ABSTRACT
With the rapid development of conductive polymers, they have shown great potential in room-temperature chemical gas detection, as their electrical conductivity can be changed upon exposure to oxidative or reductive gas molecules at room temperature. However, due to their relatively low conductivity and high affinity toward volatile organic compounds and water molecules, they always exhibit low sensitivity, poor stability, and gas selectivity, which hinder their practical gas sensor applications. In addition, inorganic sensitive materials show totally different advantages in gas sensors, such as high sensitivity, fast response to low concentration analytes, high surface area, and versatile surface chemistry, which could complement the conducting polymers in terms of the sensing characteristics. It seems to be a win-win choice to combine inorganic sensitive materials with polymers for gas detection due to their synergistic effects, which has attracted extensive interests in gas-sensing applications. In this review, we summarize the recent development in polymer-inorganic nanocomposite based gas sensors. The roles of inorganic nanomaterials in improving the gas-sensing performances of conducting polymers are introduced and the progress of conducting polymer-inorganic nanocomposites including metal oxides, metal, carbon (carbon nanotube, graphene), and ternary composites are presented. Finally, a conclusion and a perspective in the field of gas sensors incorporating conducting polymer-inorganic nanocomposite are summarized.
Graphical Abstract

1. Introduction
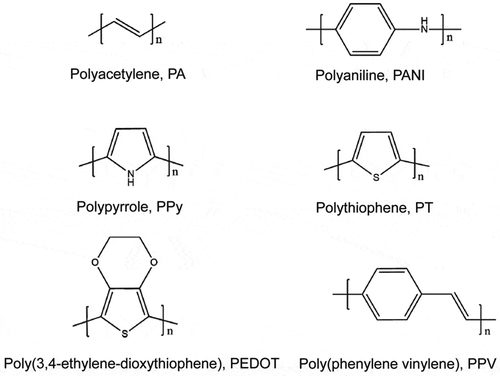
Conducting polymers have undergone rapid development and shown great potential in room-temperature chemical gas sensors since their electrical conductivity can be changed upon exposure to oxidative or reductive gas molecules at room temperature [Citation1,Citation2]. In general, the conducting polymers with typical π-conjugated structures show p-type conductive behaviors. Upon interaction with gas molecules, they behave either as an electron donor or an electron acceptor, which can result in the increase or decrease of carrier concentration, hence the change in electrical conductivity or resistance of the sensing polymers. Conducting polymers reported as sensitive materials in the literature mainly include polyacetylene (PA), polyaniline (PANI), polypyrrole (PPy), polythiophene (PT), poly(3,4-ethylenedioxythiophene) (PEDOT), poly(phenylene vinylene) (PPV) and their derivatives (). In the undoped state, conducting polymers are either electrical insulators or semiconductors. To increase their conductivity, a doping process such as protonic acid doping or redox doping is applied to polymers, which is accompanied by removing the electrons on the backbones. Positive charges remained in the backbone act as the charge carriers, which could increase the conductivity from the low level of an insulator or semiconductor (10−10-10−5 S/cm) to the conducting level (1–105 S/cm). The unique tunable electrical properties, together with easy synthesis, structural diversity, facial functionalization, and flexibility of these conducting polymer materials enable diverse energy and electrical device applications [Citation3]. Particularly, this unique doping/dedoping process allows conducting polymers to be explored as promising candidates for room-temperature gas sensor applications.
