 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Two highly active and stable Pd-based intermetallic nanocrystals with early d-metals Pd3Ti and Pd3Zr have been developed. The nanocrystals are synthesized by co-reduction of the respective salts of Pd and Ti/Zr. Hard X-ray photoemission Spectroscopy (HAXPES) analysis of the nanocrystals indicates that the electronic properties of Pd are modified significantly, as evident from the lowering of the d-band center of Pd. The intermetallic nanocrystals dispersed in Vulcan carbon, Pd3Ti/C and Pd3Zr/C, exhibit improved electrocatalytic activity towards methanol and ethanol oxidation in an alkaline medium (0.5 M KOH), compared to those of commercially available catalysts such as Pd/C, Pt/C, and Pt3Sn/C. In addition, Pd3Ti/C and Pd3Zr/C show significantly higher activity towards the oxidation of formic acid in an acidic medium (0.5 M H2SO4), compared to those of Pd/C and Pt/C. The modification of the d-band center of Pd as a result of the alloying of Pd with the early d-metals Ti and Zr may be responsible for the enhanced catalytic activity.
Graphical Abstract

1. Introduction
Direct liquid fuel cells, such as direct alcohol fuel cells (DAFC) and direct formic acid fuel cells (DFAFC), have emerged as a promising energy conversion technology because of their high efficiency and low pollution [Citation1–Citation3]. Pt and Pt-based alloys are the most extensively studied and best catalysts for DAFC and DFAFC [Citation4–Citation8]. However, the use of Pt is limited due to its high cost of Pt, slow reaction kinetics, low stability, and poisoning by CO, which is formed during the electro-oxidation of alcohols or acids [Citation9–Citation13]. Therefore, developing low-cost catalysts with higher activity and stability is of paramount importance for the commercialization of DAFC or DFAFC.
Palladium has attracted increasing attention due to its lower cost, greater abundance, and excellent electrocatalytic activity towards the oxidation of small organic molecules, particularly in alkaline media [Citation14–Citation19]. Alloying Pd with other metals, such as Ag, Au, Sn, Pb, Ni, Cu, etc., is an efficient way to improve the activity of Pd by modifying the structural and electronic properties of the active sites [Citation16,Citation20–Citation30]. Density functional theory (DFT) calculations suggest that the electronic properties of Pd (d-band center) can be modified or even matched with those of Pt by alloying the former with early d-metals such as Ti, Zr, and Ta [Citation31,Citation32]. Thus, alloys of Pd with early d-metals are expected to exhibit performances close to or even better than that of Pt in various catalytic reactions. However, developing such alloys is challenging due to the highly oxyphilic nature of the early d-metal precursors [Citation33–Citation35]. Herein, we present our attempt to develop a new class of intermetallic nanocrystals consisting of Pd and early d-metals, or Pd3X (where X = Ti and Zr), in addition to detailed characterization of these catalysts. The Pd3X nanocrystals are demonstrated to be efficient catalysts for the oxidation of methanol and ethanol in an alkaline medium. These catalysts also exhibit enhanced catalytic activity toward the oxidation of formic acid, promoting the favorable direct dehydrogenation pathway in an acidic medium. HAXPES measurements suggest that the d-band center of Pd is lowered. The modification of the electronic properties of Pd may be responsible for the enhanced activity of Pd3X nanocrystals in both alkaline and acidic media.
2. Experimental details
2.1 Reagents
We used anhydrous palladium (II) acetate, (Aldrich), anhydrous titanium(IV) chloride tetrahydrofuran complex (TiCl4 · 2THF, Aldrich,97%), zirconium (IV) chloride (ZrCl4, Aldrich, 99%), diglyme (anhydrous, 99.8%, Aldrich), LiBH(C2H5)3 (super-hydride, 1 M in THF, Aldrich), hexane (anhydrous, 95%, Aldrich), acetonitrile (99.8%, Aldrich), sodium metal (Aldrich) and naphthalene.
2.2. Synthesis of sodium naphthalide
Sodium naphthalide solution was prepared by dissolving 22.9 mg of metallic sodium and 129.4 mg of naphthalene in dry diglyme. The reaction mixture was stirred overnight under an argon atmosphere.
