Abstract
In this work, CsPbBr3 and PbSe nanocomposites were synthesized to protect perovskite material from self-enlargement during reaction. UV absorption and photoluminescence (PL) spectra indicate that the addition of Se into CsPbBr3 quantum dots modified the electronic structure of CsPbBr3, increasing the band gap from 2.38 to 2.48 eV as the Cs:Se ratio increased to 1:3. Thus, the emission color of CsPbBr3 perovskite quantum dots was modified from green to blue by increasing the Se ratio in composites. According to X-ray diffraction patterns, the structure of CsPbBr3 quantum dots changed from cubic to orthorhombic due to the introduction of PbSe at the surface. Transmission electron microscopy and X-ray photoemission spectroscopy confirmed that the atomic distribution in CsPbBr3/PbSe composite clusters is uniform and the composite materials were well formed. The PL intensity of a CsPbBr3/PbSe sample with a 1:1 Cs:Se ratio maintained 50% of its initial intensity after keeping the sample for 81 h in air, while the PL intensity of CsPbBr3 reduced to 20% of its initial intensity. Therefore, it is considered that low amounts of Se could improve the stability of CsPbBr3 quantum dots.
1. Introduction
Nowadays, much of the research concerning halide perovskites is focused on the new generation of solar cells, light-emitting diodes, transistors, lasers, and memristors with thin film, nanowire, and nanorod structures [Citation1–6]. The power conversion efficiency of lead halide perovskite-based thin film photovoltaic cells has improved fast, from 3.8% to over 20% in a decade [Citation7]. Among organic and inorganic perovskite materials, cesium lead halide has shown better stability than organic perovskite materials, suggesting great potential for use in opto-electronic devices.
Research on the morphology of perovskites shows that nano-size materials display superior properties in comparison with bulk-size materials, such as higher quantum yield, narrower emission bandwidth, and tunable color [Citation8,9]. Recently, it was reported that cesium lead halide quantum dots have promisingly high color purity for light-emitting diodes [Citation10,11]. Furthermore, it was shown that the growth rate, structure and size of CsPbBr3 dots can be controlled by changing the length of carbon chains of acids and amines in ligands during synthesis [Citation12]. This allows the possibility for finding the optimal structure and size for cesium lead halide quantum dots. However, the thermal and electrical stability of cesium lead halide quantum dots is still limited.
One particular organo-halide perovskite material, CH3NH3PbI3, has been used as the shell of PbS quantum dots. It was reported that PbS/CH3NH3PbI3 core/shell quantum dots can increase the performance of sensitized solar cells because CH3NH3PbI3 covered the PbS core as a passive shell, improving the quantum efficiency and air stability [Citation13]. Perovskite CH3NH3PbI3 was grown to cover the PbS quantum dots, which had been formed on a substrate. PbI2 was used as the Pb source of core PbS and the shell CH3NH3PbI3 layer. Nanocomposite synthesis with same base atom was reported in case of Nd2Sn2O7–SnO2, Dy2Sn2O7–SnO2, Nd2Zr2O7–ZrO2, and Ho2O3–SiO2 [Citation14–21]. The recent reports about protecting perovskite materials were summarized in Table [Citation22–27].
Table 1. Recent methods to protect perovskite materials.
A nanocomposite material of CsPbBr3 and PbSe was synthesized to protect perovskite material from self-enlargement during reaction. As a core material, CsPbBr3 quantum dots were chosen because CsPbBr3 is the most stable among cesium lead halides. It is reported that materials with low lattice mismatch could easily form the core/shell structure such as CdSe/CdS (3.9% lattice mismatch) [Citation28] or CdSe/ZnS (12% lattice mismatch) [Citation29]. The lattice constant of cubic phase CsPbBr3 is known to be 5.87 Å and that of PbSe is reported to be 6.1 Å [Citation30,31]. Therefore, the lattice mismatch between CsPbBr3 and PbSe is approximately 4%. The same source of Pb2+ in an organic solvent could be used to produce CsPbBr3 and PbSe. Furthermore, trioctylphosphine selenide can be used as an easy source for the PbSe shell layer. It is therefore expected that CsPbBr3/PbSe composites could be synthesized to improve the stability of inorganic perovskite quantum dots.
2. Experimental details
2.1. Materials
Lead bromide (PbBr2) 98%, cesium carbonate (Cs2CO3) 98%, octadecene (ODE) 90%, oleic acid 90% technique grade (OA), and oleyl amine (OAm) 95% were purchased from Sigma-Aldrich and used without further purification.