With significant efforts in the past decades, there has been a great research progress in conducting polymers based room-temperature gas sensors. However, due to their relatively low conductivity and high affinity toward volatile organic compounds (VOCs) and water molecules, they always exhibit low sensitivity, poor stability, and gas selectivity, which hinder their practical gas sensor applications. Many efforts have been made for sensing performance enhancement including increasing the active surface area, redox doping, functionalization, etc. For example, Huang et al. [Citation4] demonstrated a highly porous sensing layer composed of one-dimensional (1D) PANI nanofibers with superior sensing performances including high sensitivity, selectivity, and rapid response owing to its higher surface area compared with bulk films. Such gas-sensing enhancement effects arising from the low-dimensional structures are also reported in other conducting polymers including PPy, PT, etc. Nevertheless, although the sensing responses are greatly improved, the gas-sensing behaviors are not highly reversible and reproducible, which is a main concern for practical sensor applications. Zhang et al. [Citation5] demonstrated (+)-camphor-10-sulfonic acid (HCSA) doped polyaniline nanofibers by electrospinning methods, which exhibited greatly improved response/recovery behaviors to 500 ppm NH3. Moreover, Kwon et al. [Citation6] demonstrated that PPy functionalized with the carboxyl groups (-COOH) showed specific selectivity to dimethyl methyl phosphonate (DMMP) gas due to the intermolecular interactions between -COOH groups and phosphoryl groups of DMMP molecules. However, although the gas sensing performances were improved, their limitations such as low sensitivity, reversibility, and selectivity remained a challenge for practical use in gas sensors.
In contrast to conducting polymers, inorganic sensitive materials show totally different advantages in gas sensors, i. e. metal oxides always show high-sensitivity due to oxygen stoichiometry and active surface charge [Citation7]; however, the requirement of high temperature for operation always hinders its wide applications; metal nanostructures are adopted as sensitivity promoters by chemical and electronic sensitization effects [Citation8]; One-dimensional (1D) or two-dimensional (2D) carbon materials exhibit a fast response to low concentration analytes at room temperature owing to their low electronic noise, high surface area, and versatile surface chemistry, which could complement the conducting polymers in terms of the sensing characteristics [Citation9,Citation10]. The use of conducting polymer-inorganic nanocomposite may result in high-performance gas sensors due to their synergistic effects, which has attracted extensive interest in gas-sensing applications. In the nanocomposite system, the host organic and guest inorganic phases are interacted by weak van der Waals or hydrogen bonding or covalent or ionic covalent bonding, which could provide enhanced or novel chemical and physical functionalities. Such synergetic/complementary effects in the nanocomposite could help to eliminate their inherent drawbacks and also utilize the advantages of their individual constituents in gas-sensing fields, which could result in high-performance sensitive materials and gas sensors. In this review, we will summarize the recent development in polymer-inorganic nanocomposite toward high-performance gas sensors. In section 2, we first introduce the roles of inorganic nanomaterials in improving the gas-sensing performances of conducting polymers. In section 3, we mainly describe the progress of conducting polymer-inorganic nanocomposites including metal oxides, metal, carbon (carbon nanotube, graphene), and ternary composites, respectively. Finally, we give a conclusion and a perspective in the field of gas sensors incorporating conducting polymer-inorganic nanocomposites.
2. Comprehensive roles of inorganic nanomaterials in gas-sensing enhancement effects
2.1. Constructing P-N or Schottky heterojunctions
The gas-sensing responses of the conducting polymer-inorganic nanocomposite-based sensors are obtained by recording the time-dependent film resistance change as a function of target gas concentration. The film resistance value changes upon the exposure of reducing/oxidizing gas, and gradually restores to the original state when the target gas flow is removed. The gas-sensing response arises from the physical absorption of target analyte molecules onto the sensing films and the electron capture/donation process of the polymer matrix embedded with the inorganic nanomaterials, which is enhanced by the junction effects at the conducting polymer-inorganic interfaces.
When the conducting polymer matrix is embedded with inorganic nanomaterials, a P-N or Schottky heterojunction is formed at the conducting polymer/inorganic interfaces, depending upon the nature of inorganic nanomaterials [Citation11–16]. Taking it for example, when n-type metal oxide nanostructures are introduced into the polymer matrix, a P-N heterojunction is formed at the polymer-metal oxide interfaces, accompanying with the formation of the depletion region in both polymer and metal oxides [Citation16]. The interaction of the target gas molecules and the nanocomposite film surface lead to the decrease/increase of electrons in the polymers and therefore the change in the width of the depletion region, which could narrow/widen the conductive pathway of the polymers. Considering the concentration of doped metal oxide nanostructure to conducting polymers is low, usually in the order of 0.1–10 wt.%, the nanocomposite film resistance is mainly controlled by the conducting polymers. The synergistic effects of the changed conductivity and conductive pathway of polymers in the nanocomposite films result in the enhanced sensitivity of the nanocomposite films toward the target analyte.
