?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Titanium dioxide nanoparticles were synthesized by laser pyrolysis, their surface and electronic properties were modified by gold and/or nitrogen. These materials were characterized by different techniques like X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR). Time resolved conductivity (TRMC) was used to study the charge separation of electron/hole pairs. Altogether (XPS, EPR, TRMC), the physicochemical characterizations are well correlated with chemical photoactivity of the different samples. Their photocatalytic activity was evaluated for the degradation of linear carboxylic acids (C2-C3) under UV and visible illumination. The decomposition rate of acids was measured, it shows that the modification with gold increases the photoactivity while the presence of nitrogen slows down the process. Such observations are in good agreement with evolution of TRMC signals. A degradation pathway has been determined by identification of intermediate products by chromatography and EPR, results show different intermediate species. In particular EPR confirms the presence of NO2− paramagnetic centers and shows two novel N centered paramagnetic centers. A decrease of the degradation rate is observed with increase of carboxylic acid chain length.
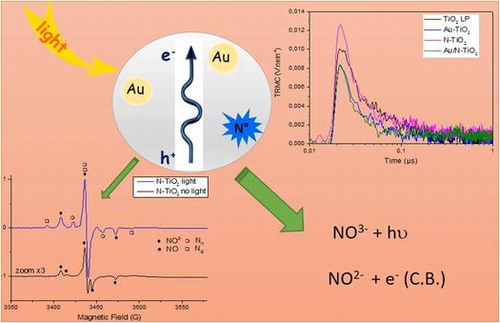
1. Introduction
Many studies deal with the fabrication and characterization of the photocatalytic activity of various materials exhibiting efficiency both in the visible and UV range [Citation1–3]. In particular, materials based on TiO2 are intensively studied due to their high activity under UV illumination. The main drawbacks are high recombination rate and lack of activity under visible light. Improving photocatalytic efficiency under visible illumination would allow taking advantage of solar irradiation that is a free, abundant and non-polluting energy [Citation4–7]. Different ways to enhance the visible photoactivity are doping by hetero-elements (N, S, F,…) or introducing metallic structures (Au, Ag…) with an absorption in the visible range and/or able to improve the charge separation [Citation8–14]. In this context, we have previously demonstrated that the laser pyrolysis is an efficient method for the one step synthesis of TiO2-based nanomaterials (Au/TiO2, N-TiO2 and co-modified Au/N-TiO2) [Citation15]. Under UV illumination, these samples were very efficient for the degradation of formic acid by comparison with commercial TiO2 P25. In particular gold modification of titanium dioxide enhances transfer of electrons. Interestingly, a significant activity was observed under visible illumination for our both materials containing nitrogen [Citation15].
In parallel to materials development, advanced characterization methods are used to study their characteristics (crystallization in preferred directions, surface states,…) and correlate them to photocatalytic properties [Citation14,16,17]. Previous EPR studies have shown the existence of paramagnetic nitrogen species interacting with the TiO2 lattice. These paramagnetic species are involved in the photo-induced electron transfer from the solid to an electron scavenger such as molecular oxygen [Citation3,17,18]. Time Resolved Microwave Conductivity (TRMC) was used to demonstrate that Au nanoparticles can act as electron scavengers retarding recombination in Au-TiO2 samples [Citation14,19] In this paper, we apply TRMC to characterize the ability of our sample to study charge carrier dynamics under UV or visible illumination. Based on TRMC and EPR results giving information on the presence of paramagnetic centers, we establish a qualitative classification in our family of samples towards their ability to separate the charges. Indeed, it is well known that charge separation is of major importance in the efficiency of photocatalyst materials [Citation20]. In combination with other methods (such as X-ray photoelectron spectroscopy (XPS), EPR and photoluminescence [Citation15]), we use the information obtained from TRMC to correlate the physicochemical properties to photocatalytic activity, i.e. ability of these samples to decompose various linear (C1 – C3) acids under UV or visible light. Indeed, by using different excitation wavelengths, TRMC gives information on the number of exciton created under UV or visible illumination for one type of sample and therefore predict their relative photocatalytic efficiency under both types of illumination.
2. Experimental details
2.1. Chemicals
Titanium tetraisopropoxide (TTIP, 97% purity) and hydrogen tetrachloroaurate (III) HAuCl4•3H2O were respectively used as titanium source for synthesis of TiO2 nanoparticles, as gold source. Ammoniac (NH3) gas was supplied by Messer. These reactants and the carboxylic acids CH3COOH, and CH3CH2COOH were obtained from Sigma-Aldrich and used without further purification. Commercial reference TiO2 P25 was obtained from Evonik.
2.2. Synthesis
Modified nanoparticles (NPs) were synthesized through laser pyrolysis method by using HAuCl4•3H2O dissolved in TTIP and NH3 as precursors. Briefly, this method is based on the interaction between a flow of precursors and a high power CO2 laser beam. The reactants are heated and dissociated with appearance of a flame. Homogeneous nucleation occurs; nanoparticles grow in the hot zone and are collected downstream on metallic filters. More details were reported in our previous work [Citation15].
The obtained powders were annealed at 400 °C under air flow (650 cm3 min−1) in an oven (Pyrox) during 3 h to remove the residual carbon due to the decomposition of reactants (TTIP). All the results presented here concern annealed powders.
2.3. Characterization methods
The identification of crystallite phase of the synthesized photocatalysts was carried out by X-ray Diffraction measurements (XRD, Siemens D5000 diffractometer with Cu-Kα radiation (λ = 1.54184 A°)). The 2θ angle ranged from 20° to 80° with a step size of 0.04° and a counting time of 7s/step. The average TiO2 crystallite size of the sample was calculated from the Scherrer equation. The chemical environment of nitrogen atoms in TiO2 was deduced by X-ray photoelectron spectra (XPS) (Kratos Analytical Axis Ultra DLD spectrometer, Al Kα X-ray) on the powders.