2.2. Synthesis of CsPbBr3 quantum dots
The synthesis of CsPbBr3 follows the previously reported procedures [Citation32]. 70 mg of PbBr2 was loaded into a 100 mL flask along with 10 mL of ODE, 1 mL of OA, and 0.5 mL of OAm. The solution was then heated up to 120 °C under N2 to completely disperse PbBr2. Cesium oleate was prepared by loading 0.814 g of Cs2CO3 along with 30 mL of ODE and 2.5 mL of OA into a 100 mL flask. The temperature of the solution was increased to 120 °C for the completion of cesium oleate. After heating the PbBr2 solution to 190 °C, 0.4 mL of cesium oleate heated at 100 °C was injected to complete the synthesis of CsPbBr3 quantum dots. The mixture was cooled down in an ice water bath. Quantum dots were precipitated and washed using hexane and butanol with a 1:1 volume ratio. After that, quantum dots were dispersed into toluene for further use.
2.3. Synthesis of CsPbBr3/PbSe nanocomposite
The selenide source was prepared by loading 10 mg of selenium in 0.5 mL of trioctylphosphine (TOP) and 0.5 mL of ODE. The mixture was heated up to 140 °C in 1 h. The 0.2 mL of TOPSe was quickly added into the solution of PbBr2 with cesium oleate. The solution was kept at 190 °C for 1 h. Then, the color of the solution changed from light green to dark green. The materials were precipitated and washed using hexane and 2-butanol. The synthesized materials were re-dispersed in toluene for further use. In order to investigate the effect of synthesis temperature and the concentration of Se on the properties of the CsPbBr3/PbSe nanocomposite, samples were produced at synthesis temperatures of 150, 170, and 190 °C, and using 0.2, 0.4, and 0.6 mL of TOP-Se to match a Cs:Se mol ratio of 1:1, 1:2, and 1:3, respectively.
2.4. Characterization
A JASCO V-670 UV-vis spectrophotometer with a xenon arc lamp and PMT-1527 Hamamatsu photomultiplier was used to measure UV-visible absorbance and photoluminescence (PL) of the materials. X-ray diffraction (XRD, D8-Advance/Bruker-AXS), field-emission scanning electron microscopy (FE-SEM, SIGMA/Carl Zeiss), and transmission electron microscopy (TEM, JEOL-2100F, Japan) were applied to measure the structures and sizes of CsPbBr3 and CsPbBr3/PbSe composites. Synchrotron radiation photoemission spectroscopy (SRPES) experiments were also performed in an ultra-high-vacuum chamber (base pressure of ~10−10 Torr) at the 4D beam line of the Pohang Acceleration Laboratory. The onset of photoemission, corresponding to the vacuum level at the sample surface, was measured using an incident photon energy of 250 eV with a negative bias on the sample. The results were corrected for charging effects using Au 4f as an internal reference.
3. Results and discussion
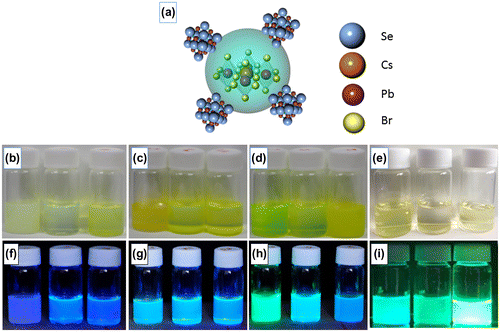
The expected structure of CsPbBr3/PbSe composite is schematically drawn in Figure (a). Figure (b)–(i) show photographs of CsPbBr3/PbSe composites and pristine CsPbBr3 in hexane solution under normal light ((b)–(e)) and under a UV lamp ((f)–(i)). For comparison, the pristine CsPbBr3 is synthesized at 150, 170, and 190 °C from left to right as shown in Figure (e) and (i). The ratio of Cs:Se in photographs of CsPbBr3/PbSe composites is 1:1, 1:2, and 1:3 from left to right. The synthesis temperatures are 150 °C ((b), (f)), 170 °C ((c), (g)), and 190 °C ((d), (h)). The wavelength of the UV lamp was 365 nm. The colors of the composite solutions synthesized at 150 °C are all blue under UV illumination. At 170 °C, the solutions emitted light of cyan color under UV, suggesting that the light shifted to higher wavelength as the synthesis temperature increased. Composite materials synthesized at 190 °C emitted green light under UV illumination and the color of emitted light changed from green to cyan as the concentration of Se increased. These results indicate that the emission wavelength of CsPbBr3/PbSe composites under UV illumination increased as the synthesis temperature increased. Furthermore, the increase of Se concentration in CsPbBr3/PbSe shortened the wavelength of emitted light under UV illumination. The synthesis of perovskite quantum dots via solution methods is quite sensitive. A large size was simple to achieve due to the easy bonding of anion and cation. According to previous reports, the CsPbBr3 quantum dot solution was cooled down immediately after finishing the reaction [Citation32,33]. In our case, the CsPbBr3 quantum dot solution was heated for 1 h after adding TOPSe to make CsPbBr3/PbSe composites. However, luminescence was detected under UV illumination as shown in Figure . This means that the crystal structure of CsPbBr3 was maintained even after a longer heating process and CsPbBr3 was protected by PbSe.
