Abstract
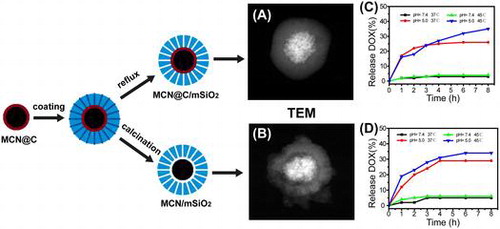
We report the preparation of magnetic mesoporous silica (MMS) nanoparticles with the potential multifunctionality of drug delivery and magnetic hyperthermia. Carbon-encapsulated magnetic colloidal nanoparticles (MCN@C) were used to coat mesoporous silica shells for the formation of the core-shell structured MMS nanoparticles (MCN@C/mSiO2), and the rattle-type structured MMS nanoparticles (MCN/mSiO2) were obtained after the removal of the carbon layers from MCN@C/mSiO2 nanoparticles. The morphology, structure, magnetic hyperthermia ability, drug release behavior, in vitro cytotoxicity and cellular uptake of MMS nanoparticles were investigated. The results revealed that the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles had spherical morphology and average particle sizes of 390 and 320 nm, respectively. The MCN@C/mSiO2 nanoparticles exhibited higher magnetic hyperthermia ability compared to the MCN/mSiO2 nanoparticles, but the MCN/mSiO2 nanoparticles had higher drug loading capacity. Both MCN@C/mSiO2 and MCN/mSiO2 nanoparticles had similar drug release behavior with pH-controlled release and temperature-accelerated release. Furthermore, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles showed low cytotoxicity and could be internalized into HeLa cells. Therefore, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles would be promising for the combination of drug delivery and magnetic hyperthermia treatment in cancer therapy.
1. Introduction
Chemotherapy is often used for cancer therapy, but the side-effect of toxic free anticancer drugs is very serious to the body.[Citation1] Therefore, the use of carriers for drug delivery is of great important and promising in cancer therapy.
To date, a variety of carriers including organic and inorganic materials have been studied for anticancer drug delivery.[Citation2] Recently, mesoporous silica nanoparticles (MSNs) have been intensively studied as nanocarriers for drug delivery owing to their high surface area, tunable particle size and mesopore size, good biocompatibility and facial surface functionalization.[Citation3–5] Compared to organic carriers such as liposomes, micelles and biodegradable polymers, MSNs can be free from various biochemical attacks. Furthermore, MSNs can load a high amount of drugs and control drug release behavior. For example, Niu et al. [Citation6] developed a kind of core-shell MSN with smaller mesopore in the shell and larger mesopore in the core, which could achieve a three-step release behavior controlled by tuning the shell thickness from 5 nm to 60 nmCitation[6]. Li et al. [Citation7] designed PEGylated mesoporous silica nanorattles as nanocarriers for docetaxel delivery to liver cancer, and the docetaxel-loaded mesoporous silica nanorattles showed increased tumor inhibition ability compared to the clinical formulation of docetaxelCitation[7].
However, cancer recurrence often occurs when only chemotherapy is used to treat the tumors, although the use of carriers can allow targeted drug delivery and controlled drug release. Therefore, much effort has been made to enhance cancer therapeutic efficacy by combining other efficient approaches, such as gene therapy, immunotherapy, radiation therapy, and magnetic hyperthermia, to achieve a synergistic effect.[Citation8–10] Among them, magnetic hyperthermia is considered an effective treatment option for cancer without adverse side effects. This method involves raising the temperature to 43–48°C to deactivate cancer cells,[Citation11,12] where the temperature increase (or heat generation) is caused by magnetic nanoparticles (MNPs) due to the hysteresis loss and/or Néel and Brownian relaxations of MNPs under an alternating magnetic field.[Citation10] Heat can increase the efficacy of different chemotherapeutic drugs in the hyperthermia temperature range.[Citation13] On the other hand, studies demonstrated that MNPs-based hyperthermia treatment may induce anticancer immunity, and can also be used for controlled drug delivery.[Citation14] The synergistic therapeutic effects may be achieved by the combination of chemotherapy with magnetic hyperthermia.[Citation15–17] However, the critical issue is to prepare a multifunctional platform with the simultaneous controlled anticancer drug release and magnetic hyperthermia ability.