The charge-carrier lifetimes in TiO2 after UV and visible illumination were determined by TRMC [Citation21,22] .The TRMC technique is based on the measurement of the change of the microwave power reflected by a sample, ΔP(t), induced by its laser pulsed illumination. The relative difference ΔP(t)/P can be correlated, for small perturbations of conductivity, to the difference of the conductivity Δσ(t) considering the following equation:
where Δni(t) is the number of excess charge-carriers i at time t and μi their mobility. The sensitivity factor A is independent of time, but depends on different factors such as the microwave frequency or the dielectric constant. Considering that the trapped species have a small mobility, which can be neglected, Δni is reduced to mobile electrons in the conduction band and holes in the valence band. And in the specific case of TiO2, the TRMC signal can be attributed to electrons because their mobility is much larger than that of the holes [Citation23].
The incident microwaves were generated by a Gunn diode of the Kα band at 30 GHz. Pulsed light source was an optical parametric oscillator (OPO, EKSPLA, NT342B) tunable from 225 to 2000 nm. It delivers 8 ns pulses (full width at half maximum) with a frequency of 10 Hz. The light energy densities received by the sample were respectively 1.2, 3.4, and 7.0 mJ∙cm−2 at 355, 410, and 450 nm. The main data provided by TRMC are given by the maximum value of the signal (Imax), which reflects the number of the excess charge-carriers created by the pulse and the decay of the signal I(t), which is due to the decrease of the excess electrons controlled by recombination and trapping.
Low-temperature continuous-wave EPR spectra were recorded with a Bruker EMX spectrometer operating at X-band (9.65 GHz) frequency and equipped with an ER-4116 dual mode cavity and an Oxford Instruments ESR-900 flow cryostat. For the study of photoirradiated dry photocatalyst powders, in situ irradiation of samples in the EPR cavity was achieved using a fibered Schott KL1500 halogen UV-visible lamp. For the study of degradation of carboxylic acids (0.1 M) in aqueous dispersion of photocatalyst (4 g L−1), the samples were frozen in liquid nitrogen and irradiated in a Rayonet UVA photoreactor (8 mW cm−2.) for 30 min at T = 77 K in a quartz cold finger, then transferred into the EPR spectrometer at T = 60 K.
2.4. Photocatalytic experiments
The photocatalytic efficiency of samples was evaluated by following the degradation of linear acids (1086 μmol L−1) in water and 181 μmol L−1 corresponding to a similar concentration of carbon. The photocatalytic tests were carried out in a Pyrex photoreactor. For UV irradiation, a mercury lamp Phillips HPK 125 W with optical filters 7.6 and 0.52 (Corning) were employed to obtain an emission peak centered at λ = 365 nm. The radiant flux at 4.2 mW cm−2 (7.7 1015 photons s−1 cm−²) was measured using a VLX-3 W radiometer with 365 nm sensor. For visible light illumination in the region (400–800 nm), LED lamp was used; the photon flux was measured around 85 10−3 μmol s−1. Both lamps were placed at the bottom of the reactor; the illuminated area was 12.5 cm². Circulating water between lamp and reactor maintain the solution temperature at 20 °C. Before irradiation, the system with TiO2 concentration of 1 g L−1 was always stirred in the dark for 30 min, to reach the adsorption-desorption equilibrium of carboxylic acids on surfaces.
At regular time intervals of irradiation, aliquots of each acid suspension was collected, filtered using 0.45 μm Millipore filters before analysis. The concentrations of acids remaining after adsorption and during the photocatalytic degradation process were determined by high-performance liquid chromatography (HPLC) analysis. VARIAN Prostar HPLC apparatus equipped with a single-wavelength UV-vis detector and a 300 mm x 7.8 mm carbohydrate analysis column (COREGEL-87H3) was used. The mobile phase was H2SO4 solution (5 10−3 mol L−1) and the flow rate was fixed at 0.7 cm3 min−1. The detection wavelength was set at 210 nm.
3. Results and discussion
Based on our previous synthesis and photocatalytic results [Citation15], four samples were selected for this study. The samples are TiO2 LP, Au/TiO2, N-TiO2 and co-modified Au/N-TiO2 and their main characteristics are presented in Table S1. The samples were synthesized by laser pyrolysis in similar conditions, except the choice of the reactive mixture injected in the laser beam. The TiO2 LP was chosen as a reference and for comparison with TiO2 P25, Au/TiO2 sample was selected to demonstrate the effect of the metallic additive. N-TiO2 was chosen because its visible activity is well known [Citation24,25]. In addition the co-modified Au/N-TiO2 sample exhibiting significant activity in the visible while maintaining good activity under UV illumination is part of the study [Citation26,27].
3.1. XRD and XPS characterization of materials
XRD patterns of pure and modified TiO2 powders [Citation15] show a major anatase crystallite phase with less than 5% of rutile (Figure S1), this ratio was estimated from the peaks located at 2θ = 25.32°(anatase) and at 2θ = 27.45° (rutile). The anatase crystallite size was evaluated using the Scherrer equation. The mean crystallite size deduced from XRD measurements is in the range 7–9 nm for all these samples, in quite good agreement with the grain size deduced from transmission electron microscopy measurements (Table S1).