Figure 1. (a) Schematic of the expected CsPbBr3/PbSe nanocomposite structure. Photographs of CsPbBr3/PbSe nanocomposite in hexane solution under normal ((b)–(d)) and UV ((f)–(h)) illumination with Cs:Se ratios of 1:1, 1:2, and 1:3 synthesized at 150 °C ((b), (f)), 170 °C ((c), (g)) and 190 °C ((d), (h)) from left to right, respectively. For comparison, the photographs of pristine CsPbBr3 under (e) normal and (i) UV illumination are also shown.
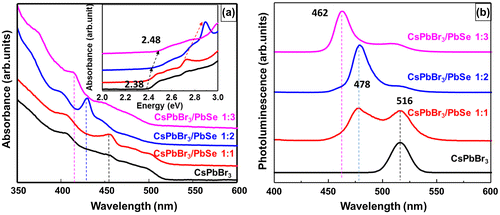
To investigate the effects of the Se content of CsPbBr3/PbSe on its optical properties, UV-vis and PL spectra were measured. Figure shows (a) UV-vis absorption spectra and (b) PL spectra of CsPbBr3 quantum dots and composites of CsPbBr3/PbSe with different Cs:Se ratios (1:1, 1:2, and 1:3). The synthesis temperature of the CsPbBr3/PbSe composites was fixed at 190 °C. The absorption peak appeared at 455 nm in CsPbBr3 quantum dots and in CsPbBr3/PbSe composites with a 1:1 Cs:Se ratio, which corresponds to an optical band gap of 2.38 eV as shown in the inset of Figure (a). In the case of CsPbBr3/PbSe composites with a 1:2 Cs:Se ratio, the absorption peak shifts to 429 nm, indicating that the band gap is increased to 2.42 eV. A Cs:Se ratio of 1:3 shifts the absorption peak to 414 nm, further increasing the band gap to 2.48 eV. These results indicate that the addition of Se into CsPbBr3 quantum dots modifies the electronic structure of CsPbBr3. This result is in agreement with the PL spectra as shown in Figure (b). The CsPbBr3 quantum dots emit green color with a wavelength between 500 and 550 nm. The PL spectra of CsPbBr3/PbSe composites with a 1:1 Cs:Se ratio indicate that their cyan color consists of a blue peak at 478 nm and a green peak at 516 nm. As the Cs:Se ratio increased from 1:1 to 1:3, the PL peak shifted to 462 nm. These results indicate that the emission color of CsPbBr3 perovskite quantum dots could be modified from green to blue by adjusting the Se ratio in composites.
Figure 2. (a) UV-visible absorbance, and (b) photoluminescence spectra of bare CsPbBr3 quantum dots and CsPbBr3/PbSe nanocomposites. Spectra are vertically shifted for clarity.
