 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Estimation of structure stability is an essential issue in materials design and synthesis. Global instability index (GII) based on bond-valence method is applied as a simple indication, while density functional theory calculation is adopted for accurate evaluation of formation energy. We compare the GII and total energy of typical ABO3-type perovskite oxides and rationalize their relationship, proposing that the criteria for empirically unstable structures (GII > 0.2 valence unit) correspond to the difference in total energy of 50–200 meV per formula unit.
1. Introduction
Structure stability is an essential factor for evaluating and predicting materials including hypothetical compounds. For ionic compounds, ionic radii [Citation1] and the radius ratios of cation to anion are primary indices to categorize and predict stable coordination polyhedra in crystal. Goldschmidt tolerance factor t [Citation2] is extensively adopted to evaluate structure stability of perovskite structure: t = , where r
A
, r
B
, and r
X
are ionic radii of A, B, and X ions in ABX
3 perovskite, respectively. When t is close to the unity, cubic perovskite (space group Pm
m) is stable at ambient conditions. When t is larger or smaller than the unity, distorted perovskite structures, typically GdFeO3-type orthorhombic perovskite, or phases other than perovskite (e.g. ilmenite) are expected instead of undistorted cubic perovskite. This index also serves to interpret systematic trends in structures and various properties for ABO3 oxides [Citation3–6].
Bond valence method is widely applied to verify adequacy of structure refinement and also to estimate oxidation states of constituent ions [Citation7,8]. Bond valences (s
ij
) are calculated by a formula: . l
ij
is a bond length, l
0 is a bond valence parameter empirically determined using experimental room-temperature structure data, and b is a parameter concerning bond softness [Citation9]. The bond valence sum (BVS) is calculated as the sum of bond valences around the relevant ion: BVS =
. BVS is approximately consistent with oxidation number of ion, thus enabling estimation of valences of constituent elements and cation/anion orderings in complex metal oxides [Citation10–12]. BVS-based indices are utilized for evaluation of structural distortions; the bond discrepancy factor (d) is defined as the deviation of BVS from formal valence: d
M
= BVS(M) – V
M
, where V
M
is the formal valence of M ion. The d
M
serves to estimate bond strains. When d
M
> 0, the bonds around M ion are under compression stress (overbonding), whereas d
M
< 0, under tensile stress (underbonding). The global instability index (GII) [Citation13] is defined as root mean square of bond discrepancy index in the unit cell:
, where N is the number of ions. GII is adopted for comparing structure instability in various perovskite forms including hypothetical phases [Citation14,15]; GII = 0 means perfectly stable structures without steric distortions although structures with GII > 0.2 valence unit (v.u.) are uncommon [Citation8]. The GII-based structure prediction program, SPuDS, developed by Lufaso et al. can calculate the degree and order of structure instability for various perovskite structures [Citation15,16], as well as the tolerance factor. The close relationship between GII and tolerance factor was demonstrated by Zhang et al. [Citation17]. The indices d and GII are also utilized for interpretation of structure-property relationship. Quadruple perovskite oxides RCu3Fe4O12 (R: rare-earth metals) undergo two distinct electronic phase transitions, charge disproportionation (8Fe3.75+ → 5Fe3+ + 3Fe5+) and charge transfer (3Cu2+ + 4Fe3.75+ → 3Cu3+ + 4Fe3+) at low temperatures [Citation18,19], the type of which is predominantly determined by strains on R–O and Fe–O bonds [Citation20].
The above-mentioned indices derived from crystal structures are helpful because of their concise procedures, but precise evaluation of structure stability needs first-principle calculations. The most stable structure at the ground can be estimated by comparing total energies of polymorphs and even structural phase transition pressures are also available by using common tangent in total energy versus volume curves [Citation21,22]. The stability of a crystal structure is evaluated by performing phonon calculations with harmonic approximation [Citation23]. The presence of imaginary phonon modes tells the structure is dynamically instable, and their eigenvectors in the reciprocal space gives us possible transition pathway [Citation24]. However, these procedures are rather complicated and require enormous computational time.