2.2. Modulating film morphology
In addition to the interfacial interactions, efficient gas molecule absorption is also an important aspect to achieve a high sensing response because the physical absorption of gas molecules onto the film is the first step for gas detection. A highly porous layer with a large surface area, high pore volume, and desired pore size could introduce more active sites and increase the gas molecule absorption. By introducing inorganic nanomaterials, the film morphology of the nanocomposite films and the gas-sensing performances can be adjusted freely [Citation13,Citation15–20]. The inorganic nanomaterials could be a template for the polymerization of conducting polymers, where the nanocomposite morphology could be determined by the inorganic nanomaterials and polymerization methods. Therefore, with a nanocomposite development effort, an efficient methodology for performance enhancement could be employed to design new nanocomposite material systems and develop new synthetic strategies to well control the morphology of the nanocomposite films.
2.3. Improving electrical conductivity
The electrical conductivity of the sensitive films is also important for achieving a high sensing response. For achieving a high sensing response, charge carriers generated upon absorption of gas molecules should migrate easily and be collected at the electrode. Due to the low conductivity of organic materials, few charge carriers reach the electrodes, which results in poor sensing response. The doping methods have been reported to improve the electrical conductivity of conducting polymers. Particularly, a nanocomposite of conducting polymers and conducting inorganic nanomaterials is a simple and effective technique to improve the intra- and inter-chain mobility of charge carriers in the polymer chains [Citation21–24]. Proper selection of inorganic nanomaterials can modify the electrical conductivity to the desired level for high sensing response. The inorganic nanomaterials with high electrical conductivity could compensate for the low conductivity of conducting polymers to prevent loss of electrical signals, thus obtaining a large sensing response. For example, Shirsat et al. decorated PANI nanowires with Au nanoparticles (NPs) (~70-120 nm) to achieve enhanced gas-sensing behaviors [Citation24]. The PANI-Au nanocomposite chemiresistive sensor exhibited a significantly enhanced detection limit with good selectivity and reproducibility, which has been ascribed to the reaction between H2S molecules and Au and enhanced conductivity of PANI induced by the electron transfer from PANI (donor) to Au (acceptor).
3. Conducting polymer-based nanocomposite gas sensors
3.1. Metal oxide-conducting polymer nanocomposites
Conducting polymers are widely used as effective species for gas detection due to their excellent electrochemical and electronic properties, as well as the advantages of low cost, long-term stability, and easy synthesis. However, the disadvantages of their performance such as low sensitivity, slow response, and recovery process, poor thermal stability, and selectivity also limit their further applications in gas sensors. Fortunately, scientists have verified that polymer/metal oxide nanocomposites can not only reduce the defects of polymer or metal oxide but also effectively improve their sensitivity, thermal stability, and response speed. Through in-depth scientific research, people have a higher understanding of the mechanism responsible for the gas sensing performance enhancements. It is believed that the change of micromorphology and the formation of P-N junctions can effectively promote their sensing process.
With the attempt of different kinds of preparation methods, a variety of conducting polymer/metal oxide nanocomposites with form of thin films [Citation25], particles [Citation26–29], fibers [Citation30–33], irregular shapes [Citation34], sheets [Citation35], short rods [Citation36], tubes [Citation37], flowers [Citation38], networks [Citation39–41] and core@shells [Citation42,Citation43] have been synthesized and applied to gas sensors successively.