In order to confirm the formation of PbSe on the surface of CsPbBr3 quantum dots, XRD measurements were performed. Figure shows the XRD patterns of CsPbBr3 quantum dots and CsPbBr3/PbSe composites with different Cs:Se ratios (1:1, 1:2, and 1:3). The synthesis temperature of the CsPbBr3/PbSe composites was fixed at 190 °C. The CsPbBr3 quantum dots show two peaks at 15.16 and 30.6°, indicating that the synthesized CsPbBr3 quantum dots have a cubic structure with (1 0 0) and (2 0 0) planes. This result agrees well with previous reports on CsPbBr3 quantum dots synthesized at above 140 °C [Citation32,34,35]. In the CsPbBr3/PbSe composite with a 1:1 Cs:Se ratio, new peaks appeared at 12.8 and 29.1° which correspond to the (1 0 0) and (2 0 0) planes of PbSe [Citation36,37]. Furthermore, the (1 0 0) and (2 0 0) peaks of cubic CsPbBr3 disappeared and new peaks at 14 and 16° appeared, which are assigned to the (1 0 0) and (1 0 1) planes of orthorhombic CsPbBr3 [Citation35]. This means that the structure of CsPbBr3 quantum dots changed from cubic to orthorhombic due to the introduction of PbSe at the surface. In the case of the CsPbBr3/PbSe composite with a 1:2 Cs:Se ratio, the peaks corresponding to PbSe (1 0 0) and (2 0 0) planes shifted to higher angles of 13 and 29°, respectively, suggesting that the plane distance of PbSe increased following the increase in Se. It is reported that PbSe has a cubic rocksalt structure with a lattice parameter of 6.12 Å and CsPbBr3 has perovskite structure with a lattice parameter of 5.87 Å [Citation30,31]. Therefore, it is considered that PbSe was compressed due to the smaller lattice parameter of CsPbBr3 and that the lattice parameter of PbSe was restored as Se content increased. It is reported that orthorhombic phase has higher band gap than cubic phase. However, the photoluminescence is inactive in cubic-to-orthorhombic crystal transformation [Citation26]. But, PL peak shift was found as shown in Figure . Therefore, band gap enlargement could be explained by Se doping. The (2 0 0) peak of CsPbBr3 was absent in XRD spectra. However, the (1 0 0) peak of PbSe and peaks of orthorhombic CsPbBr3 and (1 0 0) cubic CsPbBr3 appeared in XRD patterns with weak intensity. The full width at half maximum of PbSe and CsPbBr3 peaks also increased, indicating the size becomes smaller. Therefore, it is considered that the structure of cubic CsPbBr3 is broken as the ratio of Se increased. This result agrees with the data of absorbance and photoluminescence as shown in Figure .
Figure 3. X-ray diffraction patterns of bare CsPbBr3 and CsPbBr3/PbSe nanocomposites with Cs:Se ratios of 1:1, 1:2, and 1:3 synthesized at 190 °C. The peaks of orthorhombic CsPbBr3 confirm the distortion of the lattice when forming PbSe beside the CsPbBr3 quantum dots in the 1:1 Cs:Se ratio sample.
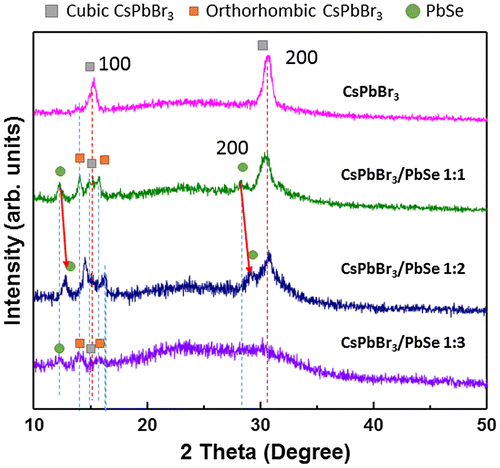
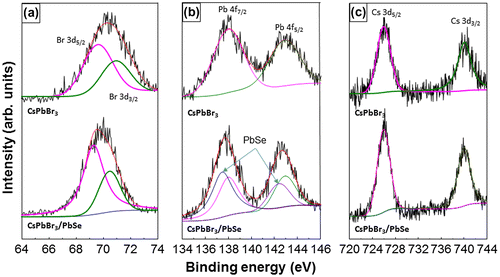
Figure shows SRPES spectra of CsPbBr3 quantum dots and CsPbBr3/PbSe composite synthesized at 190 °C with a Cs:Se ratio of 1:3. The two peaks of Cs 3d5/2 and Cs 3d3/2 are shown at 726.1 and 740 eV, respectively. The Br 3d5/2 and Br 3d3/2 peaks are also shown at 69.3 and 70.4 eV, respectively. No peak change was observed in Cs 3d and Br 3d after the formation of PbSe at the surface of CsPbBr3. In the case of the Pb 4f peak, two peaks are shown at 138.1 and 143 eV in CsPbBr3 quantum dots, which correspond to Pb 4f7/2 and Pb 4f5/2, respectively. Additional peaks appeared at 137.5 and 142.4 eV in the CsPbBr3/PbSe composite, which indicates the formation of Pb-Se bonds. These values are compatible with previous research on CsPbBr3 and PbSe nanocrystals [Citation38–40]. These results indicate that PbSe was formed well on the surface of CsPbBr3 quantum dots.