To solve this critical issue, magnetic mesoporous silica (MMS) nanoparticles are proposed to be a multifunctional platform for drug delivery and magnetic hyperthermia. Mesoporous silica can deliver drugs owing to the high drug loading in mesoporous channels.[Citation3–5] MNPs can be embedded in mesoporous silica, which is useful for the enhancement of the stability of MNPs and the decrease of potential toxicity effect of MNPs on cells.[Citation18] Therefore, preparation of MMS nanoparticles for potential drug delivery or magnetic hyperthermia has gained more attention.[Citation19–30] Gai et al. [Citation19] prepared fibrous-structured magnetic mesoporous particles (Fe3O4/FMSMs), which showed a sustained drug release behavior and excellent magnetic responsivity to external magnetic field. Furthermore, the Fe3O4/FMSMs carriers could deliver DOX into the cell cytoplasm and cell nucleusCitation[19]. Shi and coworkers [Citation20–22] reported a series of Fe3O4/Fe2O3@mSiO2 nanocapsules with a single or double mesoporous silica shell, and these nanocapsules had sufficient magnetic responsivity and high anticancer drug loading capacityCitation[20], [21], [22]. Also, the DOX-loaded nanocapsules showed greater cytotoxicity to induce MCF-7 cell death than free DOX. Julian-Lopez et al. [Citation23] for the first time reported mesoporous maghemite-organosilica microspheres with the multifunctionality of MR imaging and hyperthermia therapyCitation[23]. Vallet-Regí and coworkers [Citation24,25] synthesized magnetic γ-Fe2O3-encapsulated mesoporous silica microspheres, and these magnetic microspheres showed the controlled release of therapeutic drugs and good magnetic heating capacity under a low-frequency alternating magnetic fieldCitation[24], [25]. Lu et al. [Citation26] prepared magnetic iron oxide-loaded hollow mesoporous silica nanocapsules with a particle size of 100 nm, and they found that magnetic nanocapsules induced heating upon exposure to magnetic field and remotely triggered drug releaseCitation[26]. However, there are limited studies on investigating MMS nanoparticles for drug delivery with synergistic magnetic hyperthermia until now.
Recently, we prepared Fe3O4/SiO2 MMS nanoparticles by encapsulating Fe3O4 nanoparticles in mesoporous silica using a sol-gel method, which had pH-controlled DOX release behavior and magnetic heating capacity under an alternating magnetic field.[Citation27] Using DNA to cap the mesopore openings of MMS nanoparticles, the DOX-loaded nanosystem showed potential temperature-controlled DOX release induced by magnetic heating.[Citation28] However, the Fe3O4 amount was limited in the prepared Fe3O4/SiO2 MMS nanoparticles, which results in the lower magnetic heating capacity.
In this study, we report a kind of MMS nanoparticle as a multifunctional platform for drug delivery with synergistic magnetic hyperthermia ability. Preparation of MMS nanoparticles involves the synthesis of carbon-encapsulated magnetic colloidal nanoparticles (MCN@C) by solvothermal treatment [Citation31] and subsequently coating mesoporous silica on MCN@C to form the core-shell structured MCN@C/mSiO2 nanoparticles. Here, the carbon layer could enhance the ability to protect the magnetic colloidal nanoparticles from oxidation or dissolution in an acidic environment. After the treatment of the MCN@C/mSiO2 nanoparticles by calcination to remove the carbon layers, rattle-type MCN/mSiO2 nanoparticles were obtained. Furthermore, magnetic hyperthermia ability, drug release behavior, biocompatibility and cellular uptake of the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were investigated.
2. Experimental details
2.1. Materials
Ferrocene (Fe(C5H5)2, ≥98%), hydrogen peroxide (H2O2, 30%), acetone (C3H6O, ≥99%), cetyl trimethyl ammonium bromide (CTAB), triethanolamine (TEA) and tetraethyl orthosilicate (TEOS) were of analytic grade from the Sinopharm Chemical Reagent (Shanghai) Co., Ltd, Shanghai, China. Doxorubicin hydrochloride (DOX) was purchased from Sangon Biotech (Shanghai) Co., Ltd (Shanghai, China). Dodecane was obtained from TCI, Shanghai, China. All chemicals were used as received without further purification.
2.2. Preparation of carbon-encapsulated magnetic colloidal (MCN@C) nanoparticles
MCN@C nanoparticles were synthesized according to the previous report after minor modification.[Citation31] Typically, 0.30 g of ferrocene was completely dissolved in 30 ml of acetone, and 1.50 ml of H2O2 was slowly added into the above solution to form the precursor solution. After the vigorous magnetic stirring for 30 min, the precursor solution was transferred to a Teflon-lined stainless autoclave, and then heated to 230°C for 48 h. After cooling to room temperature, the precipitates were collected by a magnet and washed with ethanol several times. Finally, the black products were dried at room temperature in a vacuum oven to obtain MCN@C nanoparticles.