2.3. Synthesis of Pd3Ti/C NPs
Intermetallic Pd3Ti and NPs were synthesized by co-reduction of the metal precursors in diglyme.Palladium (II) acetate (0.13 mmol) and TiCl4 · 2THF (0.043 mmol) were weighed and transferred to a round-bottom flask containing the strong reducing agent sodium naphthalide. 54 mg of Vulcan carbon was added to the reaction mixture. The reaction mixture was then transferred to a stainless-steel pressure vessel and heated at 200°C in an oil bath for 2 h under an argon pressure of 0.5 MPa [Citation34,Citation35]. The product was then transferred to a centrifuge tube under an argon atmosphere. The precipitate was separated from diglyme by centrifuging at 6000 rpm for 5 min. The product was washed several times with hexane and acetonitrile to remove the byproducts, and then dried under vacuum for 1 h. The washing solvents were carefully selected to minimize interaction with the oxyphilic metal and hence, leaching or dissolution of the metal, resulting in a non-uniform composition. The as-prepared product was annealed at 1000°C for 15 h under vacuum to achieve the desired intermetallic Pd3Ti phase.
2.4. Synthesis of Pd3Zr/C NPs
Intermetallic Pd3Zr NPs were synthesized by co-reduction of the metal precursors in diglyme.Palladium (II) acetate (0.33 mmol) and ZrCl4 (0.11 mmol) were weighed in a stainless-steel pressure vessel. Then, 30 ml of diglyme were added to the vessel, and the mixture was stirred for 20 min to dissolve the reactants. Next, 1 ml of super-hydride was added to the reaction mixture, which was heated at 200°C in an oil bath for 2 h under an argon pressure of 0.5 MPa [Citation34,Citation35]. The product was then transferred to a centrifuge tube under an argon atmosphere. The precipitate was separated from diglyme by centrifuging at 6000 rpm for 5 min. The product was washed several times with hexane and acetonitrile to remove the byproducts and dried under vacuum for 1 h. The as-prepared product was then mixed with Vulcan carbon to form Pd3Zr/C. The as-prepared product Pd3Zr/C was annealed at 1000°C for 15 h under vacuum to obtain intermetallic Pd3Zr/C.
2.5 Synthesis of bulk Pd3Ti and Pd3Zr
Polycrystalline bulk samples of intermetallic Pd3Ti and Pd3Zr were synthesized with an arc furnace in a pure Ar atmosphere (99.9999%). Prior to the synthesis, the arc furnace was evacuated to a vacuum level lower than 10 mPa and back-filled with pure Ar. All of the starting materials were purchased from Furuya Kinzoku Co. An aliquot of 1 g of Pd powder (99.9%) was pelletized with a stainless-steel die and melted into an ingot using an arc furnace. Ti/Zr (ingot, 99%) was used as received. The ingots of Ti/Zr and Pd were weighed such that the molar ratio was Ti/Zr:Pt = 1:3 and melted together in an arc furnace to obtain the desired intermetallic Pd3Ti/Pd3Zr. The product was finally annealed in vacuum at 1000°C for 72 h.
3. Characterization
3.1. Powder X-ray diffractometry (pXRD)
pXRD measurements were performed using Cu Kα radiation (Panalytical X’Pert PRO; λ = 0.1548 nm) in the range of diffraction angles from 20 to 100 degrees, with increments of 0.02 degrees. An obliquely finished Si crystal (non-reflection Si plate) was used as a sample holder to minimize the background.
3.2. Hard X-ray photoemission spectroscopy (HAXPES)
HAXPES measurements were performed using X-rays with a photon energy of 5.95 keV, at the undulator beamline BL15XU of SPring-8, Japan. Samples for the HAXPES measurements were prepared by mixing the sample solution (in THF) with carbon black (Vulcan XC-72, Cabot Co. Ltd.) to avoid charging effects. 10 µl of the sample was dropped onto a carbon substrate (Nilaco Co., Ltd.) and dried under vacuum. The core-level states of the samples were examined at room temperature in ultrahigh vacuum (UHV) using a hemispherical electron energy analyzer (VG SCIENTA R4000). The total energy resolution was set to 220 meV. The binding energy was referenced to the Fermi edge of an Au thin film.
3.3. Transmission electron microscopy
A 200 kV transmission electron microscope (TEM and/or STEM, JEM-2100 F, JEOL) was used. It was equipped with two aberration correctors (CEOS GmbH) for the image- and probe-forming lens systems and an X-ray energy-dispersive spectrometer (JED-2300 T, JEOL) for compositional analysis. Both the aberration correctors were optimized to realize point-to-point resolutions of 1.3 and 1.1 Å for TEM and scanning transmission electron microscopy (STEM), respectively. A probe convergence angle of 29 mrad and a high-angle annular-dark-field (HAADF) detector with an inner angle greater than 100 mrad were used for HAADF-STEM observation. An UHV-STEM (TECNAI G2) was used to monitor the morphology and particle size of the materials. The samples for UHV-STEM were prepared by dropping a THF suspension of the sample powder onto a commercial TEM grid coated with a collodion film. The sample was thoroughly dried in vacuum prior to observation.