Figure 4. SRPES spectra of CsPbBr3 quantum dots and CsPbBr3/PbSe nanocomposites. The formation of PbSe peaks can be seen in the Pb 4f spectrum of the CsPbBr3/PbSe nanocomposites. The Br 3d and Cs 3d peaks do not show large changes, suggesting that the perovskite structure was unchanged by the PbSe growth.
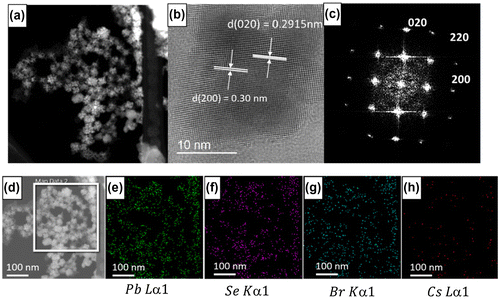
The size of CsPbBr3 quantum dots and CsPbBr3/PbSe composite with a 1:3 Cs:Se ratio was measured using FE-SEM (Figure ) and TEM (Figure ). It is shown that the average size of CsPbBr3 quantum dots is approximately 15 nm and that of CsPbBr3/PbSe composite is approximately 20–25 nm. This suggests that PbSe is attached to the surface of CsPbBr3, increasing the size by approximately 5–8 nm. The magnified images shown in the insets of Figure (a) and (b) indicate that CsPbBr3 is mono-dispersed well and CsPbBr3/PbSe forms a cluster. Figure (c) shows the size distribution of CsPbBr3/PbSe composite clusters according to synthesis temperature. The average size of particles synthesized at 150, 170, and 190 °C is 6–8, 8–10, and 16–22 nm, respectively. These results support the conclusion that the emission wavelength of CsPbBr3/PbSe composites under UV illumination increased as the synthesis temperature increased (see Figures and ). The CsPbBr3/PbSe composite clusters were examined by TEM as shown in Figure . The (0 2 0) plane distance of CsPbBr3 was measured as 0.29 nm, and the (2 0 0) plane distance of PbSe had a similar value of 0.30 nm. Therefore, it is considered that the (0 2 0) plane of CsPbBr3 connects with the (2 0 0) plane of PbSe, forming the CsPbBr3/PbSe composite clusters. Energy dispersive X-ray spectroscopy (EDS) mapping of Pb, Se, Br, and Cs atoms confirmed that the atomic distribution in CsPbBr3/PbSe composite clusters is uniform and the composite materials were well-formed.
Figure 5. FE-SEM images of (a) CsPbBr3 quantum dots and (b) CsPbBr3/PbSe nanocomposite. Monodispersed CsPbBr3 quantum dots have a diameter of approximately 10 nm and nanocomposite clusters are approximately 20–23 nm in size. The scale bars are 100 nm. (c) Size distribution of CsPbBr3/PbSe synthesized at 150, 170, and 190 °C.
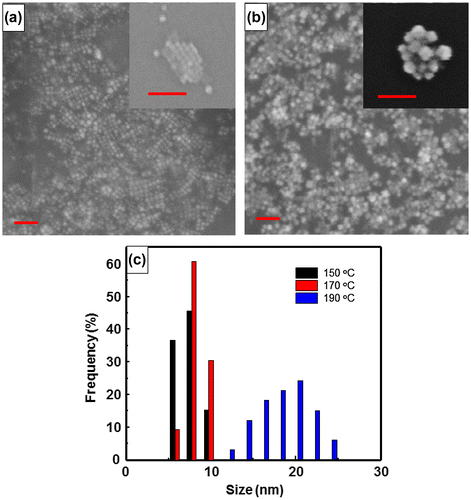
Figure 6. ((a), (d)) TEM images of CsPbBr3/PbSe nanocomposite, (b) high-resolution TEM (HRTEM) image, (c) selected area electron diffraction (SAED) pattern, and EDS atomic mapping of (e) Pb, (f) Se, (g) Br, and (h) Cs. The HRTEM and SAED patterns show the (0 2 0) plane of CsPbBr3 and the (2 0 0) plane of PbSe with plane distances of approximately 0.3 nm.