2.3. Preparation of MMS nanoparticles
MMS nanoparticles were prepared via a one-pot biphase stratification approach after some modification.[Citation32] Typically, 0.2 g of MCN@C nanoparticles was added into 60 ml of water, and followed the ultrasonication for 30 min to disperse MCN@C nanoparticles. Subsequently, the MCN@C suspension was transferred into a 100-ml round bottom flask including 0.5 g of CTAB and 0.36 g of TEA. After stirring gently at 60°C for 1 h, 20 ml of TEOS solution (5% v/v in dodecane) was carefully added to the flask. The reaction was kept at 60°C with continuous stirring for 8 h. Then, the colloidal nanoparticles were separated by centrifugation, and washed with ethanol several times. Finally, the obtained colloidal nanoparticles were treated in ammonium nitrate (NH4NO3) ethanol solution (0.6 wt%) at 60°C for 6 h thrice to remove the surfactant, which was denoted as MCN@C/mSiO2 nanoparticles. Rattle-type MMS nanoparticles were obtained by the calcination of the MCN@C/mSiO2 nanoparticles at 540°C for 7 h, which was denoted as MCN/mSiO2 nanoparticles.
2.4. Characterization
The wide-angle X-ray diffraction (WAXRD) patterns were performed on a D8 ADVANCE powder diffractometer (Bruker, Germany) using Cu Kα1 radiation (1.5405 Å). Scanning electron microscope (SEM) observations were carried out with a FEI Quanta 450 field emission setup. Scanning transmission electron microscopy (STEM) images were obtained with a Tecnai G2 F30 electron microscope (FEI, Netherlands) operated at an acceleration voltage of 300 kV. UV-vis analysis was performed on a NanoDrop2000C spectrophotometer (Thermo Fisher Scientific, USA). N2 sorption measurements were carried out on a Micromeritics Tristar 3020 automated surface area and pore size analyzer (Micromeritics, USA) at 77 K under continuous adsorption conditions. Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods were used to determine the surface area and pore size distribution. Magnetization curves were carried out using a LakeShore 7407 vibrating sample magnetometer (VSM, Lake Shore, USA) at 298 K.
2.5. Drug loading and release from MMS nanoparticles
In this study, anticancer drug (doxorubicin hydrochloride, DOX) was introduced into the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles to verify drug release behavior. For drug loading, DOX was dissolved into PBS to obtain DOX solution with a concentration of 0.5 mg ml–1. Subsequently, 60 mg of MCN@C/mSiO2 or MCN/mSiO2 nanoparticles was dispersed into 12 ml of DOX solution. The mixture solution was agitated for 24 h under dark conditions, and then collected by centrifugation to obtain the DOX-loaded MCN@C/mSiO2 or MCN/mSiO2 nanoparticles. The DOX-loaded nanoparticles were washed with PBS twice to remove DOX adsorbed on the surfaces. The DOX loading amount was determined by measuring the initial and residual DOX solutions using UV-vis analysis at the wavelength of 481 nm.
The DOX release behaviors from the MCN@C/mSiO2 or MCN/mSiO2 nanoparticles were performed as follows: 60 mg of the DOX-loaded MCN@C/mSiO2 or MCN/mSiO2 nanoparticles was dispersed in 10 ml of the release medium (pH 5.0 or pH 7.4 at 37°C and pH 5.0 or pH 7.4 at 45°C). At the predetermined time intervals, 20 μl of the released solution was withdrew and centrifuged for UV-vis analysis. After the UV-vis analysis, the residual solution was returned to the release system.
2.6. Magnetic hyperthermia ability of MMS nanoparticles
The evaluation of magnetic hyperthermia ability of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles was carried out on a DM100 System (NanoScale Biomagnetics, Spain). The MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were dispersed in H2O, and the concentration was 30 mg ml–1, respectively. Subsequently, 1.0 ml of the nanoparticles suspension was put in the test vessel and then fixed under an alternating magnetic field. The magnetic field strength and frequency were set to be 180 Gauss and 409 kHz, respectively. The specific absorption rate (SAR) was calculated to evaluate the magnetic hyperthermia ability of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles.
2.7. Cell culture
In this study, HeLa cells lines were used to test in vitro cytotoxicity and cellular uptake of MMS nanoparticles. HeLa cell lines were maintained in MEM medium containing 10% fetal bovine serum, 100 units ml–1 penicillin, and 100 mg ml–1 streptomycin. Cells were cultured with the complete medium in 5% CO2 at 37°C.