To our knowledge, most of structure stability evaluation studies are performed using either BVS-based indices or first-principle calculations. Complementary evaluation and integration of multiple indices improve structure prediction and resulting materials design. The aim of this paper is to evaluate structural stability of typical perovskite oxides by comparing GII and total energy. The empirical criteria (GII < 0.2 v.u. in stable structure) correspond to energy scale of < 50–200 meV per formula unit (f.u.) for perovskite structure using first-principles calculations.
2. Computational procedure
Since consideration of magnetic structures and spin states in density functional theory (DFT) calculation may prevent simple evaluation of structure stability, we selected typical nonmagnetic oxides for calculations as follows: CaTiO3, SrTiO3, BaTiO3, CaZrO3, SrZrO3, BaZrO3, NaTaO3, and LaAlO3. BVSs were calculated by using the following parameters: l 0 (Å) = 1.651 for Al3+, 2.29 for Ba2+, 1.967 for Ca2+, 2.172 for La3+, 1.80 for Na+, 2.118 for Sr2+, 1.920 for Ta5+, 1.815 for Ti4+, 1.937 for Zr4+, and b = 0.37 Å for all [Citation9]. Structure optimizations using GII were performed; the BVSs were calculated by changing lattice constant a by 0.05 Å step around the optimized unit cell size (a = a 0) with minimum GII value.
Single-point energy calculations for SrTiO3 and BaZrO3 in cubic perovskite structure were performed using cubic unit cell of length sa exp with fixed internal coordinates, where a exp is the experimental lattice constant, and s is the scaling factor changed by 0.001 step around unity. The calculations were performed using the plane-wave-based augmented projector wave (PAW) method as implemented in the VASP code [Citation25–29]. The exchange–correlation interaction was treated as a generalized gradient approximation (GGA) using the function formulated by Perdew, Burke, and Ernzerhof [Citation30]. The PAW potential data-set with radial cutoffs of 1.45, 2.3, 2.8, 2.5, 2.8, 1.9, 2.8, 2.5, 2.9 and 1.52 Bohr for Na, Ca, Ba, Sr, La, Al, Ti, Zr, Ta and O were employed, respectively, where Na-3s, 3p, Ca-3s, 3p, 4s, Ba-5s, 5p, 6s, Sr-4s, 4p, 5s, La-5s, 5p, 6s, 5d, Al-3s, 3p, Ti-3d, 4s, Zr-4s, 4p, 5s, 4d, Ta-6s, 5d, and O-2s, 2p were described as valence electrons. The plane wave cut-off energy was set to 550 eV, and 12 × 12 × 12 k-points mesh with the Monkhorst-Pack scheme was used for integration in the reciprocal space. The minimum value was set to zero in the relative total energy.
3. Results and discussion
Figure shows calculated GII for the selected ABO3-type oxides. Only two oxides, SrTiO3 and BaZrO3, had the minimum GII values close to zero, whereas others did not reach near zero (< 0.01 v.u.). SrTiO3 (t = 1.001) and BaZrO3 (t = 1.004), which crystallize in cubic perovskite at ambient conditions, displayed linear behavior in GII versus a. LaAlO3 (t = 1.009) exhibited an almost linear curve down to the minimum value (GII = 0.027 v.u.), whereas it crystallizes in the rhombohedral perovskite structure at ambient conditions. This implies that the cubic perovskite phase is nearly stable for this oxide, as expected from the t value close to the unity. NaTaO3 (t = 0.967, orthorhombic perovskite at ambient conditions), which had a smaller GII value (0.102 v.u.) next to LaAlO3, demonstrated slightly nonlinear behavior. Other oxides with larger GII values (>0.1 v.u.) at the minima showed quadratic-like dependences and the deviations from a linear curve were apparently enhanced as the minimum GII increased. These arguments imply that the GII values hardly apply to these oxides because the energy is proportional to square of GII in principle [Citation8].