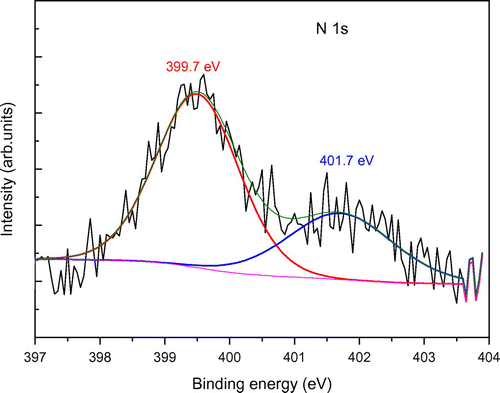
The XPS spectra of all nitrogeN-doped and gold modified TiO2 have been recorded. The spectra corresponding to Ti 2p and O 1s levels are shown in Figure S2. As usually observed for the Ti 2p spectra, two peaks are present at 458.5 eV and 464.3 eV assigned to Ti 2p3/2 and Ti 2p1/2 in agreement with the presence of Ti4+ in a TiO2 environment. The O 1s binding energies of all powders were located at energies slightly higher than 530 eV, attributed to O2− in the TiO2 lattice. Let us note the Au could not be detected, probably due to the low content below the detectivity level of XPS. In the N-doped samples (N-TiO2 and Au/N-TiO2), the N local environment has also been studied. The spectra and fitting curves are presented in Figure (The spectrum of Au/N-TiO2 sample is very similar and is therefore not shown here). Two peaks are present; their binding energies at 399.7 and 401.7 eV as already observed in the literature and are assigned to O-Ti-N bonds (interstitial N) [Citation18,28,29] and to molecularly adsorbed N species like NO or NO2 molecules on the particle surface [Citation30], respectively. It is therefore important to note here that a significant part of our nitrogen is located at the surface of nanoparticles.
3.2. EPR study of charge carriers and paramagnetic species
N-doped and Au-modified samples (Au/TiO2, N-TiO2 and Au/N-TiO2) were also studied by EPR at low temperature (60 K) in the dark as well as in the presence of UV-visible light irradiation (Figure ).
Figure 2. EPR spectra observed for dry photocatalysts powder without (dark curves) and with (blue curves) in situ UV-visible (halogen-lamp) irradiation at T = 60 K. (see exp. section for details). (a) Au modified sample, (b) N-doped sample.
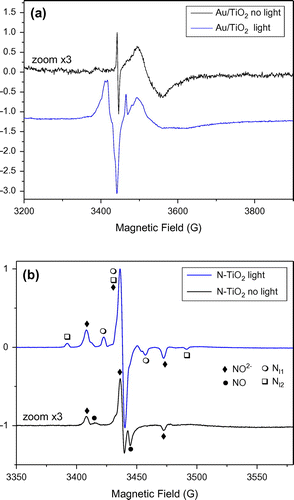
Looking at the EPR spectrum recorded for Au/TiO2 samples, (Figure (a)), the signal in the dark is of weak intensity. It is composed of a broad part due to trapped electrons (Ti3+ in rutile and surface Ti3+, Table ) and a sharp line at g = 2.002 tentatively attributed to bulk anion vacancy defects [Citation31]. Such signals were previously reported in EPR studies of Au/TiO2 catalysts prepared by deposition/precipitation methods [Citation31]. Under UV-visible light irradiation, this sample exhibits much more intense EPR signals due mostly to the presence of charge carriers. Trapped electrons including Ti3+ in rutile, Ti3+ in anatase and Ti3+ in surface sites are observed at g values lower than 2. Holes trapped in anatase and or rutile phases are also observed with signals at g = 2.021 and 2.018 (Table and [Citation32]). The remaining smaller features at g > 2 can be attributed to O2− stabilized at anatase surface or Ti4+O2−Ti4+O°− sites [Citation31,32].
Table 1. EPR parameters of species observed for dry photocatalysts powders.
The EPR spectrum recorded for N-TiO2 (Figure (b)) in the dark is much more intense than the spectrum of Au/TiO2 in the dark, as also shown by the signal to noise ratio, and different species are present. By numerical simulation of the EPR spectrum (Figure S3), it was possible to identify four main species with important contributions in the EPR spectrum : surface Ti3+ with similar g tensor as reported for C-doped TiO2 [Citation33], a second surface Ti3+ site similar to the one observed for Au/TiO2, NO° radicals [Citation34,35] and a last paramagnetic center exhibiting nitrogen hyperfine splitting. This signal was attributed to a nitrogen center labeled as Nb° in many papers in the literature [Citation18,35,36], then attributed to interstitial or substitutional N atom in TiO2 [Citation16] and finally identified as paramagnetic NO2− species trapped in bulk TiO2 [Citation17,37]. When the sample is irradiated in situ with halogen lamp, the spectrum changes drastically (Figure (b)). The contributions of Ti3+ in rutile, surface Ti3+ and NO° radical vanish. Signal from holes is no more observed. The signal due to NO2− increases by a factor of five and two new paramagnetic centers exhibiting different N hyperfine coupling appear (named Nl1° and Nl2° in Table ).
The increase of EPR signal attributed to NO2− centers in N-TiO2 samples under visible or UV-visible irradiation has been already observed [Citation37,38] and was rationalized by Barolo et al. [Citation37]. These authors report the presence in the synthesized nanoparticles of a diamagnetic center (NO3−). This species is responsible of the visible absorption in N-TiO2 materials. It does not create [electron-hole] pairs in N-TiO2, but rather promote an electron from diamagnetic NO3− intraband gap states to the conduction band, thus creating more paramagnetic NO2− intraband gap states.
The produced electron is transferred onto the scavenger (O2) to form O2°−
On the other hand to the best of our knowledge the two other paramagnetic centers exhibiting different N hyperfine coupling have not been described in the literature. They disappear when the sample is warmed up to room temperature and were observed only during or just after irradiation at low temperature, so they will be named Nl1° and Nl2° hereafter (the subscript ‘l’ stands for ‘light’).
The EPR spectrum recorded for Au/N-TiO2 (Figure S4) in the dark exhibits only NO2− paramagnetic centers. No contribution from NO neither from Ti3+ was observed. Under UV-visible irradiation the EPR spectrum is comparable to this observed for N-TiO2 but approximately twice less intense: the same paramagnetic centers NO2−, Nl1° and Nl2° are observed under UV-vis irradiation.
Finally, by comparing the EPR spectra recorded with UV-visible irradiation of Au/TiO2, N-TiO2 and Au/N-TiO2 in exactly the same conditions, one can see that nitrogen doping induces drastic changes. UV-visible photoirradiated Au/TiO2 exhibits both EPR signals due to holes and electrons as observed in classical TiO2 samples. On the opposite, no EPR signal from holes nor photogenerated electrons could be observed in N-TiO2 and Au/N-TiO2. So according to these results N containing samples produce much less electron and holes than Au/TiO2 under UV-visible irradiation. Instead of producing holes, photoirradiation promotes mainly the formation of paramagnetic NO2− species which may induce a less good reactivity for the photooxidation of carboxylic acids.