2.8. In vitro cytotoxicity of MMS nanoparticles
In vitro cytotoxicities of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were evaluated using Cell Counting Kit-8 (CCK-8) assay (Dojindo, Japan). Before cell seeding, the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were dispersed in MEM medium with a centration of 1 mg ml–1. HeLa cells were seeded into a 96-well plate at a density of 5000 cells in each well. Subsequently, the nanoparticles suspension were added in each well, and the final concentrations of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were 0, 25, 50, 75 and 100 μg ml–1 with the medium volume of 100 μl in each well. After 24 h incubation of cells, 10 μl of CCK-8 solution was added into each well, and the cells were incubated for another 3 h. The optical density (OD) was then measured by the absorbance at 450 nm using a microplate reader (Bio-Rad 680, California, USA).
2.9. Cellular uptake of MMS nanoparticles
For observation of cellular uptake of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles, three kinds of nanoparticles were labeled with rhodamine B isothiocyanate (RBITC) to form RBITC-labeled nanoparticles. Typically, 1.0 × 105 cells were seeded in a 35-mm Petri dish. After the cells were attached to the bottom of Petri dish they were washed twice with PBS. Subsequently, RBITC-labeled nanoparticles were added to the Petri dishes (100 μg ml–1). After another 4 h incubation, the cells were washed with PBS three times to remove the remaining nanoparticles and dead cells. Then, the nuclei of cells were stained for 25 min using Hoechst 33342 solution (0.5 μg ml–1). After washing with PBS to remove the residual dye molecules, the cells were fixed with 4% of paraformaldehyde for 20 min. Finally, the cells were washed with PBS for three times, and observed under a confocal laser scanning microscopy (SP5, Leica, Hamburg, Germany).
3. Results and discussion
3.1. Characterization of MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles
The wide-angle XRD patterns of MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles are shown in Figure (A). MCN@C nanoparticles were synthesized by the previously reported method,[Citation31] and the XRD pattern indicated that the MCN@C nanoparticles mainly had magnetite (JCPDS file 19-0629) or maghemite (JCPDS file 39-1346). For MCN@C/mSiO2 nanoparticles, the XRD pattern showed a broad reflection at 2θ = 20–25°, suggesting an amorphous structure of the mesoporous silica coating; but other diffraction peaks were the same as those of MCN@C nanoparticles, indicating that the crystalline structure of the MCN@C nanoparticles did not change after the coating of mesoporous silica and the removal of surfactant. But for MCN/mSiO2 nanoparticles, several diffraction peaks indexed to hematite (JCPDS file 33-664) were observed on the XRD pattern, except for the peaks for magnetite or maghemite, which suggests that some magnetite or maghemite in MCN@C nanoparticles have converted to hematite in MCN/mSiO2 nanoparticles due to the calcination treatment for the removal of carbon and surfactant. On the other hand, compared to the MCN@C and MCN@C/mSiO2 nanoparticles, the width of the diffraction peaks of the MCN/mSiO2 nanoparticles became much narrower, which suggested that the calcination treatment of the MCN@C/mSiO2 nanoparticles induced the formation of larger crystals of magnetite or maghemite. According to Scherrer’s formula, the magnetic nanocrystal size was increased from 16.1 nm in the MCN@C/mSiO2 nanoparticles to 18.2 nm in the MCN/mSiO2 nanoparticles.
Figure 1. The wide-angle XRD patterns (A) and magnetization curves measured at room temperature (B) of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles.
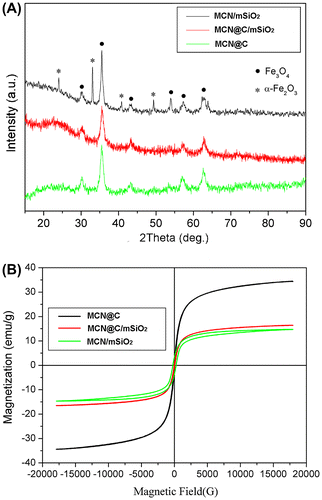
Figure (B) shows the magnetization curves of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles measured at 298 K with a vibrating sample magnetometer (VSM). Similar to the previous reports,[Citation31] the magnetic property of the MCN@C nanoparticles demonstrated a very small remanence and reveals its superparamagnetic nature at 298 K, and the magnetization saturation value (Ms) of the MCN@C nanoparticles was estimated to be 34.4 emu g–1. It can also be observed that a very small hysteresis loop is for the MCN@C/mSiO2 nanoparticles and the Ms decreased to be 16.5 emu g–1. This indicated that the MCN@C nanoparticles still maintained superparamagnetic behavior. The decrease in Ms was attributed to the non-magnetic mesoporous silica coating on the MCN@C nanoparticles. However, a relatively obvious hysteresis loop was observed in the magnetization curve of the MCN/mSiO2 nanoparticles. It might be that the larger crystals of magnetite or maghemite and hematite formation contributed the ferromagnetic behavior of the MCN/mSiO2 nanoparticles. On the other hand, the Ms of the MCN/mSiO2 nanoparticles decreased to be 14.7 emu g–1 owing to the formation of some hematite crystals from magnetite or maghemite during the calcination treatment. Compared to our previously reported MMS nanoparticles (Ms of 4.2–8.1 emu g–1),[Citation27] the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles exhibited much higher magnetization saturation, which might enhance the magnetic heating capacity.