3.4. Electrochemical experiment
Electrochemical measurements were performed with a three-electrode system on a HSV-100 electrochemical apparatus. Ag/AgCl (4 M) and a Pt wire were used as the reference and counter electrodes, respectively. A glassy carbon (GC) electrode (PINE, 5 mm diameter) was polished with Gamma Micropolish Alumina (Baikalox, Type 0.05 µm CR) and thoroughly cleaned before use. 4 mg of the catalysts were dispersed in ultrapure water+isopropanol+5% Nafion (v/v/v = 4/1/0.04) with sonication. 45 µl of the suspension was then drop-cast on the cleaned GC electrode and dried at 60°C for 20 min. Prior to the electrochemical measurements, the electrolytes (0.5 M KOH/0.5 M H2SO4, Fluka) were degassed by bubbling Ar gas for 30 min. Cyclic voltammetry (CV) measurements were performed at a sweep rate of 20 mVs−1, with 1 M methanol, ethanol and formic acid present in the electrolyte. Commercial Pt/C NPs (20 wt%, Fuel Store) and Pt3Sn/C (Premetek Co.) were used as the control.
Electrochemical active surface area (ECSA) was obtained from the CV of each of the catalysts in 0.5 M KOH/H2SO4 by measuring the coulombic charge obtained from the area under the Pd-O reduction (Qo) curve, assuming that the charge required for the reduction of Pd-O is 0.405 mC.cm−2, using the following equation [Citation36–Citation38].
For Pt/C and Pt3Sn/C, ECSA was calculated using the following equation [Citation39]
4. Results and discussion
Figure 1. (a) pXRD profile of intermetallic Pd3Ti/C. (b) Structural model showing the atomic arrangement in Pd3Ti. (c,d)pXRD profile of Pd3Zr/C and structural model of intermetallic Pd3Zr. Simulated pXRD profiles of intermetallic Pd3Ti and Pd3Zr are also shown as references.
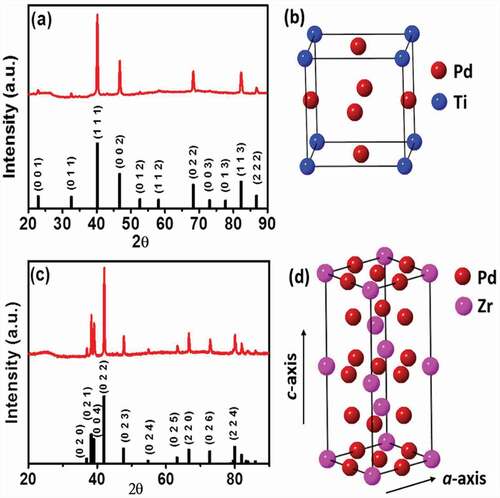
presents the powder X-ray diffraction (pXRD) profile of Pd3Ti/C. The pXRD profile of Pd3Ti shows intense reflection peaks at 40.2°, 46.7°, 68.3° and 82.3°, corresponding to the 111, 002, 022, and 113 reflections of the face-centered cubic (fcc) structure of Pd. However, these peaks are shifted to higher diffraction angles compared to the reflections of pure Pd, which suggests that the smaller atoms have been incorporated into the fcc crystal lattice of Pd. More importantly, the appearance of the less intense peaks at 22.9°, 32.6°, 52.3° and 58.1° clearly indicates the formation of the intermetallic phase. None of these reflections originates from the fcc-type structure instead from an atomically ordered, intermetallic Pd3Ti phase. Indeed, the pXRD profiles of Pd3Ti/C are in good agreement with the simulated pXRD pattern of Pd3Ti (Cu3Au structure, Pmm, a = 3.888 Å) [Citation40]. The atomic arrangements of Pd and Ti in the crystal are shown in . shows the pXRD pattern of Pd3Zr/C. The pXRD profile of Pd3Zr/C indicates that the crystal structure of Pd3Zr is different from the cubic structure of Pd. The peaks at 37.0°, 38.3°, 39.1°, 54.9°, and 63.2° are assigned to the 020, 021, 004, 024, and 025 reflections, respectively, of an atomically ordered intermetallic Pd3Zr. The experimentally observed pXRD pattern is fully consistent with intermetallic Pd3Zr with a hexagonal crystal structure (space group P63/mmc, a = 5.6119 Å, c = 9.2316 Å, ) [Citation41].
Figure 2. HAXPES spectra of the Pd 3d- (a) and Ti 2p- (b) regions of the Pd3Ti NPs and those of the Pd 3d- (c) and Zr 3d- (d) regions of the Pd3Zr NPs. HAXPES spectra of the bulk samples of Pd, Ti, Zr, Pd3Ti and Pd3Zr are shown as references.