Figure 1. Global instability index for typical ABO3-type metal oxides in cubic perovskite structure.
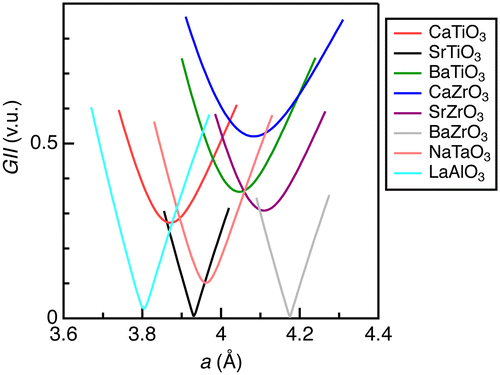
Figure illustrates GII and d M as a function of a for ATiO3 (A = Ca, Sr, and Ba) and BaZrO3. For any A, B, and O ions in all the oxides, bond discrepancy monotonically increased when a decreased. This corresponds to overbonding in A–O and B–O bonds in compression stress (or underbonding in tensile stress). GII converged to almost zero (0.006 v.u.) at a 0 = 3.930 Å for SrTiO3, as well as near zero values for d Sr, d Ti, and d O. This result indicates that the optimized crystal structure could be obtained for this oxide, which is in agreement with the literature [Citation31]. Also, BaZrO3 displayed similar behavior with SrTiO3; GII = 0.003 v.u. at a 0 = 4.175 Å when the structure optimization was achieved. The a 0 values obtained at GII minima were almost identical with the experimental ones for room-temperature cubic perovskite phases (Table ).
Figure 2. Global instability index and discrepancy factors for selected ABO3-type metal oxides in cubic perovskite structure; (a) SrTiO3, (b) BaZrO3, (c) CaTiO3, and (d) BaTiO3.
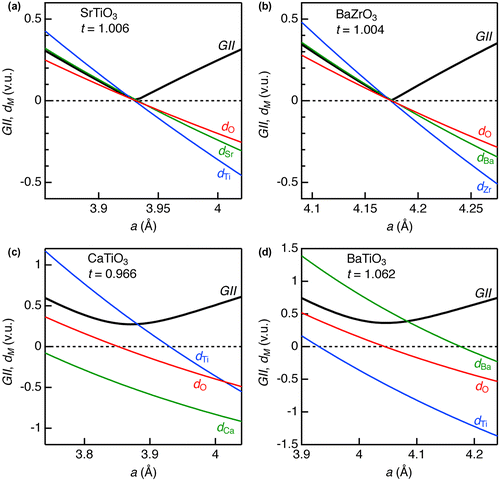
Table 1. Tolerance factor (t), ionic radius of A- and B-ions, GII for cubic (GII cubic) and distorted perovskite structures (GII exp), a 0, and crystal structure at room temperature determined by experiment for selected ABO3-type oxides.
Since tolerance factors for these two oxides are almost the unity, the GII is simple index for stable structures. When t is smaller than the unity [e.g. t = 0.966 in CaTiO3, see Table and Figure (c)], the structure optimization via GII could not be well conducted in cubic perovskite structure. The minimum GII = 0.273 v.u. was obtained at a 0 = 3.870 Å for CaTiO3, which is much larger than the empirically determined threshold value (0.2 v.u.). The structural instability of this oxide is explained by underbonding of Ca–O bonds (d Ca = −0.500 v.u.) and overbonding of Ti–O (d Ti = 0.338 v.u.) in the GII-optimized structure, whereas O ions are in insignificant stress (d O = −0.054 v.u.). As a result, structure transformations including rotations of the TiO6 octahedra occur, as evidenced by the fact that CaTiO3 crystallizes in GdFeO3-type orthorhombic perovskite structure at ambient conditions (room temperature and atmosphere pressure). BaTiO3, whose t is larger than the unity, also did not converge into stable cubic perovskite structure. Unlike CaTiO3, overbonding of Ba–O bonds (d Ba = 0.570 v.u.) and underbonding of Ti–O bonds (d Ti = −0.576 v.u.) were predominant in the GII-optimized structure. This oxide actually crystallizes in tetragonal perovskite structure at ambient conditions. The above results demonstrate that GII and t are consistent, confirming their usefulness as structure instability indices.
