at implantation. Otology & Neurotology, 41(6): 758–766. doi:10.1097/MAO.0000000000002640.
- Leigh, J., Dettman, S., Dowell, R., Briggs, R. 2013. Communication development in children who receive a cochlear implant by 12 months of age. Otology & Neurotology, 34(3): 443–450. doi:10.1097/MAO.0b013e3182814d2c.
- Li, G., Zhao, F., Tao, Y., Zhang, L., Zheng, Y. 2023. Trajectories of receptive and expressive vocabulary in mandarin speaking children under 4 years of age fitted with cochlear implants: A 12-month longitudinal study. International Journal of Audiology, 62(7): 626–634. doi:10.1080/14992027.2022.2071769.
- Li, J., Shi, L., Du, H., Chen, W., Wang, Q., Kang, S., Yang, S. 2024. A 10-year in-depth follow-up of post-lingual hearing loss patients with Chinese domestic cochlear implants. Acta Oto-Laryngologica, 181–186. doi:10.1080/00016489.2024.2355216.
- Li, W., Dai, C., Li, H., Chen, B., Jiang, Y. 2016. Factors impacting early cochlear implantation in Chinese children. European Archives of Oto-Rhino-Laryngology, 273(1): 87–92. doi:10.1007/s00405-015-3492-1.
- Looi, V., Lee, Z.Z., Loo, J.H.Y. 2016. Quality of life outcomes for children with hearing impairment in Singapore. International Journal of Pediatric Otorhinolaryngology, 80: 88–100. doi:10.1016/j.ijporl.2015.11.011.
- Loundon, N., Simon, F., Aubry, K., Bordure, P., Bozorg-Grayeli, A., Deguine, O., et al. 2020. The French cochlear implant registry (EPIIC): Perception and language results in infants with cochlear implantation under the age of 24 months. European Annals of Otorhinolaryngology, Head and Neck Diseases, 137: S11–S18. doi:10.1016/j.anorl.2020.07.010.
- Lyu, J., Kong, Y., Xu, T.Q., Dong, R.J., Qi, B.E., Wang, S., et al. 2019. Long-term follow-up of auditory performance and speech perception and effects of age on cochlear implantation in children with pre-lingual deafness. Chinese Medical Journal, 132(16): 1925–1934. doi:10.1097/CM9.0000000000000370.
- Mauch, H., Kaur, J., Irwin, C., Wyss, J. 2021. Design, implementation, and management of an international medical device registry. Trials, 22(1): 845. doi:10.1186/s13063-021-05821-5.
- Muller, L., Goh, B.S., Cordovés, A.P., Sargsyan, G., Sikka, K., Singh, S., et al. 2023. Longitudinal outcomes for educational placement and quality of life in a prospectively recruited multinational cohort of children with cochlear implants. International Journal of Pediatric Otorhinolaryngology, 170: 111583. doi:10.1016/j.ijporl.2023.111583.
- Nikolopoulos, T.P., Archbold, S.M., Gregory, S. 2005. Young deaf children with hearing aids or cochlear implants: Early assessment package for monitoring progress. International Journal of Pediatric Otorhinolaryngology, 69(2): 175–186. doi:10.1016/j.ijporl.2004.08.016.
- Omar, M., Qatanani, A., Kaleem, S.Z., McKinnon, B.J. 2022. Sociodemographic disparities in pediatric cochlear implantation access and use: A systematic review. The Laryngoscope, 132(3): 670–686. doi:10.1002/lary.29716.
- Qiu, J., Yu, C., Ariyaratne, T.V., Foteff, C., Ke, Z., Sun, Y., et al. 2017. Cost-effectiveness of pediatric cochlear implantation in rural China. Otology & Neurotology, 38(6): e75–e84. doi:10.1097/MAO.0000000000001389.
- R Core Team. 2021. A language and environment for statistical computing. In R Foundation for Statistical Computing. https://www.R-project.org/.
- Sanderson, G., Ariyaratne, T.V., Wyss, J., Looi, V. 2014. A global patient outcomes registry: Cochlear paediatric implanted recipient observational study (cochlear™ P-IROS). BMC Ear, Nose and Throat Disorders, 14(1): 10. doi:10.1186/1472-6815-14-10.
- Sarant, J.Z., Blamey, P.J., Dowell, R.C., Clark, G.M., Gibson, W.P. 2001. Variation in speech perception scores among children with cochlear implants. Ear and Hearing, 22(1): 18–28. doi:10.1097/00003446-200102000-00003.
- Sarant, J., Harris, D., Bennet, L., Bant, S. 2014. Bilateral versus unilateral cochlear implants in children: A study of spoken language outcomes. Ear & Hearing, 35(4): 396–409. doi:10.1097/AUD.0000000000000022.
- Saunders, G.H., Christensen, J.H., Gutenberg, J., Pontoppidan, N.H., Smith, A., Spanoudakis, G., Bamiou, D.-E. 2020. Application of Big data to support evidence-based public health policy decision-making for hearing. Ear & Hearing, 41(5): 1057–1063. doi:10.1097/AUD.0000000000000850.
- Sininger, Y.S., Grimes, A., Christensen, E. 2010. Auditory development in early amplified children: Factors influencing auditory-based communication outcomes in children with hearing loss. Ear & Hearing, 31(2): 166–185. doi:10.1097/AUD.0b013e3181c8e7b6.
- van Hasselt, A., Sung, J.K.K., Tong, M.C.F. 2019. Overcoming developing world challenges of cochlear implantation: Chinese perspective. Current Opinion in Otolaryngology & Head & Neck Surgery, 27(3): 193–197. doi:10.1097/MOO.0000000000000529.
- Warner-Czyz, A.D., Nelson, J.A., Kumar, R., Crow, S. 2022. Parent-reported quality of life in children with cochlear implants differs across countries. Frontiers in Psychology. doi:10.3389/fpsyg.2022.966401.
- World Medical Association. 2013. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20): 2191–2194. doi:10.1001/jama.2013.281053.
- Wu, S.S., Sbeih, F., Anne, S., Cohen, M.S., Schwartz, S., Liu, Y.C., Appachi, S. 2023. Auditory outcomes in children who undergo cochlear implantation before 12 months of age: A systematic review. Otolaryngology – Head and Neck Surgery. doi:10.1002/ohn.284.
- Yang, Y., Gao, J., Du, H., Geng, L., Li, A., Zhao, N., et al. 2022. Influence of cochlear implants on hearing-related quality of life: Results from Chinese children with cochlear implants entering mainstream education. International Journal of Pediatric Otorhinolaryngology, 160: 111228. doi:10.1016/j.ijporl.2022.111228.
- Yoshinaga-Itano, C., Sedey, A.L., Wiggin, M., Mason, C.A. 2018. Language outcomes improved through early hearing detection and earlier cochlear implantation. Otology & Neurotology, 39(10): 1256–1263. doi:10.1097/MAO.0000000000001976.