3.3. Charge carrier dynamics
The electronic properties of the samples were studied by TRMC technique considering both UV and visible regions. The excitation wavelengths were 355 and 450 nm. Let us note that such comparison of the samples is possible because all the samples were obtained by the same method in similar conditions
3.3.1. UV excitation (355 nm)
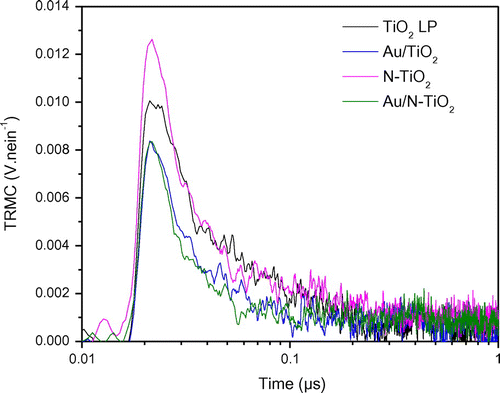
Figure presents the TRMC signals recorded for the different samples after UV excitation. In Table , column 2 gives the maximum value of the signal proportional to the number of excitons created under illumination while column 3 allows comparison of the short range decay behavior of the different samples. Column 4 presents the KD adimensional parameter related to long-term lifetime of charge carriers (after 100 ns), higher KD corresponds to faster decays of the TRMC signal. This parameter is obtained using the I = ID × t−kD expression as proposed in the paper of Meichtry et al. [Citation40]. Figure shows similar behavior for all the samples : a classical anatase signal achieving a maximum value at about 20–25 ns followed by an anatase long decay with recombination phenomena at short-time range (0 to 40 ns) and trapping phenomena at longer-time range (after 40 ns) and mainly noise after 100 ns [Citation41,42]. The 0.18 and 0.19 kD values obtained for samples containing gold show that there is almost no slow processes (after 100 ns) in these samples. Considering the TiO2 LP and N-TiO2 samples, the higher kD values indicate the presence of phenomena occurring at long-time range such as decay of excess electrons [Citation40] in good agreement with the higher number of electrons in theses samples (Figure ).The influence of the incorporated gold can be also observed by comparing the signals measured on TiO2 and Au/TiO2 materials (Figure ). First, at short-time range, gold acts like an impurity. It can be observed by the reduction of Imax and a lower value of I40 ns/Imax (0.34 compared to 0.27 in Table ), corresponding to a faster decay of the electrons population in Au/TiO2 sample. This decay can be associated either to recombination phenomena or transfer of charges. In this case, photoluminescence experiments have already indicated an electronic transfer from TiO2 to gold, therefore the TRMC result can be safely attributed to the transfer of electrons to gold and is in good agreement with an improved photocatalytic activity of Au/TiO2 in comparison with TiO2 LP as already observed for the degradation of formic acid (FA). Indeed, the effect of Au and/or Cu metallic additives on P25 was studied by TRMC [Citation19]. It induced a faster transfer of electrons to the surface correlated to an improved photocatalytic efficiency similar to our present work.
Table 2. TRMC parameters for pure and modified TiO2 obtained by irradiation at 355 nm.
If we now turn to the N-doped sample, a first effect can be seen at short-time range. Imax is slightly higher for the N-doped compound (84 mV vs 69 mV for the TiO2 sample) indicating a better charge-carrier creation. However, like previously, this effect is accompanied by a faster loss of electrons (lower I40 ns/Imax, 0.22 compare to 0.34) and no long-time range effects are detected: TRMC signals of pure and doped compounds are equal after 40 ns. In the N-TiO2 samples the faster loss of electrons can be related to the presence of nitrogen in surface states as shown by XPS (Figure ). Indeed, such surface states are known to act as recombination centers where the electrons are lost. Therefore, the N doping induces recombination which is in good agreement with the less efficient photocatalytic activity (FA degradation) observed for this sample under UV illumination [Citation15].
The compound modified with Au and N shows the lowest I40 ns/Imax value, i.e. the fastest electron loss (0.16 vs 0.34 for TiO2). Indeed, in this sample, both N and Au tend to decrease the electron population both by transfer to gold and charge recombination at the surface, explaining this observation. However, it is not possible to quantify the respective contribution of these effects and explain the efficiency of Au/N-TiO2 by comparison to TiO2.
Finally, these results tend to indicate that the most active sample is Au/TiO2, the less active N-TiO2 while TiO2 and Au/N-TiO2 are in between but their respective efficiency cannot be anticipated. Taking into account the very low Au amount in the powder in Au/N-TiO2, it could expected that the negative recombination effects due to N at the surface will be dominant, and explaining the lower photoactivity (degradation of FA) of Au/N-TiO2 compared to TiO2 [Citation15].
3.3.2. Visible excitation (450 nm)
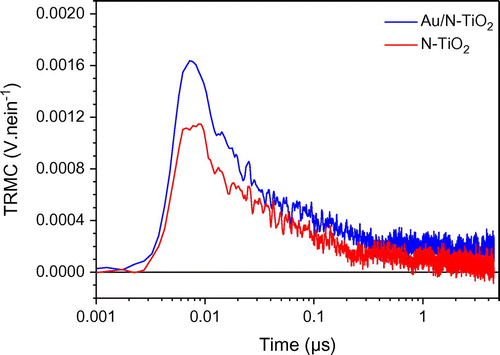
Using the photons delivered by solar light in the visible range to increase the efficiency of photocatalytic processes remains a challenge, and N doping is known to induce some photocatalytic activity in the visible range [Citation3,43] .In a previous study, we have shown that both N-TiO2 and Au/N-TiO2 samples are efficient for the degradation of FA under visible illumination. In the same way than previously, but using visible excitation, the creation and decay of exciton was measured by TRMC for our two N-doped samples exhibit some activity under visible range [Citation15], Figure shows the TRMC signals of N-doped and Au/N co-modified TiO2.