Figure shows comparison between GII 2 and relative total energy (E total in eV/f.u.) for SrTiO3 and BaZrO3. The optimized lattice constants of SrTiO3 and BaZrO3 by DFT calculations with minimum total energy were overestimated from experimental value by about 1.5%. This is mainly ascribed to the GGA used in the calculation to express the exchange-correlation energy. However, the change of total energy induced by the lattice strain can be calculated in better accuracy because significant part of the errors in exchange-correlation energy introduced by GGA is canceled out. In both oxides, GII 2 and E total could be fitted by using the quadratic function fitting: GII 2 = k 1(a – a 0)2 + G 0 2 and E total = k 2(a – a 0)2, where a 0 is the unit cell edge when GII 2 or E total is the minimum, and G 0 is the minimum GII value. Fitting range were limited within the GII < 0.2 v.u. (GII 2 < 0.04 v.u.2). The obtained coefficients k 1 and k 2, and resulting k 2/k 1 are listed in Table . The k 1 values for SrTiO3 and BaZrO3 are almost identical, and k 2 also similar. The k 2/k 1 values are approximately 1.3 eV/(v.u.2 f.u.). Note that this is the first example of the conversion coefficient between GII and E total in cubic perovskite structure, enabling total energy estimation as E total = ~1.3 × GII 2 eV/f.u.
Figure 3. Square of global instability index (red circles) and relative total energy (blue squares) as a function of a for (a) SrTiO3 and (b) BaZrO3 in cubic perovskite structure. The curves represent the fitting results using quadratic function.
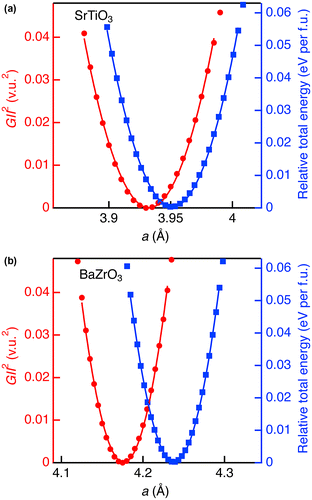
Table 2. Fitting results of GII 2 and relative total energy using quadratic function for SrTiO3 and BaZrO3.
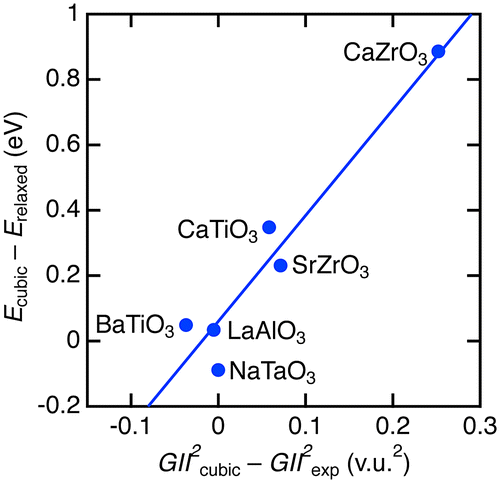
Furthermore, we tentatively evaluated the instability for severely unstable structures. The instability of hypothetical cubic perovskite structure in Figure was estimated from the differences in GII 2 between the minimum values in cubic structure (GII 2 cubic) and values for experimentally observed structures (GII 2 exp) [Citation32–39]. The instability in total energy was estimated by the difference between the energy at the minimum for cubic structure (E cubic) and the energy at relaxed non-cubic perovskite structure by DFT. Figure demonstrates the correlation between the DFT- and GII-based instability. Rough estimation by linear fitting represents a relationship of E total = 0.06(5) + 3.2(5) × GII 2 eV/f.u. The coefficient is about 2.5 times larger than those obtained by the analysis of stable cubic phases in Figure , but their scales are at the same level. Large-scale data analysis is needed to improve the total energy-GII relationship, but it is beyond the aim of the present study.