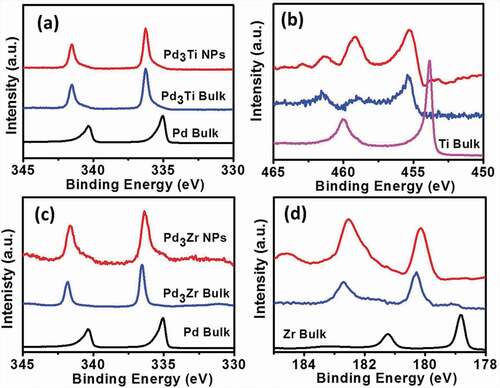
HAXPES measurements were used to probe the elemental states of Pd, Ti and Zr in Pd3Ti/C and Pd3Zr/C. and show HAXPES spectra of the core levels of Pd and Ti, respectively, in Pd3Ti NPs. The binding energy (BE) corresponding to the 3d core level of Pd is shifted to a higher energy, indicating the formation of Pd-Ti bonds in Pd3Ti NPs. This is further confirmed by the shift in the BE of the Ti core level to higher energies (). HAXPES analysis also suggests that a finite amount of oxides of Ti was also present in the Pd3Ti NPs (Figure S1). A similar trend is observed in the case of Pd3Zr. The emissions from the core levels, corresponding to the metallic state of Pd and Zr, are shifted to higher energies, indicative of Pd-Zr bonds in the intermetallic compounds (,, and S1). The composition of the Pd3Zr NPs was calculated from the intensities of the Pd 3d5/2 and Zr 3d5/2 emissions as Pd:Zr = 3.0 ± 0.1: 1.2 ± 0.3 [Citation42–Citation45]. The shift of BE to a higher energy arises as a result of the diminished screening of the nuclear charge. The shifts in the BEs of the core levels clearly demonstrate the modification of the electronic structure of Pd, Ti and Zr. The BEs of the core levels of Pd, Ti, and Zr in their respective nanoparticles/bulk samples are tabulated in Tables S1 and S2.
Figure 3. (a)TEM image and (b) selected area diffraction pattern of Pd3Ti/C, showing the zone axis of [1 1 0]. (c) STEM image of Pd3Ti/C. EDS mapping of (d) Pd, (e) Ti and the composite image for the Pd3Ti/C (f). (g) Size distribution of Pd3Ti NPs.
![Figure 3. (a)TEM image and (b) selected area diffraction pattern of Pd3Ti/C, showing the zone axis of [1 1 0]. (c) STEM image of Pd3Ti/C. EDS mapping of (d) Pd, (e) Ti and the composite image for the Pd3Ti/C (f). (g) Size distribution of Pd3Ti NPs.](/cms/asset/c0bab7cd-7ff9-4ca2-8b9b-8105cb20e476/tsta_a_1789437_f0003_oc.jpg)
shows a TEM image of Pd3Ti/C, which consists of spherical particles with a size of 100 nm dispersed on Vulcan carbon. The bigger particles are formed as a result of thermal annealing at high temperature. Selected area electron diffraction (SAED) recorded on an individual nanoparticle is shown in (inset: Pd3Ti/C nanoparticle). The obtained pattern fits well with the [1 1 0] zone axis, which further supports the pXRD analysis and suggests the formation of Pd3Ti intermetallic nanoparticles. Energy-dispersive spectroscopy (EDS) combined with TEM was used to determine the distribution of elements as well as the composition of the nanoparticles. As evident from , the elements Pd and Ti are distributed uniformly over the nanoparticles. However, trace amounts of Ti oxides are also observed. shows TEM images of Pd3Zr/C nanoparticles. The average size of the particles is estimated to be 100 nm. The Pd3Zr nanoparticles are uniformly distributed over the carbon matrix. SAED recorded on the single particles matches the [11 0] zone axis (, inset Pd3Zr nanoparticle). The EDS analysis suggests that the distribution of Pd and Zr is uniform within the nanoparticles, with a small amount of oxides on the surface (). Compositional analysis with EDS shows that the Pd to Ti/Zr atomic ratio is 3:1 for Pd3Ti/C or Pd3Zr/C (Figures S2, S3).
Figure 4. (a)TEM image and (b) selected area diffraction pattern of Pd3Zr/C, showing the [1 1 0] zone axis. (c) STEM image of Pd3Zr/C. EDS mapping of (d) Pd, (e) Zr and the composite image for the Pd3Zr/C (f) are also shown. (g) The statistical distribution of Pd3Zr NPs.