The main point of these data is the very low number of excitons created after visible excitation compared to UV excitation (0.001 vs 0.01 V.nein−1). The low number of excitons is easily correlated to the existing but weak efficiency observed for degradation of FA.
However, it clearly illustrates that the efficiency of our materials under visible excitation cannot be considered significant and this point will not be studied any more in the present paper. Moreover in good agreement with this low efficiency, monochromatic visible irradiation is known in literature to produce electron and not holes [Citation37].
3.4. Comparison of EPR and TRMC under UV irradiation
UV-visible photoirradiated Au/TiO2 exhibits both EPR signals due to holes and electrons as observed in classical TiO2 samples. In agreement with a high number of electrons created under UV irradiation, the TRMC signal is of high intensity. Therefore, both techniques tend to indicate efficient surface activity.
The EPR signal observed with N-modified sample is mainly attributed to paramagnetic NO2− species. In TRMC, although the charge carrier creation is the highest in N-TiO2 sample, the electrons are rapidly lost by recombination with surface traps. In fact, the NO2− species seen by EPR under UV-visible irradiation (Figure ), can act as traps for the electrons explaining the low efficiency of these samples.
3.5. Photocatalytic results
We have seen that, all our samples are significantly more active that TiO2 P25 when degradation of FA is considered [Citation15]. In view of addressing more realistic pollutants, our interest was to understand if this result could be extended to more complex reactants while remaining in the same family (i.e. carboxylic acids). Therefore, the degradation of carboxylic acids with more carbon atoms (up to 3) was studied.
3.5.1. Adsorption studies
Reaction at the surface of nanoparticles is a key mechanism in photocatalysis, it is therefore fundamental to investigate the evolution of the adsorption in the dark of the different reactants on the different materials. The adsorption results are presented in Table . It shows that for Laser synthesized powders, adsorption is significantly higher for FA compared to other acids. The difference is not so large for the TiO2 P25 reference sample. Table shows pKa and pH of each acid (pH was measured in solution without nanoparticles). Based on these data the ratio of ionized [A−] to neutral [AH] species is much higher for FA than for acetic or propionic acid. It can explain the very different adsorption of FA compared to acetic and propionic acids (257 μmol g−1 compared 35 or 66 μmol g−1 for example in TiO2 LP). These results are also in agreement with the study by Serpone et al. [Citation44] relating the adsorption behavior on TiO2 to the degree of ionization of acids. Indeed, the lower the pKa, the highest is the degree of ionization thereby favoring adsorption. Serpone et al. also predict a decrease of activity with the increment of the length of the carbon chain.
Table 3. Adsorbed quantities of carboxylic acids at equilibrium under dark for the different reactants and catalysts (μmol g−1).
Table 4. Pka and pH of carboxylic acids (C1, C2, and C3).
3.5.2. Photocatalytic degradation
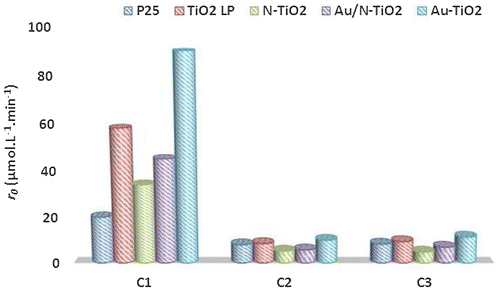
Figure presents the evolution of the concentration of C2 and C3 acids under UV irradiation in presence of modified TiO2 samples. In both cases, similar tendencies can be observed; the curves show that according to their behavior, the powders can be divided into two groups. The first group is composed of N-doped TiO2 samples; they are less active than TiO2 LP. TRMC, XPS and EPR results have shown the presence of nitrogen species that could act as recombination center. Therefore, the lower photocatalytic result is in good agreement with behavior expected from characterization. The second group is composed of P25, TiO2 LP and Au/TiO2 powders presenting a similar photoactivity. As in our previous study, the modification with Au has a positive effect on the photocatalytic efficiency: (Au/N-TiO2 and Au/TiO2 are more active than N-TiO2 and TiO2 LP) [Citation15]. Finally, the photocatalytic activity follows the order: Au/TiO2 > TiO2 LP ≈ P25 > Au/N-TiO2 > N-TiO2 (Figure ).
Figure 5. Photocatalytic decomposition of acetic acid (a) and propionic acid (b) under UV illumination for pure and modified TiO2 samples (the lines are only guides for the eyes).
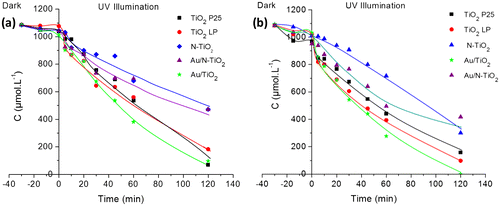
In comparison with our previous results on the degradation of formic acid under UV [Citation15], kinetic decomposition of acetic acid (AA) and propionic acid (PA) is slower as illustrated in Figure . The kinetics of degradation of AA and PA are quite comparable, this seems correlated to adsorption very similar for most samples when these reactants are considered. In the same way, adsorption is significantly smaller for these two reactants compared to FA and the kinetics of degradation are slower, illustrating again the importance of surface reaction in the photocatalytic process. Moreover, the disappearance rates of FA, AA and PA molecules are correlated to the rate constants of OH• attack radicals which are 1.4 108, 1.six 107 and five.7 106 L.mol−1.s−1, respectively [Citation45].
3.6. Intermediate species and mechanism
One interest of using longer carbon chain corresponding to smaller degradation rates is the possibility to observe intermediate species giving information on the mechanisms involved in the degradation processes. Considering both formic and acetic acids, no peak could observed in the chromatograms (HPLC) at the end of the degradation process. Therefore, EPR analyses were carried out to obtain further insight into the nature of intermediates species and to characterize the local environment of the paramagnetic species.