Figure 4. Comparison of structure instability between total energy and GII. The line represents the linear fitting result.
It should be noted that the GIIs were calculated using bond-valence parameters, which were empirically determined by the structures at room temperature. On the other hand, total energies obtained calculated by DFT represent the internal energies at 0 K; the temperature dependence of internal energy and entropy term in the Helmholtz free energy are not taken into account. The changes of free energy introduced by lattice strain can be estimated quantitatively by performing phonon calculations under quasi-harmonic approximation if a target compound is dynamically stable [Citation24,40]. Reflecting the thermal expansion of a crystal, the equilibrium volume at finite temperature slightly shifted from that at 0 K. The curvature of energy-volume curve (similar to k 2 in this manuscript) also changes as increasing the temperature. However, the changes of these quantities at room temperature from those at 0 K are insignificant (order of 0.1%).
We verified adequacy of structure instability obtained from GII and E total calculations. It is known that stable structures with GII > 0.2 v.u. are rare. This GII value corresponds to energy instability by ~50 meV/f.u according to the relationship obtained from cubic perovskites (SrTiO3 and BaZrO3) in Figure , whereas ~200 meV/f.u. was obtained from the analysis of non-cubic perovskites (CaTiO3, BaTiO3, CaZrO3, SrZrO3, NaTaO3, and LaAlO3) in Figure . To our knowledge, there are no established criteria to judge instability in total energy but energy differences among perovskite polymorphs are usually obtained by the order of several ten meV [Citation41–44]. Therefore, GII calculations can reasonably exclude unstable phases at low cost.
4. Conclusions
We compared GII on bond-valence model and total energy by DFT calculations for cubic perovskite oxides. The obtained conversion coefficient is in roughly agreement with the energy scale in the previous DFT calculations, supporting the adequacy of GII as structure instability index. As well as ionic radii and Goldschmidt tolerance factor, GII can be adopted for structure-instability evaluation and prediction. For further study, integration of the above indices based on large-scale data analysis is expected to achieve fast and reliable materials design and exploration.
Funding
This work was supported by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, the Ministry of Education, Culture, Sports, Science and Technology of Japan [grant numbers 26106518; 16H04220; 16H00893; 17K19182].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Shannon RD . Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A Found Adv. 1976;32:751–767.10.1107/S0567739476001551
- Goldschmid VM . Die Gesetze der Krystallochemie. Naturwissenschaften. 1926;14:477–485.10.1007/BF01507527
- Alonso JA , Martínez-Lope MJ , Casais MT , et al . Metal−insulator transitions, structural and microstructural evolution of RNiO3 (R = Sm, Eu, Gd, Dy, Ho, Y) perovskites: evidence for room-temperature charge disproportionation in monoclinic HoNiO3 and YNiO3 . J Am Chem Soc. 1999;121:4754–4762.10.1021/ja984015x
- Torrance JB , Lacorre P , Nazzal AI , et al . Systematic study of insulator-metal transitions in perovskites RNiO3 (R = Pr, Nd, Sm, Eu) due to closing of charge-transfer gap. Phys Rev B. 1992;45:8209–8212.10.1103/PhysRevB.45.8209
- Akaogi M , Kojitani H , Yusa H , et al . High-pressure transitions and thermochemistry of MGeO3 (M = Mg, Zn and Sr) and Sr-silicates: systematics in enthalpies of formation of A2+B4+O3 perovskites. Phys Chem Miner. 2005;32:603–613.10.1007/s00269-005-0034-1
- Martinez J-R , Mohn CE , Stolen S , et al . What can a ‘quantum black-box’ do for the inorganic thermochemist? PCCP. 2006;8:2036–2039.10.1039/B600485G
- Brown ID , Altermatt D . Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater. 1985;41:244–247.10.1107/S0108768185002063
- Brown ID . Recent developments in the methods and applications of the bond valence model. Chem Rev. 2009;109:6858–6919.