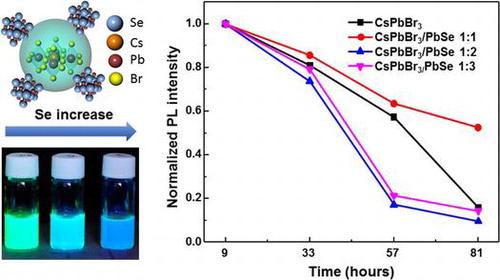
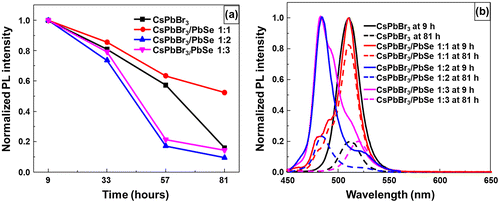
In order to test their stability, CsPbBr3 quantum dots and CsPbBr3/PbSe composites synthesized at 190 °C were dispersed in hexane and kept in air. Figure (a) shows the change in normalized PL as a function of time. The PL was measured every 24 h from the starting point of measurement. After 33 h, the PL intensity had degraded to around 70–80% of its initial intensity regardless of samples. After 57 h, PL values of CsPbBr3/PbSe samples with Cs:Se ratios of 1:2 and 1:3 dramatically decreased to approximately 20% of its initial intensity, suggesting that PbSe could not elongate CsPbBr3 stability. In the case of CsPbBr3 quantum dots, the PL intensity reduced to 20% of its initial value after 81 h. However, CsPbBr3/PbSe with a 1:1 Cs:Se ratio still maintained 50% of its initial intensity even after 81 h, suggesting that low amounts of Se could improve the stability of CsPbBr3 quantum dots. Figure (b) shows normalized PL spectra after storage for 9 and 81 h. The reduction of photoluminescence after keeping samples in room condition was found even though the peak position was maintained. However, the shift peak to higher wavelength was found in CsPbBr3/PbSe composite synthesized at 190 °C with a Cs:Se ratio of 1:3. This result indicated that large amount of PbSe with CsPbBr3 could not protect perovskite quantum dots. We suppose the effect of PbSe with CsPbBr3 due to the stress of lattice mismatch. CsPbBr3 bonded due to the halide bonding that lead easily degrading in an ionic solvent or a humidity condition [Citation32,41]. The high concentration of Se could cause the high stretch leading the degradation of CsPbBr3. With low concentration, the low stretch is not effect on CsPbBr3 and also protect the itself enlargement then improve the stability.
Figure 7. Stability of materials in hexane solution, stored under ambient air, as evaluated by photoluminescence measurements: (a) pristine and composite perovskites compared at 9, 33, 57, and 81 h; (b) normalized photoluminescence spectra of CsPbBr3 and CsPbBr3/PbSe 1:1, 1:2, 1:3 kept for 9 and 81 h.
4. Conclusions
CsPbBr3/PbSe composite clusters were synthesized by adding TOPSe into a solution of PbBr2 with cesium oleate. As the ratio of Cs:Se increased from 1:1 to 1:3, CsPbBr3 partially transformed from cubic structure to orthorhombic structure and bonded with PbSe crystal, forming the composite cluster. The addition of Se into CsPbBr3 quantum dots modified the electronic structure of CsPbBr3, increasing the band gap from 2.38 to 2.48 eV as the Cs:Se ratio increased to 1:3. Therefore, the emission color of CsPbBr3 perovskite quantum dots was modified from green to blue by increasing the Se ratio in composites. The average size of CsPbBr3 quantum dots was approximately 15 nm and that of CsPbBr3/PbSe composite with a 1:3 Cs:Se ratio was approximately 20–23 nm. According to SEM and TEM images, CsPbBr3/PbSe composite exists in cluster form and each atom is uniformly distributed in the whole composite. The PL intensity of a CsPbBr3/PbSe sample with a 1:1 Cs:Se ratio maintained 50% of its initial intensity after keeping the sample for 81 h in air, while the PL intensity of CsPbBr3 quantum dots reduced to 20% of their initial intensity. These results suggested that low amounts of Se could improve the stability of CsPbBr3 quantum dots. Therefore, it is considered that this synthesis method for CsPbBr3/PbSe composites could be used for other perovskite materials to increase the stability.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research was supported in part by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) [grant number 2015K1A3A1A59073839] and in part by the Chung-Ang University Research Grants in 2016.
References
- Li G , Rivarola FWR , Davis NJLK , et al . Highly efficient perovskite nanocrystal light-emitting diodes enabled by a universal crosslinking method. Adv Mater. 2016;28:3528–3534.10.1002/adma.201600064
- Li X , Wu Y , Zhang S , et al . CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv Func Mater. 2016;26:2435–2445.10.1002/adfm.v26.15
- Jeon NJ , Noh JH , Kim YC , et al . Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat Mater. 2014;13:897–903.10.1038/nmat4014
- Xiao ZG , Huang JS . Energy-efficient hybrid perovskite memristors and synaptic devices. Adv Electron Mater. 2016;2:1600100.