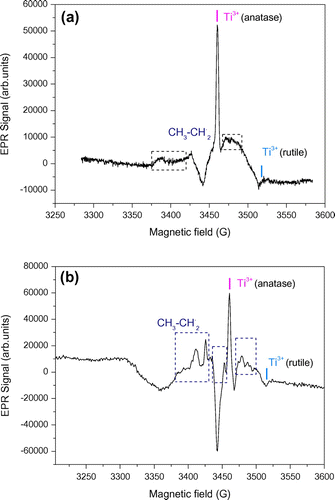
Figure shows the EPR spectra obtained with Au/TiO2 and N-TiO2 samples in the presence of acetic acid, recorded at 60 K and previously irradiated in a quartz cold finger at 365 nm and 77 K. Two intermediates were well identified, they can be assigned to methyl radical ◦CH3 with a proton hyperfine interaction (aH,CH3 = 22.9G), and to carboxymethyl radical ◦CH2-COOH (aH,CH2 = 21.1G). Four other narrow EPR lines (green labels in Figure ) were observed and could be rationalized by a free radical containing two non-equivalent protons (aH1 = 23.2 G, aH2 = 11.4 G). To the best of our knowledge this last species was not reported to date in the literature of carboxylic acid photooxidation by TiO2, and no satisfying hypothesis could be found yet to rationalize it.
Figure 7. EPR spectra of (a) Au/TiO2 and (b) N-TiO2 in presence of acetic acid recorded at 77 K, and irradiated at λ = 365 nm.
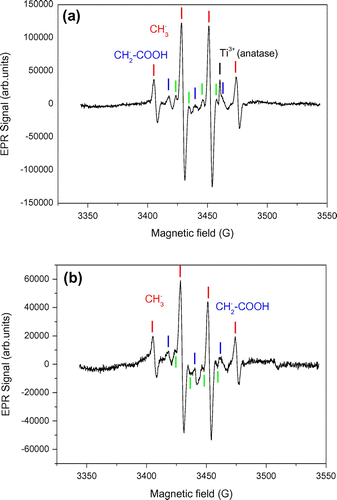
The observation of methyl and carboxymethyl radicals is consistent with the results reported by Kaise et al. [Citation46] who explain their formation by the attack of photogenerated hole of TiO2 and then enter in the steps of photo-Kolbe reaction (Equation (Equation1(1)
(1) )). The same radicals were found by Nosaka et al. [Citation47]. The formation of methyl radical is easily explained by the reaction of AA with a hole at the surface of the particle following reaction (Equation1
(1)
(1) ).
(1)
(1)
The resulting methyl radical can in turn react with AA to form carboxymethyl radical
(2)
(2)
Another possible reaction can generate the carboxymethyl radical (Equation(Equation3(3)
(3) )) [Citation48]
(3)
(3)
These radicals can follow reaction path with O2 to product alcohol, then aldehyde and finally CO2 in the case of ∘CH3 or glycolic acid, glyoxylic acid, formaldehyde and then CO2 in the case of ∘CH2COOH [Citation48].
During propionic acid photodegradation, only acetic acid was detected by HPLC. In this case, again EPR was used to detect eventual intermediates. Figure shows the EPR spectra of Au/TiO2 and N-TiO2 samples in presence of propionic acid, recorded at 77 K and irradiated at 365 nm. Both of spectra show the typical signals corresponding to electron (Ti3+ species) in anatase and rutile environment [Citation32]. Let us note that in this case the narrow Ti3+ signal is easily seen by contrast to the broader features observed in the spectrum. Some of these features could be attributed to ∘CH2 – CH3 radicals by comparison with observation by Shkrob and Chemerisov [Citation49] and appeared more clearly in the experiment performed with N-TiO2. Similarly to the observation by Shkrob and Chemerisov, the hyperfine splitting of ∘CH2 – CH3 free radicals are not well resolved due to their anisotropy. Sakata et al. [Citation50] proposed reaction path that explain the photocatalytic degradation of propionic acid (Equations (Equation4(4)
(4) -Equation7
(7)
(7) )).
Figure 8. EPR spectra of (a) Au/TiO2 and (b) N-TiO2 in presence of propionic acid recorded at 77 K, and irradiated at λ = 365 nm.
(4)
(4)
(5)
(5)
(6)
(6)
(7)
(7)
After these cascade reactions, the final product (acetic acid) will undergo the same reaction which was seen previously.
Finally, when HPLC could not give information (acetic acid), EPR study provides us the detection of intermediate species in the degradation mechanism in good agreement with the literature [Citation47,49]. Considering propionic acid, EPR radicals support the presence of species identified by HPLC and confirm results of the literature [Citation50].
4. Conclusions
The surface modification of titania by gold and/or nitrogen was successfully performed by using laser pyrolysis method. The as-synthesized materials show higher activity compared to TiO2 P25 under UV illumination for degradation of model organic pollutants (formic, acetic and propionic acids). However, co-modification of laser synthesized TiO2 with N and Au induces a decrease of the degradation rate of carboxylic acids in comparison with single modification by Au. TRMC results showed that under irradiation with UV light, the modification by Au improves photocatalytic efficiency of TiO2 due to more efficient transfer of electrons to the surface. On the contrary, presence of N causes recombination of charges, and thus gives rise to lower photoactivity in both Au/N-TiO2 and N-TiO2 samples. EPR investigations confirmed the presence of two radical species in N-TiO2 and Au/N-TiO2 samples. That result has never been observed with N-TiO2 synthesized by other methods, it seems to be specific to nanoparticles obtained by laser pyrolysis. As a complement, the intermediate species detected by EPR and HPLC allow proposing a reaction mechanism involved in the photo-oxidation of aliphatic organic acids. In order to improve photo-efficiency, it would be interesting to increase the size of gold nanoparticles and take advantage of surface plasmon resonance favoring activity in the visible range. In the same way, a better control of N doping favoring interstitial or substitutional doping would allow decreasing recombination of charges.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
The supplemental material for this paper is available online at https://doi.org/10.1080/14686996.2017.1379858
sup_data_200617.docx
Download MS Word (101.9 KB)References
- Pelaez M, Nolan NT, Pillai SC, et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B Environ. 2012;125(1):331–349. 10.1016/j.apcatb.2012.05.036
- Lan Y, Lu Y, Ren Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy. 2013;2(5):1031–1045.