- Brese NE , Okeeffe M . Bond-valence parameters for solids. Acta Crystallogr Sect B Struct Sci Cryst Eng Mater. 1991;47:192–197.10.1107/S0108768190011041
- Kolitsch U , Giester G . Elyite, Pb4Cu(SO4)O2(OH)4·H2O: crystal structure and new data. Am Mineral. 2000;85:1816.10.2138/am-2000-11-1226
- Azuma M , Takata K , Saito T , et al . Designed ferromagnetic, ferroelectric Bi2NiMnO6 . J Am Chem Soc. 2005;127:8889–8892.10.1021/ja0512576
- Oka K , Oh-ishi K . Observation of anion order in Pb2Ti4O9F2 . Inorg Chem. 2015;54:10239–10242.10.1021/acs.inorgchem.5b01496
- Salinassanchez A , Garciamunoz JL , Rodriguezcarvajal J , et al . Structural characterization of R2BaCuO5 (R = Y, Lu, Yb, Tm, Er, Ho, Dy, Gd, Eu and Sm) oxides by X-ray and neutron-diffraction. J Solid State Chem. 1992;100:201–211.10.1016/0022-4596(92)90094-C
- Byeon S-H , Lufaso MW , Parise JB , et al . High-pressure synthesis and characterization of perovskites with simultaneous ordering of both the A- and B-site cations, CaCu3Ga2M2O12 (M = Sb, Ta). Chem Mater. 2003;15:3798–3804.10.1021/cm034318c
- Lufaso MW , Barnes PW , Woodward PM . Structure prediction of ordered and disordered multiple octahedral cation perovskites using SPuDS. Acta Crystallogr B. 2006;62:397–410.10.1107/S010876810600262X
- Lufaso MW , Woodward PM . Prediction of the crystal structures of perovskites using the software program SPuDS. Acta Crystallogr Sect B. 2001;57:725–738.10.1107/S0108768101015282
- Zhang H , Li N , Li K , et al . Structural stability and formability of ABO3-type perovskite compounds. Acta Crystallogr B. 2007;63:812–818.10.1107/S0108768107046174
- Long YW , Hayashi N , Saito T , et al . Temperature-induced A-B intersite charge transfer in an A-site-ordered LaCu3Fe4O12 perovskite. Nature. 2009;458:60–63.10.1038/nature07816
- Etani H , Yamada I , Ohgushi K , et al . Suppression of intersite charge transfer in charge-disproportionated perovskite YCu3Fe4O12 . J Am Chem Soc. 2013;135:6100–6106.10.1021/ja312015j
- Yamada I , Etani H , Tsuchida K , et al . Control of bond-strain-induced electronic phase transitions in iron perovskites. Inorg Chem. 2013;52:13751–13761.10.1021/ic402344 m
- Limpijumnong S , Jungthawan S . First-principles study of the wurtzite-to-rocksalt homogeneous transformation in ZnO: a case of a low-transformation barrier. Phys Rev B. 2004;70:054104.10.1103/PhysRevB.70.054104
- Li L , Yu W , Jin C . First-principles study of pressure-induced phase transition in the strongly correlated compound YCrO4 . Phys Rev B. 2006;73:174115.10.1103/PhysRevB.73.174115
- Parlinski K , Li ZQ , Kawazoe Y . First-principles determination of the soft mode in cubic ZrO2 . Phys Rev Lett. 1997;78:4063–4066.10.1103/PhysRevLett.78.4063
- Togo A , Tanaka I . First principles phonon calculations in materials science. Scripta Mater. 2015;108:1–5.10.1016/j.scriptamat.2015.07.021
- Kresse G , Hafner J . Ab initio molecular dynamics for open-shell transition metals. Phys Rev B. 1993;48:13115–13118.10.1103/PhysRevB.48.13115
- Blöchl PE . Projector augmented-wave method. Phys Rev B. 1994;50:17953–17979.