Figure shows SEM and STEM images of MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles. The MCN@C nanoparticles had a narrow particle size distribution, and the average particle size was about 170 nm (Figure (A)). STEM images revealed that the MCN@C nanoparticles had an obvious core-shell structure, similar to the previous reports,[Citation31] and magnetic cores were composed of small magnetite or maghemite nanocrystals and thin carbon shells were coated on the surfaces of magnetic cores. After coating with mesoporous silica on the MCN@C nanoparticles, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles still had spherical morphology, but the average particle size increased to about 390 and 320 nm, respectively. STEM images showed different structures for the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles. The MCN@C/mSiO2 nanoparticles had the core/shell structure, while the MCN/mSiO2 nanoparticles had a rattle-type structure, e.g. iron oxide nanocrystals were encapsulated in hollow cores of the MCN/mSiO2 nanoparticles. On the other hand, dendritic mesoporous channels can be clearly observed on both MCN@C/mSiO2 and MCN/mSiO2 nanoparticles, indicating the possibility of drug loading in the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles for drug delivery.
Figure 2. SEM and STEM images of the MCN@C (A, D); MCN@C/mSiO2 (B, E); and MCN/mSiO2 (C, F) nanoparticles.
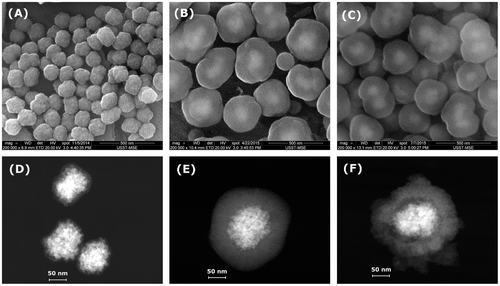
Figure shows N2 adsorption–desorption isotherms of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles and their corresponding pore size distribution curves. The MCN@C nanoparticles had much lower N2 adsorption and no mesopore size distribution, the BET surface area (SBET) was about 70 m2 g–1. However, both isotherms of the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles belong to type IV isotherm, indicating the mesoporous structure of the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles. The SBET of the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were 606 and 709 m2 g–1, respectively. The pore volumes (VP) at P/P0 = 0.97 for the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were 0.95 and 1.08 cm3 g–1, respectively (Table ). However, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles exhibited the similar pore size distributions peaked at 3.4 and 3.2 nm. Therefore, the coating of mesoporous silica on the MCN@C nanoparticles provides high surface area and mesoporous channels for loading a high amount of drugs. Furthermore, the MCN/mSiO2 nanoparticles created hollow cores and might contribute much higher drug loading capacity.
Figure 3. N2 adsorption-desorption isotherms (A) of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles and their corresponding pore size distribution curves (B).
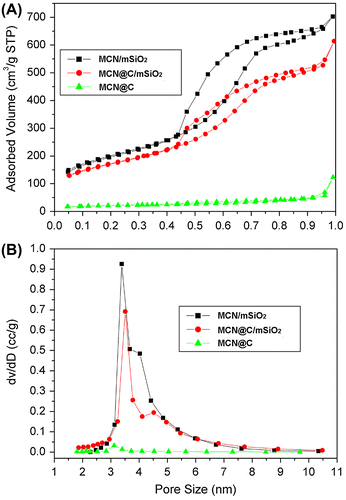
Table 1. The structure parameters, DOX loading capacities and SAR values of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles.