- Zhu H , Fu Y , Meng F , et al . Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat Mater. 2015;14:636–642.10.1038/nmat4271
- Chin XY , Cortecchia D , Yin J , et al . Lead iodide perovskite light-emitting field-effect transistor. Nat Commun. 2015;6:7383–7391.10.1038/ncomms8383
- Lee MM , Teuscher J , Miyasaka T , et al . Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science. 2012;338:643–647.10.1126/science.1228604
- Swarnkar A , Chulliyil R , Ravi VK , et al . Colloidal CsPbBr3 perovskite nanocrystals: luminescence beyond traditional quantum dots. Angew Chem Int Ed. 2015;54:15424–15428.10.1002/anie.201508276
- Zhang Y-X , Wang H-Y , Zhang Z-Y , et al . Photoluminescence quenching of inorganic caesium lead halides perovskite quantum dots (CsPbX3) by electron/hole acceptor. Phys Chem Chem Phys. 2017;19:1920–1926.10.1039/C6CP04083G
- Zhang X , Lin H , Huang H , et al . Enhancing the brightness of caesium lead halide perovskite nanocrystal based green light-emitting devices through the interface engineering with perfluorinated ionomer. Nano Lett. 2016;16:1415–1420.10.1021/acs.nanolett.5b04959
- Du X , Wu G , Cheng J , et al . High-quality CsPbBr3 perovskite nanocrystals for quantum dot light-emitting diodes. RSC Adv. 2017;7:10391–10396.10.1039/C6RA27665B
- Pan A , He B , Fan X , et al . Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: the role of organic acid, base, and caesium precursors. ACS Nano. 2016;10:7943–7954.10.1021/acsnano.6b03863
- Seo G , Seo J , Ryu S , et al . Enhancing the performance of sensitized solar cells with PbS/CH3NH3PbI3 core/shell quantum dots. J Phys Chem Lett. 2014;5:2015–2020.10.1021/jz500815 h
- Morassaei MS , Zinatloo-Ajabshir S , Salavati-Niasari M . Simple salt-assisted combustion synthesis of Nd2Sn2O7–SnO2 nanocomposites with different amino acids as fuel: an efficient photocatalyst for the degradation of methyl orange dye. J Mater Sci Mater Electron. 2016;27:11698–11706.10.1007/s10854-016-5306-7
- Zinatloo-Ajabshir S , Mortazavi-Derazkola S , Salavati-Niasari M . Simple sonochemical synthesis of Ho2O3–SiO2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminant. Ultrason Sonochem. 2017;39:452–460.10.1016/j.ultsonch.2017.05.016
- Zinatloo-Ajabshir S , Morassaei MS , Salavati-Niasari M . Facile fabrication of Dy2Sn2O7–SnO2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminants. J Colloid Interface Sci. 2017;497:298–308.10.1016/j.jcis.2017.03.031
- Zinatloo-Ajabshir S , Zinatloo-Ajabshir Z , Salavati-Niasari M , et al . Facile preparation of Nd2Zr2O7–ZrO2 nanocomposites as an effective photocatalyst via a new route. J Energy Chem. 2017;26:315–323.10.1016/j.jechem.2016.11.005
- Razi F , Zinatloo-Ajabshir S , Salavati-Niasari M . Preparation, characterization and photocatalytic properties of Ag2ZnI4/AgI nanocomposites via a new simple hydrothermal approach. J Mol Liq. 2017;225:645–651.10.1016/j.molliq.2016.11.028
- Morassaei MS , Zinatloo-Ajabshir S , Salavati-Niasari M . New facile synthesis, structural and photocatalytic studies of NdOCl-Nd2Sn2O7–SnO2 nanocomposites. J Mol Liq. 2016;220:902–909.10.1016/j.molliq.2016.05.041
- Zinatloo-Ajabshir S , Mortazavi-Derazkola S , Salavati-Niasari M . Sono-synthesis and characterization of Ho2O3 nanostructures via a new precipitation way for photocatalytic degradation improvement of erythrosine. Int J Hydrogen Energy. 2017;42:15178–15188.10.1016/j.ijhydene.2017.04.252
- Zinatloo-Ajabshir S , Salavati-Niasari M , Zinatloo-Ajabshir Z . Nd2Zr2O7–Nd2O3 nanocomposites: New facile synthesis, characterization and investigation of photocatalytic behaviour. Mater Lett. 2016;180:27–30.10.1016/j.matlet.2016.05.094
- Huang C-Y , Zou C , Mao C , et al . CsPbBr3 perovskite quantum dot vertical cavity lasers with low threshold and high stability. ACS Photon. 2017;4:2281–2289.10.1021/acsphotonics.7b00520
- Chen W , Hao J , Hu W , et al . Enhanced stability and tunable photoluminescence in perovskite CsPbX3/ZnS quantum dot heterostructure. Small. 2017;13: 1604085.