![Figure 4. (a)TEM image and (b) selected area diffraction pattern of Pd3Zr/C, showing the [1 1 0] zone axis. (c) STEM image of Pd3Zr/C. EDS mapping of (d) Pd, (e) Zr and the composite image for the Pd3Zr/C (f) are also shown. (g) The statistical distribution of Pd3Zr NPs.](/cms/asset/4cacc4d0-5b9d-40c5-9752-e159b5c58935/tsta_a_1789437_f0004_oc.jpg)
The electrocatalytic activity of Pd3Ti/C and Pd3Zr/C towards methanol and ethanol oxidation reactions (MOR and EOR, respectively) in alkaline media is tested by CV, and compared with that of commercially available catalysts Pd/C (10 wt %), Pt/C (20 wt %), and Pt3Sn/C (20 wt %). shows the CV curves obtained for the MOR during the forward scan between a potential range from −1.0 V to 0.4 V.
Figure 5. (a) CV curves of methanol oxidation on different catalysts in 1 M methanol + 0.5 M KOH at a scan rate of 20 mVs−1. (b) Variation of normalized current density at the peak maximum of different potential cycles from the first to the 300th cycle of methanol oxidation.
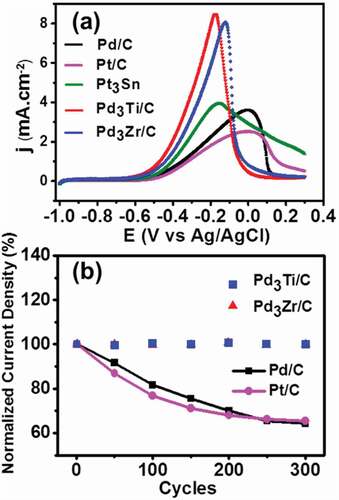
As is evident from , the specific current density of Pd3Ti/C and Pd3Zr/C is 3 and 2.5 times higher, respectively, than that of Pd/C. The current density is obtained by normalizing the current with the electrochemical surface area. It is interesting to note that both Pd3Ti/C and Pd3Zr/C behave similarly, with a slightly higher current density for Pd3Ti/C. The peak maximum for the MOR is shifted to a lower potential compared to that of Pd/C, which suggests that the incorporation of Ti or Zr into Pd facilitates the oxidation of methanol. In other words, the oxidation kinetics of methanol on Pd3Ti/C and Pd3Zr/C are different from those on Pd/C. More importantly, Pd3Ti/C and Pd3Zr/C exhibit a higher specific activity (1.8 times for Pd3Ti/C and 1.6 times for Pd3Zr/C) in comparison with that of Pt/C. Compared with Pd/C and Pt/C, the lower oxidation peak potential and higher oxidation current density indicate that Pd3Ti/C and Pd3Zr/C can be considered as promising candidates for the MOR. The long-term stability and activity of Pd3Ti/C and Pd3Zr/C for the MOR was tested by chronoamperometry at −0.2 V. The intermetallic compounds show a higher current density compared to that of Pd/C for 3600 s (Figure S4). The reproducible operation of the catalysts was further verified by accelerated durability tests (ADT) at a scan rate of 90 mVs−1. The durability was tested by comparing the peak current density for different potential cycles (, S5). As is evident from , the current density remains virtually the same even after 300 potential cycles for Pd3Ti/C and Pd3Zr/C. However, Pd/C and Pt/C lose ~40% to 50% of the initial current density after 300 cycles (Figure S5).
Figure 6. (a) CV curves of ethanol oxidation on different catalysts in 1 M ethanol + 0.5 M KOH at a scan rate of 20 mVs−1. (b) Variation of normalized current density at the peak maximum of different potential cycles from the first to the 300th cycle of ethanol oxidation.
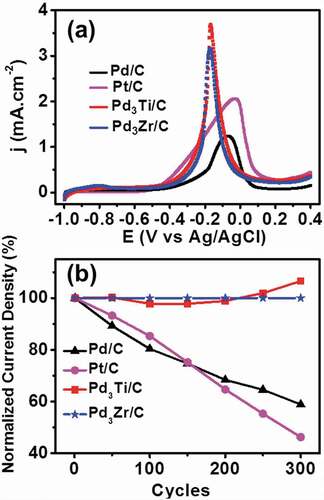
shows the CV curves of the EOR on different catalysts. The specific current density for Pd3Ti/C and Pd3Zr/C is 2.35 and 2.25 times higher, respectively, than that of Pd/C. In addition, the onset potential for Pd3Ti/C (−0. 574 V) and Pd3Zr/C (−0. 521 V) is shifted to a significantly lower potential in comparison with that of Pd/C (−0. 480 V). Importantly, the oxidation peak potential of the EOR shifts by −0.175 V for Pd3Ti/C and −0.125 V for Pd3Zr/C, in comparison with that of Pd/C (0 V). It should be noted that the activity of Pd3Ti/C and Pd3Zr/C towards the EOR is remarkably higher than that of Pt/C and Pt3Sn/C. Considering the onset potential, current density, and oxidation peak potential, Pd3Ti/C is found to be the best catalyst for the EOR. Note that the oxidation peaks for the MOR and EOR over the Pd3Ti- and Pd3Zr catalysts were narrow and sharp compared to those for the control materials, Pd/C, Pt/C or Pt3Sn/C, reflecting that the surface Pd atoms are surrounded by the Ti- and Zr-atoms to act as an isolated adsorption centre for the reaction intermediate, CO.