3.2. Magnetic hyperthermia ability of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles
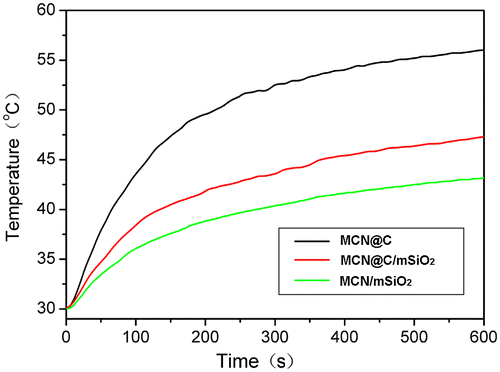
Figure shows the magnetic heating curves of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles with a concentration of 30 mg ml–1 evaluated under the same alternating magnetic field. From the temperature kinetic curve of the MCN@C nanoparticles, the temperature increased from 37 to 59°C within 5 min, suggesting that alternating magnetic field can induce the MCN@C nanoparticles to generate heat and reach the hyperthermia temperature within a very short time. For the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles, the temperature increase with time was a little slower than the MCN@C nanoparticles. But the absolute temperature increases in 5 min for the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were estimated to be 10.4 and 13.5°C, respectively. That is to say, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles can also generate heat for hyperthermia treatment. Correspondingly, the specific absorption rate (SAR) of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles, which was quantified for the magnetic heating capacity of magnetic materials, was estimated to be 54.7, 34.8 and 25.7 W g–1, respectively. The MCN@C/mSiO2 and MCN/mSiO2 nanoparticles also showed good magnetic hyperthermia ability, although the magnetic hyperthermia ability of the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles was lower than that of the MCN@C nanoparticles, owing to the coating of non-magnetic mesoporous silica on the MCN@C nanoparticles. On the other hand, the MCN@C/mSiO2 nanoparticles showed a little higher magnetic heating capacity than the MCN/mSiO2 nanoparticles, which is attributed to the maintaining of iron oxide crystals in the MCN@C/mSiO2 nanoparticles during extracting treatment, but a small amount of hematite crystals were formed in the MCN/mSiO2 nanoparticles during the calcination treatment.
3.3. Drug release behavior from the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles
Doxorubicin hydrochloride (DOX) was used as a model anticancer drug to investigate the drug release behavior from the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles in this study. The DOX loading amounts in the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were 58.5 and 96.2 μg DOX per 1 mg of the MCN@C/mSiO2 or MCN/mSiO2 nanoparticles, respectively. Here, the higher DOX loading capacity of the MCN/mSiO2 nanoparticles was attributed to its higher surface area and hollow cavity.
Figure shows the DOX release profiles from the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles in the release media with pH 7.4 and 5.0 at temperatures of 37 and 45°C. Here, the temperature of 45°C was used to evaluate the possibility of drug release in the range of hyperthermia temperature. Both MCN@C/mSiO2 and MCN/mSiO2 nanoparticles exhibited pH dependent DOX release behavior. The DOX releases from the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were very slow in pH 7.4 medium (only c.2–5% of DOX release in 8 h), but the DOX release became faster in pH 5.0 medium (c.25–30% of DOX release in 8 h). The DOX release behaviors from the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles are similar to those in previous reports on the DOX release from mesoporous silica nanoparticles.[Citation27] It has been accepted that the electrostatic interaction and hydrogen bonding between DOX molecules and the surfaces of silica nanoparticles could become weaker with lowering pH value, owing to the protonation of surface silanols. Thus, DOX molecules are easy to detach from the surfaces of silica nanoparticles in acidic environment, and thereby fasten DOX release.
Figure 5. The DOX release behaviors from the MCN@C/mSiO2 (A) and MCN/mSiO2 (B) nanoparticles in the release media of pH 7.4 and 5.0 and at temperatures of 37 and 45°C.

On the other hand, for both MCN@C/mSiO2 and MCN/mSiO2 nanoparticles, the DOX release at 45°C was slightly faster compared to that at 37°C, which might be because the increased temperature can accelerate the diffusion of DOX molecules in the mesoporous channels. In this study, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles can generate heat under an alternating magnetic field to raise temperature, and thereby stimulate drug diffusion.
Therefore, such drug release behavior could decrease the side-effect of toxic anticancer drug delivery, because anticancer drugs mostly do not release from the carriers during the circulation of the drug delivery system in the blood stream (pH 7.4). When the drug delivery system is internalized in the targeted cancer cells, faster DOX release could enhance the therapeutic efficiency owing to the acidic environment in the endosome/lysosome and cytosol of cancer cells (pH 5.0–5.5). The combination with magnetic hyperthermia could further accelerate drug release after the endocytosis of drug delivery system by cancer cells.
3.4. In vitro cytotoxicity and cellular uptake of MMS nanoparticles
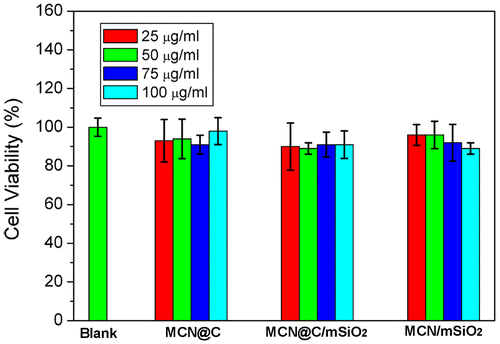
Figure shows in vitro cytotoxicities of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles against HeLa cells evaluated using CCK-8 assay. Cell viabilities of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were more than 90% even up to a concentration of 100 μg ml–1, and they had no significant difference compared to those of the control group. The results revealed that the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles are safe for drug delivery.