- Asahi R, Morikawa T, Irie H, et al. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: designs, developments, and prospects. Chem Rev. 2014;114;114(19):9824–9852. 10.1021/cr5000738
- Chatterjee D, Dasgupta S. Visible light induced photocatalytic degradation of organic pollutants. J Photochem Photobiol C Photochem Rev. 2005;6(2-3):186–205. 10.1016/j.jphotochemrev.2005.09.001
- Daghrir R, Drogui P, Robert D. Modified TiO2 for environmental photocatalytic applications: a review. Ind Eng Chem Res. 2013;52:3581–3599. 10.1021/ie303468t
- Kang QM, Yuan BL, Xu JG, et al. Synthesis, characterization and photocatalytic performance of TiO2 codoped with bismuth and nitrogen. Catal Lett. 2011;141(9):1371–1377. 10.1007/s10562-011-0629-8
- Liu H, Dong X, Li G, et al. Synthesis of C, Ag co-modified TiO2 photocatalyst and its application in waste water purification. Appl Surf Sci. 2013;271:276–283. 10.1016/j.apsusc.2013.01.181
- Gomathi Devi L, Kavitha R. Review on modified N-TiO2 for green energy applications under UV/visible light: selected results and reaction mechanisms. RSC Adv. 2014;4(54):28265–28299. 10.1039/C4RA03291H
- Mahy JG, Lambert SD, Léonard GL, et al. Towards a large scale aqueous sol-gel synthesis of doped TiO2: study of various metallic dopings for the photocatalytic degradation of p-nitrophenol. Journal Photochem Photobiol A Chem. 2016;329:189–202. 10.1016/j.jphotochem.2016.06.029
- Gnanasekaran L, Hemamalini R, Saravanan R, et al. Intermediate state created by dopant ions (Mn, Co and Zr) into TiO2 nanoparticles for degradation of dyes under visible light. J Mol Liq. 2016;223:652–659. 10.1016/j.molliq.2016.08.105
- Jafari S, Mohammadi MR, Madaah Hosseini HRM. Impact of morphology and nitrogen and carbon codoping on photocatalytic activity of TiO2 as environmental catalysts. Ind Eng Chem Res. 2016;55:12205–12212. 10.1021/acs.iecr.6b03053
- Zaleska A. Doped-TiO2: a review. Recent Patents Eng. 2008;2(3):157–164. 10.2174/187221208786306289
- Grabowska E, Marchelek M, Klimczuk T, et al. Noble metal modified TiO2 microspheres: Surface properties and photocatalytic activity under UV-vis and visible light. J Mol Catal A Chem. 2016;423:191–206. 10.1016/j.molcata.2016.06.021
- Méndez-Medrano MG, Kowalska E, Lehoux A, et al. Surface modification of TiO2 with Au nanoclusters for efficient water treatment and hydrogen generation under visible light. J Phys Chem C. 2016;120(43):25010–25022. 10.1021/acs.jpcc.6b06854
- Bouhadoun S, Guillard C, Dapozze F, et al. One step synthesis of N-doped and Au-loaded TiO2 nanoparticles by laser pyrolysis: application in photocatalysis. Appl Catal B Environ. 2015;174-175:367–375. 10.1016/j.apcatb.2015.03.022
- Livraghi S, Paganini MC, Giamello E, et al. Origin of photoactivity of nitrogen-doped titanium dioxide under visible light. J Am Chem Soc. 2006 Dec 13;128(49):15666–15671. 10.1021/ja064164c
- Livraghi S, Votta A, Paganini MC, et al. The nature of paramagnetic species in nitrogen doped TiO2 active in visible light photocatalysis. Chem Commun. 2005;4:498–500. 10.1039/b413548b
- Di Valentin C, Finazzi E, Pacchioni G, et al. N-doped TiO2: theory and experiment. Chem Phys. 2007;339(1-3):44–56. 10.1016/j.chemphys.2007.07.020
- Hai Z, El Kolli N, Uribe DB, et al. Modification of TiO2 by bimetallic Au–Cu nanoparticles for wastewater treatment. J Mater Chem A. 2013;1(36):10829. 10.1039/c3ta11684 k
- Hoffmann MR, Martin ST, Choi W, et al. Environmental applications of semiconductor photocatalysis. Am Chem Soc. 1995;95:69–96.
- Colbeau-Justin C, Kunst M, Huguenin D. Structural influence on charge-carrier lifetimes in TiO2 powders studied by microwave absorption. J Mater Sci. 2003;38(11):2429–2437. 10.1023/A:1023905102094
- Tahiri Alaoui O, Herissan A, Le Quoc C, et al. Elaboration, charge-carrier lifetimes and activity of Pd-TiO2 photocatalysts obtained by gamma radiolysis. J Photochem Photobiol A. 2012;242:34–43. 10.1016/j.jphotochem.2012.05.030
- Fonash SJ. Solar cell device physics. New York, NY: Academy Press; 1981.
- Asahi R, Morikawa T, Ohwaki T, et al. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 2001;293(5528):269–271. 10.1126/science.1061051
- Yang G, Jiang Z, Shi H, et al. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J Mater Chem. 2010;20(25):5301–5309. 10.1039/c0jm00376j
- Duta L, Popescu C, Popescu A, et al. Nitrogen-doped and gold-loaded TiO2 photocatalysts synthesized by sequential reactive pulsed laser deposition. Appl Phys A. 2014;117(1): 97–101.