Chronoamperometry measurements at −0.2 V show that Pd3Ti/C and Pd3Zr/C are superior to Pd/C, exhibiting a higher current density for 600 s (Figure S6), indicating a higher tolerance of Pd3Ti/C and Pd3Zr/C to the carbonaceous species generated during ethanol oxidation. The durability of the catalysts in the EOR was further tested with ADT at a scan rate of 90 mVs−1 (). The current density of Pd3Ti/C and Pd3Zr/C increases with an increasing number of potential cycles and then remains constant, even after 300 ADT cycles. However, the activity of Pd/C and Pt/C is decreased by 40% after 300 cycles (Figure S7). Therefore, the above results demonstrate that Pd3Ti/C and Pd3Zr/C show improved catalytic activity and stability in reproducible operation for the EOR.
Figure 7. (a) CV curves of formic acid oxidation on different catalysts in 1 M formic acid + 0.5 M H2SO4 at a scan rate of 20 mVs−1. (b) Variation of normalized current density at the peak maxima of different potential cycles from the first to the 200th cycle of formic acid oxidation.
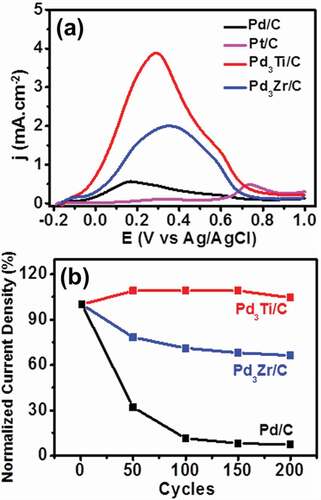
We further tested the activities of the catalysts towards formic acid electro-oxidation (FAEO) in an acidic medium (). Pd-based catalysts are known to facilitate the oxidation of formic acid through the dehydrogenation pathway. The peak current intensity for Pd3Ti/C and Pd3Zr/C is 6.8 and 3.5 times higher, respectively, than that of Pd/C. The onset potential of Pd3Ti/C (−0.118 V) is much more negative than that of Pd3Zr/C (−0.04 V) and Pd/C (−0.07 V).
The ADT experiments finally suggest that Pd3Ti/C exhibits very good tolerance to repeated operation towards formic acid oxidation. For Pd3Ti/C, the current density initially increases with the number of cycles and remains constant even after 200 cycles (104%). However, the activity of Pd3Zr/C is decreased with an increasing number of cycles (35% loss) after 200 cycles. On the other hand, Pd/C loses almost 90% of its initial activity within even 100 potential cycles, which suggests that alloying of Pd with Ti/Zr improves the stability of the catalysts (). The superior stability of Pd3Ti/C to Pd3Zr/C may be attributed to the chemical stability of titanium oxides (TiOx) layers on the catalyst surface (Figure S1), which can inhibit surface segregation or dealloying in acidic electrolytes.
The d-band centers of Pd3Ti- and Pd3Zr NPs were calculated as −3.47 eV and −3.71 eV, respectively. These values are lowered significantly compared to that of pure Pd, which is close to −3.10. More importantly, the d-band centers of Pd3Ti- and Pd3Zr NPs are even lower than that of Pt, −3.31 eV (Figure S8). The lowered d-band center of Pd3X can alter hybridization strength of one of the major reaction intermediates of FAEO, MOR and EOR, carbon monoxide that works a catalytic poison, which may result in the improved electrocatalytic performances.
5. Conclusion
We have developed two Pd-based inter-metallic nanocrystals with the early d-metals Ti and Zr. The Pd3Ti/C and Pd3Zr/C catalysts were characterized in detail using pXRD, TEM and EDS. These newly developed catalysts exhibit enhanced catalytic activity towards methanol and ethanol electro-oxidation in an alkaline medium and formic acid electro-oxidation in an acidic medium, compared to commercially available catalysts.
Supporting information
Synthesis, characterization (pXRD, HAXPES, TEM) and electrochemical data.