Figure 6. Viabilities of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles to HeLa cells after incubation for 24 h evaluated using CCK-8 assay.
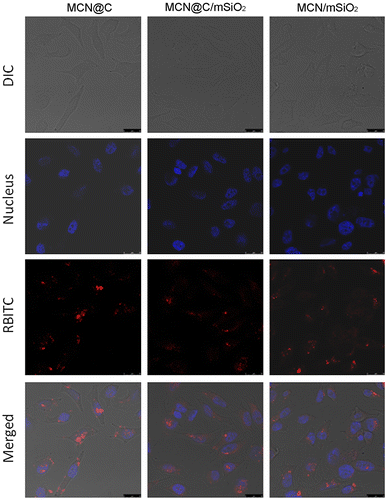
Cellular uptake of the drug carriers is very important for enhancing the drug delivery efficiency. On the one hand, cellular uptake of drug carriers could be beneficial for magnetic hyperthermia therapy owing to magnetic local heating in cancer cells. On the other hand, cellular uptake of drug carriers could also enhance the chemotherapeutic efficiency due to the intracellular delivery of drugs. In this study, RBITC-labeled MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were incubated with HeLa cells for 4 h to observe the cellular uptake. As shown in Figure , red fluorescence could be observed in the cells for each type of magnetic mesoporous nanoparticles, which suggests that the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were taken up into HeLa cells after endocytosis. However, the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles have not been internalized into the nuclei. Furthermore, the distributions of red fluorescence had no significant difference among the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles. Therefore, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles as nanocarriers for anticancer drug delivery could achieve local drug delivery and magnetic hyperthermia, and thereby enhance the cancer therapeutic efficiency.
Figure 7. Cellular uptake of the MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles in HeLa cells after 4 h of incubation with a concentration of 100 μg ml–1: DIC shows the optical photograph of HeLa cells; Nucleus shows the nuclei of HeLa cells stained with Hoechst 33342; RBITC shows the location of RBITC-labeled nanoparticles; Merged shows the overlap DIC, Nucleus and RBITC images, indicating that RBITC-labeled MCN@C, MCN@C/mSiO2 and MCN/mSiO2 nanoparticles were taken up by HeLa cells. All scale bars are 25 μm.
4. Conclusions
In this study, we have synthesized MMS nanoparticles (MCN@C/mSiO2 and MCN/mSiO2) as a multifunctional platform for potential drug delivery and magnetic hyperthermia. The MCN@C/mSiO2 nanoparticles had core-shell structure with higher magnetic heating capacity compared to the MCN/mSiO2 nanoparticles, but the MCN/mSiO2 nanoparticles had higher drug loading capacity. Both MCN@C/mSiO2 and MCN/mSiO2 nanoparticles had similar drug release behavior with pH-controlled release and temperature-accelerated release. Furthermore, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles showed low cytotoxicity and could be internalized into cells within a short period. Therefore, the MCN@C/mSiO2 and MCN/mSiO2 nanoparticles would be promising for chemotherapy with synergistic magnetic hyperthermia in cancer therapy.
Funding
The authors gratefully acknowledge the support by National Natural Science Foundation of China [number 51572172, 51102166], Shanghai Shuguang Project [number 12SG39], Program for New Century Excellent Talent in University [number NCET-12-1053] and the Hujiang Foundation of China [number B14006].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer CA Cancer. J Clin. 2011;61:183–203.10.3322/caac.v61:3
- Cho K, Wang X, Nie S. Therapeutic nanoparticles for drug delivery in Cancer Clin. Cancer Res. 2008;14:1310–1316.
- He Q, Shi JL. Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J Mater Chem. 2011;21:5845–5855. 10.1039/c0jm03851b
- Wang BX, Li CH, Peng XF. Research on stability of nano-particle suspension. J. Univ. Shanghai Sci. Technol. 2003;3:209–212.
- Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–1534.