- Kresse G , Hafner J . Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys Rev B. 1994;49:14251–14269.10.1103/PhysRevB.49.14251
- Kresse G , Furthmüller J . Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B. 1996;54:11169–11186.10.1103/PhysRevB.54.11169
- Kresse G , Furthmüller J . Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci. 1996;6:15–50.10.1016/0927-0256(96)00008-0
- Perdew JP , Burke K , Ernzerhof M . Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–3868.10.1103/PhysRevLett.77.3865
- Brown ID , Poeppelmeier KR . Bond Valences. Berlin: Springer Berlin Heidelberg; 2014.10.1007/978-3-642-54968-7
- Yashima M , Ali R . Structural phase transition and octahedral tilting in the calcium titanate perovskite CaTiO3 . Solid State Ionics. 2009;180:120–126.10.1016/j.ssi.2008.11.019
- Luo W-Q , Shen Z-Y , Li Y-M , et al . Structural characterizations, dielectric properties and impedance spectroscopy analysis of Nd x Sr1–1.5x TiO3 ceramics. J Electroceram. 2013;31:117–123.10.1007/s10832-013-9805-0
- Kwei GH , Lawson AC , Billinge SJL , et al . Structures of the ferroelectric phases of barium titanate. J Phys Chem. 1993;97:2368–2377.10.1021/j100112a043
- Stoch P , Szczerba J , Lis J , et al . Crystal structure and ab initio calculations of CaZrO3 . J Eur Ceram Soc. 2012;32:665–670.10.1016/j.jeurceramsoc.2011.10.011
- Ahtee A , Ahtee M , Glazer AM , et al . The structure of orthorhombic SrZrO3 by neutron powder diffraction. Acta Crystallogr Sect B. 1976;32:3243–3246.10.1107/S0567740876010029
- Ahmed I , Eriksson SG , Ahlberg E , et al . Proton conductivity and low temperature structure of In-doped BaZrO3 . Solid State Ionics. 2006;177:2357–2362.10.1016/j.ssi.2006.05.030
- Mitchell RH , Liferovich RP . A structural study of the perovskite series Ca1−x Na x Ti1−x Ta x O3 . J Solid State Chem. 2004;177:4420–4427.10.1016/j.jssc.2004.09.031
- Christopher JH , Brendan JK , Bryan CC . Neutron powder diffraction study of rhombohedral rare-earth aluminates and the rhombohedral to cubic phase transition. J Phys Condens Matter. 2000;12:349.
- Kuwabara A , Tohei T , Yamamoto T , et al . Ab initiolattice dynamics and phase transformations of ZrO2 . Phys Rev B. 2005;71:064301.
- Moriwake H , Kuwabara A , Fisher CAJ , et al . First-principles calculations of lattice dynamics in CdTiO3 and CaTiO3: phase stability and ferroelectricity. Phys Rev B. 2011;84:104114.10.1103/PhysRevB.84.104114
- Nielsen MB , Ceresoli D , Parisiades P , et al . Phase stability of the SrMnO3 hexagonal perovskite system at high pressure and temperature. Phys Rev B. 2014;90:214101.10.1103/PhysRevB.90.214101
- Cazorla C , Diéguez O , Íñiguez J . Multiple structural transitions driven by spin-phonon couplings in a perovskite oxide. Sci Adv. 2017;3:e1700288.10.1126/sciadv.1700288
- Paul A , Sun J , Perdew JP , et al . Accuracy of first-principles interatomic interactions and predictions of ferroelectric phase transitions in perovskite oxides: energy functional and effective Hamiltonian. Phys Rev B. 2017;95:054111.10.1103/PhysRevB.95.054111