- Bella F , Griffini G , Correa-Baena J-P , et al . Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers. Science. 2016;354:203–206.10.1126/science.aah4046
- Smith IC , Hoke ET , Solis-Ibarra D , et al . A layered hybrid perovskite solar-cell absorber with enhanced moisture stability. Angew Chem Int Ed. 2014;53:11232–11235.10.1002/anie.201406466
- Wang C , Chesman ASR , Jasieniak JJ . Stabilizing the cubic perovskite phase of CsPbI3 nanocrystals by using an alkyl phosphinic acid. Chem Commun. 2017;53:232–235.10.1039/C6CC08282C
- Lou S , Xuan T , Yu C , et al . Nanocomposites of CsPbBr3 perovskite nanocrystals in an ammonium bromide framework with enhanced stability. J Mater Chem C. 2017;5:7431–7435.10.1039/C7TC01174A
- Talapin DV , Koeppe R , Götzinger S , et al . Highly emissive colloidal CdSe/CdS heterostructures of mixed dimensionality. Nano Lett. 2003;3:1677–1681.10.1021/nl034815s
- Dabbousi BO , Rodriguez-Viejo J , Mikulec FV , et al . (CdSe)ZnS core-shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B. 1997;101:9463–9475.10.1021/jp971091y
- Jiang LQ , Guo JK , Liu HB , et al . Prediction of lattice constant in cubic perovskites. J Phys Chem Solids. 2006;67:1531–1536.10.1016/j.jpcs.2006.02.004
- Skelton JM , Parker SC , Togo A , et al . Thermal physics of the lead chalcogenides PbS, PbSe, and PbTe from first principles. Phys Rev B. 2014;89:205203.10.1103/PhysRevB.89.205203
- Protesescu L , Yakunin S , Bodnarchuk MI , et al . Nanocrystals of caesium lead halide perovskites (CsPbX(3), X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015;15:3692–3696.10.1021/nl5048779
- Liang Z , Zhao S , Xu Z , et al . Shape-controlled synthesis of all-inorganic CsPbBr3 perovskite nanocrystals with bright blue emission. ACS Appl Mater Interfaces. 2016;8:28824–28830.
- Beal RE , Slotcavage DJ , Leijtens T , et al . Caesium lead halide perovskites with improved stability for tandem solar cells. J Phys Chem Lett. 2016;7:746–751.10.1021/acs.jpclett.6b00002
- Rakita Y , Kedem N , Gupta S , et al . Low-temperature solution-grown CsPbBr3 single crystals and their characterization. Cryst Growth Des. 2016;16:5717–5725.10.1021/acs.cgd.6b00764
- Lipovskii A , Kolobkova E , Petrikov V , et al . Synthesis and characterization of PbSe quantum dots in phosphate glass. Appl Phys Lett. 1997;71:3406–3408.10.1063/1.120349
- Yong K-T , Sahoo Y , Choudhury KR , et al . Shape control of PbSe nanocrystals using noble metal seed particles. Nano Lett. 2006;6:709–714.10.1021/nl052472n
- Wang Y , Zhu Y , Huang J , et al . CsPbBr3 perovskite quantum dots-based monolithic electrospun fiber membrane as an ultrastable and ultrasensitive fluorescent sensor in aqueous medium. J Phys Chem Lett. 2016;7:4253–4258.10.1021/acs.jpclett.6b02045
- Kim S , Marshall AR , Kroupa DM , et al . Air-stable and efficient PbSe quantum-dot solar cells based upon ZnSe to PbSe cation-exchanged quantum dots. ACS Nano. 2015;9:8157–8164.10.1021/acsnano.5b02326
- Yanover D , Čapek RK , Rubin-Brusilovski A , et al . Small-sized PbSe/PbS core/shell colloidal quantum dots. Chem Mater. 2012;24:4417–4423.10.1021/cm302793 k
- Van Le Q , Park M , Sohn W , et al . Investigation of energy levels and crystal structures of caesium lead halides and their application in full-color light-emitting diodes. Adv Electron Mater. 2017;3:1600448.10.1002/aelm.201600448