Supplemental Material
Download PDF (752.3 KB)Acknowledgments
The authors are grateful to HiSOR, Hiroshima University, and JAEA/SPring-8 for the development of HAXPES at BL15XU of Spring-8.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Arico AS, Bruce P, Scrosati B, et al. Nanostructured materials for advanced energy conversion and storage devices. Nat Mater. 2005;4:366–377.
- Debe MK. Electrocatalyst approaches and challenges for automotive fuel cells. Nature. 2012;486:43–51.
- Kakati N, Maiti J, Lee SH, et al. Anode catalysts for direct methanol fuel cells in acidic media: do we have any alternative for Pt or Pt–Ru? Chem Rev. 2014;114:12397–12429.
- Stamenkovic VR, Fowler B, Mun BS, et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science. 2007;315:493–497.
- Lamy C, Belgsir EM, Leger JM. Electrocatalytic oxidation of aliphatic alcohols: application to the direct alcohol fuel cell (DAFC). J Appl Electrochem. 2001;31:799–809.
- Lamy C, Lima A, LeRhun V, et al. Recent advances in the development of direct alcohol fuel cells (DAFC). J Power Sources. 2002;105:283–296.
- Yu X, Pickup PG. Recent advances in direct formic acid fuel cells (DFAFC). J Power Sources. 2008;182:124–132.
- Choi JH, Jeong KJ, Dong Y, et al. Electro-oxidation of methanol and formic acid on PtRu And PtAu for direct liquid fuel cells. J Power Sources. 2006;163:71–75.
- Yu W, Porosoff MD, Chen JG. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem Rev. 2012;112:5780–5817.
- Kavanagh R, Cao XM, Lin WF, et al. Origin of low CO2 selectivity on platinum in the direct ethanol fuel cell. Angew Chem Int Ed. 2012;51:1572–1575.
- Silva JCM, Parreira LS, De Souza RFB, et al. PtSn/C alloyed and non-alloyed materials: differences in the ethanol electro-oxidation reaction pathways. Appl Catal B. 2011;110:141–147.
- Lima FHB, Gonzalez ER. Ethanol electro-oxidation on carbon-supported Pt–Ru, Pt–Rh and Pt–Ru–Rh nanoparticles. Electrochim.Acta. 2008;53:2963–2971.
- Wang Q, Sun GQ, Jiang LH, et al. Adsorption and oxidation of ethanol on colloid-based Pt/C, PtRu/C and Pt3Sn/C catalysts: in situ FTIR spectroscopy and online DEMS studies. Phys.Chem.Chem.Phys. 2007;9:2686–2696.
- Hu G, Nitze F, Gracia-Espino E, et al. Small palladium islands embedded in palladium–tungsten bimetallic nanoparticles form catalytic hotspots for oxygen reduction. Nat Commun. 2014;5(5253):1–9.
- Zhu C, Guo S, Dong S. PdM (M = Pt, Au) Bimetallic alloy nanowires with enhanced electrocatalytic activity for electro-oxidation of small molecules. Adv Mater. 2012;24:2326–2331.
- Mazumder V, Chi M, Mankin MN, et al. A facile synthesis of MPd (M = Co, Cu) nanoparticles and their catalysis for formic acid oxidation. Nano Lett. 2012;12:1102–1106.
- Liu W, Herrmann K, Geiger D, et al. High-performance electrocatalysis on palladium aerogels. Angew Chem Int Ed. 2012;51:5743–5747.
- Wu H, Li H, Zhai Y, et al. Facile synthesis of free-standing Pd-based nanomembranes with enhanced catalytic performance for methanol/ethanol oxidation. Adv Mater. 2012;24:1594–1597.
- Yin S, Cai M, Wang C, et al. Tungsten carbide promoted Pd–Fe as Alcohol- tolerant electrocatalysts for oxygen reduction reaction. Energy Environ Sci. 2011;4:558–563.
- Bianchini C, Shen PK. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem Rev. 2009;109:4183–4206.
- Liu D, Guo Q, Hou H, et al. PdxCoy nanoparticle/carbon nanofiber composites with enhanced electrocatalytic properties. ACS Catal. 2014;4:1825–1829.
- She Y, Lu Z, Fan W, et al. Facile preparation of PdNi/rGO and its electrocatalytic performance towards formic acid oxidation. MaterChem A. 2014;2:3894–3898.
- Cui Z, Yang M, DiSalvo FJ. Mesoporous Ti0.5Cr0.5N supported PdAgNanoalloy as highly active and stable catalysts for the electro-oxidation of formic acid and methanol. ACS Nano. 2014;8:6106–6113.
- Mao H, Huang T, Yu AS. Facile synthesis of trimetallic Cu1Au0.15Pd1.5/C catalyst for ethanol oxidation with superior activity and stability. J Mater Chem A. 2014;2:16378–16380.