- Gazsi A, Schubert G, Pusztai P, et al. Photocatalytic decomposition of formic acid and methyl formate on TiO2 doped with N and promoted with Au. Production of H2. Int J Hydrogen Energy. 2013;38(19):7756–7766. 10.1016/j.ijhydene.2013.04.097
- Amadelli R, Samiolo L, Borsa M, et al. N-TiO2 Photocatalysts highly active under visible irradiation for NOX abatement and 2-propanol oxidation. Catal Today. 2013;206(2):19–25. 10.1016/j.cattod.2011.11.031
- Wang J, Tafen DN, Lewis JP, et al. Origin of photocatalytic activity of Nitrogen-doped TiO2 nanobelts. J Am Chem Soc. 2009;131(34):12290–12297. 10.1021/ja903781 h
- Asahi R, Morikawa T. Nitrogen complex species and its chemical nature in TiO2 for visible-light sensitized photocatalysis. Chem Phys. 2007;339(1-3):57–63. 10.1016/j.chemphys.2007.07.041
- Okumura M, Coronado JM, Soria J, et al. EPR Study of CO and O2 interaction with supported Au catalysts. J Catal. 2001;203(1):168–174. 10.1006/jcat.2001.3307
- Kumar CP, Gopal NO, Wang TC. EPR investigation of TiO2 nanoparticles with temperature-dependent properties. J Phys Chem B. 2006;110:5223–5229. 10.1021/jp057053t
- Li Y, Hwang DS, Lee NH, et al. Synthesis and characterization of carbon-doped titania as an artificial solar light sensitive photocatalyst. Chem Phys Lett. 2005;404(1-3):25–29. 10.1016/j.cplett.2005.01.062
- Livraghi S, Paganini MC, Chiesa M, et al. Trapped molecular species in N-doped TiO2. Res Chem Intermed. 2007;33(8):739–747. 10.1163/156856707782169462
- Livraghi S, Chierotti MR, Giamello E, et al. Nitrogen-doped titanium dioxide active in photocatalytic reactions with visible light: a multi-technique characterization of differently prepared materials. J Phys Chem C. 2008;112(44):17244–17252. 10.1021/jp803806s
- Di Valentin C, Pacchioni G, Selloni A, et al. Characterization of paramagnetic species in N-Doped TiO2 powders by EPR spectroscopy and DFT calculations. J Phys Chem B. 2005;109(23):11414–11419.
- Barolo G, Livraghi S, Chiesa M, et al. Mechanism of the photoactivity under visible light of N-doped titanium dioxide. charge carriers migration in irradiated N-TiO2 investigated by electron paramagnetic resonance. J Phys Chem C. 2012;116(39):20887–20894. 10.1021/jp306123d
- Konstantinova EA, Kokorin AI, Lips K, et al. EPR study of the illumination effect on properties of paramagnetic centers in nitrogen-doped TiO2 active in visible light photocatalysis. Appl Magn Reson. 2009;35(3):421–427. 10.1007/s00723-009-0173-5
- Howe RF, Gratzel M. EPR observation of trapped electron in colloidal TiO2. J Phys Chem. 1985;89(21):4495–4499. 10.1021/j100267a018
- Meichtry JM, Colbeau-Justin C, Custo G, et al. TiO2-photocatalytic transformation of Cr (VI) in the presence of EDTA : comparison of different commercial photocatalysts and studies by time resolved microwave conductivity. Appl Catal B Environ. 2014;144:189–195. 10.1016/j.apcatb.2013.06.032
- Boujday S, Wünsch F, Portes P, et al. Photocatalytic and electronic properties of TiO2 powders elaborated by sol–gel route and supercritical drying. Sol Energy Mater Sol Cells. 2004;83(4):421–433. 10.1016/j.solmat.2004.02.035
- Emilio CA, Litter MI, Kunst M, et al. Phenol photodegradation on platinized-TiO2 photocatalysts related to charge-carrier dynamics. Langmuir. 2006;22(8):3606–3613. 10.1021/la051962s
- Kumar SG, Devi LG. Review on modified TiO2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J Phys Chem A. 2011;115(46):13211–13241. 10.1021/jp204364a
- Serpone N, Martin J, Horikoshi S, et al. Photocatalyzed oxidation and mineralization of C1–C5 linear aliphatic acids in UV-irradiated aqueous titania dispersions—kinetics, identification of intermediates and quantum yields. J Photochem Photobiol A Chem. 2005;169(3):235–251. 10.1016/j.jphotochem.2004.07.001
- Buxton GV, Greenstock CL, Helman WP, et al. Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (.OH/.O-) in aqueous-solution. J Phys Chem. 1988;17: 513–886.
- Kaise M, Nagai H, Tokuhashi K, et al. Electron spin resonance studies of photocatalytic interface reactions of suspended M/TiO2 (M = Pt, Pd, Ir, Rh, Os, or Ru) with alcohol and acetic acid in aqueous media. Langmuir. 1994;10(5):1345–1347. 10.1021/la00017a005
- Nosaka Y, Koenuma K, Ushida K, et al. Reaction mechanism of the decomposition of acetic acid on illuminated TiO2 powder studied by means of in situ electron spin resonance measurements. Langmuir. 1996;12:736–738. 10.1021/la9509615
- Gandhi V, Mishra M, Joshi PA. Titanium dioxide catalyzed photocatalytic degradation of carboxylic acids from waste water: a review. Mater Sci Forum. 2012;712:175–189. 10.4028/www.scientific.net/MSF.712
- Shkrob IA, Chemerisov SD. Light induced fragmentation of polyfunctional carboxylated compounds on hydrated metal oxide particles: from simple organic acids to peptides. J Phys Chem C. 2009;113(39):17138–17150. 10.1021/jp906250w
- Sakata T, Kawai T, Hashimoto K. Heterogeneous photocatalytic reactions of organic acids and water. New reaction paths besides the photo-Kolbe reaction. J Phys Chem. 1984;88(11):2344–2350. 10.1021/j150655a032