- Niu DC, Ma Z, Li YS, et al. Synthesis of core−shell structured dual-mesoporous silica spheres with tunable pore size and controllable shell thickness. J Am Chem Soc. 2010;132:15144–15147. 10.1021/ja1070653
- Li L, Tang F, Liu H, et al. In Vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy. ACS Nano. 2010;4:6874–6882. 10.1021/nn100918a
- Jia HZ, Zhang W, Zhu JY, et al. Hyperbranched–hyperbranched polymeric nanoassembly to mediate controllable co-delivery of siRNA and drug for synergistic tumor therapy. J. Controlled Release. 2015;216:9–17. 10.1016/j.jconrel.2015.08.006
- Tagliamonte M, Petrizzo A, Napolitano M, et al. Novel metronomic chemotherapy and cancer vaccine combinatorial strategy for hepatocellular carcinoma in a mouse model. Cancer Immunol Immunother. 2015;64:1305–1314. 10.1007/s00262-015-1698-0
- Kumar CSSR, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Del. Rev. 2011;63:789–808. 10.1016/j.addr.2011.03.008
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17:1–18. 10.1080/02656730150201552
- Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biothechnol J. 2011;6:1342–1347.
- Lee H, Kim S, Choi BH, et al. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int J Hyperthermia. 2011;27:698–707. 10.3109/02656736.2011.608217
- Ito A, Shinkai M, Honda H, et al. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 2005;100:1–11. 10.1263/jbb.100.1
- Taratula O, Dani RK, Schumann C, et al. Multifunctional nanomedicine platform for concurrent delivery of chemotherapeutic drugs and mild hyperthermia to ovarian cancer cells. Int J Pharm. 2013;458:169–180.
- Ren Y, Zhang H, Chen B, et al. Multifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistance. Int J Nanomed. 2012;7:2261–2269.
- Pradhan P, Giri J, Rieken F, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Controlled Release. 2010;142:108–121. 10.1016/j.jconrel.2009.10.002
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021.
- Gai S, Yang P, Ma PA, et al. Fibrous-structured magnetic and mesoporous Fe3O4/silica microspheres: synthesis and intracellular doxorubicin delivery. J Mater Chem. 2011;21:16420–16426. 10.1039/c1jm13357h
- Wu H, Liu G, Zhang S, et al. Biocompatibility, MR imaging and targeted drug delivery of a rattle-type magnetic mesoporous silica nanosphere system conjugated with PEG and cancer-cell-specific ligands. J Mater Chem. 2011;21:3037–3045. 10.1039/c0jm02863k
- Chen Y, Chen H, Zeng D, et al. Core/Shell structured hollow mesoporous nanocapsules: A potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano. 2010;4:6001–6013.
- Zhao W, Chen H, Li Y, et al. Uniform rattle-type hollow magnetic mesoporous spheres as drug delivery carriers and their sustained-release property. Adv Funct Mater. 2008;18:2780–2788. 10.1002/adfm.v18:18
- Julian-Lopez B, Boissiere C, Chaneac C, et al. Mesoporous maghemite–organosilica microspheres: a promising route towards multifunctional platforms for smart diagnosis and therapy. J Mater Chem. 2007;17:1563–1569. 10.1039/B615951F
- Ruiz-Hernandez E, Lopez-Noriega A, Arcos D, et al. Aerosol-assisted synthesis of magnetic mesoporous silica spheres for drug targeting. Chem Mater. 2007;19:3455–3463.
- Martin-Saavedra FM, Ruiz-Hernandez E, Bore A, et al. Magnetic mesoporous silica spheres for hyperthermia therapy. Acta Biomater. 2010;6:4522–4531.
- Lu F, Popa A, Zhou S, et al. Iron oxide-loaded hollow mesoporous silica nanocapsules for controlled drug release and hyperthermia. Chem Commun. 2013;49:11436–11438.
- Tao C, Zhu Y. Magnetic mesoporous silica nanoparticles for potential delivery of chemotherapeutic drugs and hyperthermia. Dalton Trans. 2014;43:15482–15490.
- Zhu Y and Tao C. DNA-capped Fe3O4/SiO2 magnetic mesoporous silica nanoparticles for potential controlled drug release and hyperthermia. RSC Adv. 2015;5:22365–22372. 10.1039/C5RA00701A
- Liu J, Qiao SZ, Hartono SB, et al. Monodisperse yolk-shell nanoparticles with a hierarchical porous structure for delivery vehicles and nanoreactors. Angew Chem Int Ed. 2010;49:4981–4985. 10.1002/anie.v49:29
- Souza KC, Mohallem NDS, Sousa EMB. Mesoporous silica-magnetite nanocomposite: facile synthesis route for application in hyperthermia. J Sol-Gel Sci Technol. 2010;53:418–427. 10.1007/s10971-009-2115-y
- Wang H, Chen QW, Yu YF, et al. Size- and solvent-dependent magnetically responsive optical diffraction of carbon-encapsulated superparamagnetic colloidal photonic crystals. J Phys Chem C. 2011;115:11427–11434. 10.1021/jp201893z
- Shen D, Yang J, Li X, et al. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett. 2014;14:923–932.