- Lu Y, Jiang Y, Gao X, et al. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J Am Chem Soc. 2014;136:11687–11697.
- Fu S, Zhu C, Du D, et al. Facile one-step synthesis of three-dimensional Pd–Ag bimetallic alloy networks and their electrocatalytic activity toward ethanol oxidation. ACS Appl Mater Interfaces. 2015;7:13842–13848.
- Lua Y, Jianga Y, Chena W. Pt Pd porous nanorods with enhanced electrocatalytic activity and durability for oxygen reduction reaction. Nano Energy. 2013;2:836–844.
- Liu M, Lu Y, Chen W. Pd Ag Nanorings supported on graphene nanosheets: highly methanol-tolerant cathode electrocatalyst for alkaline fuel cells. Adv Funct Mater. 2013;23:1289–1296.
- Zhang Z, Zhang C, Sun J, et al. Ultrafine nanoporous PdFe/Fe3O4 catalysts with doubly enhanced activities towards electro-oxidation of methanol and ethanol in alkaline media. J Mater Chem A. 2013;1:3620–3628.
- Qia Z, Gengb H, Wanga X, et al. Novel nanocrystalline PdNi Alloy catalyst for methanol and ethanol electro-oxidation in alkaline media. J Power Sources. 2011;196:5823–5828.
- Shao M, Liu P, Zhang J, et al. Origin of enhanced activity in palladium alloy electrocatalysts for oxygen reduction reaction. J PhysChemB. 2007;111:6772–6775.
- Yu TH, Sha Y, Merinov BV, et al. Improved non-Pt alloys for the oxygen reduction reaction at fuel cell cathodes predicted from quantum mechanics. J Phys ChemC. 2010;114:11527–11533.
- Abe H, Matsumoto F, Alden LR, et al. Electrocatalytic performance of fuel oxidation by Pt3Ti nanoparticles. J Am Chem Soc. 2008;130:5452–5458.
- Ramesh GV, Kodiyath R, Tanabe T, et al. NbPt3 intermetallic nanoparticles: highly stable and CO-tolerant electrocatalyst for fuel oxidation. ChemElectroChem. 2014;1:728–732.
- Ramesh GV, Kodiyath R, Tanabe T, et al. Stimulation of electro-oxidation catalysis by bulk-structural transformation in intermetallic ZrPt3 nanoparticles. ACS Appl Mater Interfaces. 2014;6:16124–16130.
- Zhang Z, More KL, Sun K, et al. Preparation and characterization of PdFeNanoleaves as electrocatalysts for oxygen reduction reaction. Chem Mater. 2011;23:1570–1577.
- Jiang L, Hsu A, Chu D, et al. Oxygen reduction reaction on carbon supported Pt and Pd in alkaline solutions. J Electrochem Soc. 2009;156(3):B370–B 376.
- Singh RN, Singh A. Anindita, electrocatalytic activity of binary and ternary composite films of Pd, MWCNT and Ni, Part II: methanol electrooxidation in 1 M KOH. Int J Hydrogen Energy. 2009;34:2052–2057.
- Kodiyath R, Ramesh GV, Koudelkova E, et al. Promoted C–C bond cleavage over intermetallic TaPt3 catalyst toward low-temperature energy extraction from ethanol. Energy Environ Sci. 2015;8:1685–1689.
- Evans J, Harris IR, Guzei LS. An investigation of some palladium-titanium and some palladium-titanium-hydrogen alloys. J Less-Common Met. 1979;4:39–57.
- Harris IR, Norman M. Observations on the lattice spacings of some α Pd-X solid solutions and some Pd3X phases. J Less-Common Met. 1970;22:127–130.
- Yeh JJ, Lindau I. Atomic subshell photoionization cross sections and asymmetry parameters: 1 « Z « 103. Atomic Data Nucl Data Tables. 1985;32:1–155.
- Scofield, J H. Mon. “Theoretical photoionization cross sections from 1 to 1500 keV”. United States. doi:https://doi.org/10.2172/4545040. https://www.osti.gov/servlets/purl/4545040.
- Trzhaskovskaya MB, Nefedov VI, Yarzhemsky VG. Photoelectron angular distribution parameters for elements Z=1 to Z=54 in the photoelectron energy range 100–5000 eV. Atomic Data Nucl Data Tables. 2001;77:97–159.
- Trzhaskovskaya MB, Nikulin VK, Nefedov VI, et al. Non-dipole second order parameters of the photoelectron angular distribution for elements Z=1–100 in the photoelectron energy range 1–10keV. Atomic Data Nucl Data Tables. 2006;92:245–304.