Figure 2. (a) An optical image of PANI/TiO2 nanocomposite-based sensor, (b) SEM images of TiO2 microfibers and (c) PANI/TiO2 nanocomposites. Reprinted with permission from [Citation30]. Copyright 2020 American Chemical Society
![Figure 2. (a) An optical image of PANI/TiO2 nanocomposite-based sensor, (b) SEM images of TiO2 microfibers and (c) PANI/TiO2 nanocomposites. Reprinted with permission from [Citation30]. Copyright 2020 American Chemical Society](/cms/asset/2f4af3a8-4813-4aba-b42e-6c17de0b4155/tsta_a_1820845_f0002_oc.jpg)
Taking PANI-based nanocomposites for example, as early as 2007, Jiang et al. [Citation25] successfully prepared gas sensors for NH3 detection using hybrid PANI/TiO2 nanocomposites. They observed that compared with mono-phase PANI-based sensors, hybrid nanocomposites based sensors showed higher sensitivity, quicker responsivity, better stability, and shorter recovery time. After that, different kinds of nanostructured metal oxides including SnO2 [Citation44–48], TiO2 [Citation30], Fe2O3 [Citation32,Citation49], GeO2 [Citation28,Citation50], ZnO [Citation34,Citation51,Citation52], WO3 [Citation38,Citation39,Citation41,Citation43], Nb2O5 [Citation53] and MoO3 [Citation36] are tried to be combined with PANI for gas detection. In 2010, sensors based on PANI/TiO2 nanofiber structures were firstly reported by Gong et al. [Citation30] for dilute NH3 detection (). They obtained the Ti4+ containing microfiber precursor by electrospinning method and then calcined the precursor at 600°C for 4 h to prepare TiO2 microfibers. After that, the P-N heterojunction nanohybrids with PANI nano-grain enchased TiO2 microfibers were formed through a polymerized reaction. The PANI/TiO2 based sensors showed high sensitivity to 50 ppt of NH3. In the nanocomposites, the PANI NPs embedded on the surface of TiO2 microfibers acted as nano-switches. When the NH3 molecules were adsorbed onto the PANI/TiO2 nanocomposites, the PANI NPs can turn off the current loop, and when the gas molecules were desorbed, the circuit was reconnected. The NH3 gas sensitivity was significantly improved with the rapid increase of sensor resistance. The next year, Wang et al. [Citation28] fabricated NH3 gas sensors using core-shell CeO2@PANI structure. The sensors exhibited a high response of 6.5 to 50 ppm under NH3 detection and great long-term stability. The internal mechanism of device performance improvement was analyzed in detail. They demonstrated that the increased sensitivity and stability benefitted from the P-N heterojunction of nanohybrids. The electron-donating NH3 changed the original space charge region at the equilibrium condition, which decreased the hole concentration in the PANI, and enlarged the depletion region from Wp to Wp-NH3. The conduction paths were thus reduced from the thicker emeraldine salt (ES) formed shell painted in green to the thinner emeraldine base (EB) formed shell painted in blue. Since the inherent resistance of CeO2 was extremely high, the entire resistance of the hybrid system increased finally ().
Figure 3. Schematic diagram about synergic effect of CeO2@PANI under NH3 gas. The inset was schematic diagram of P − N junction in equilibrium state. Reprinted with permission from [Citation28]. Copyright 2014 American Chemical Society
![Figure 3. Schematic diagram about synergic effect of CeO2@PANI under NH3 gas. The inset was schematic diagram of P − N junction in equilibrium state. Reprinted with permission from [Citation28]. Copyright 2014 American Chemical Society](/cms/asset/8c8ebbde-9750-40a4-b804-d25da96edc05/tsta_a_1820845_f0003_oc.jpg)
In recent years, the fabrication of electronic devices based on organic/inorganic hybrid systems on flexible/ductile substrates has become a new research hotspot. Compared with the traditional electronics, flexible electronics can adapt to different working environments to a certain extent, and meet the deformation requirements of the equipment.
In 2017, Bai et al. [Citation36] reported nanorod-like α-MoO3/PANI-based triethylamine (TEA) sensors. The sensors were fabricated on a flexible polyethylene terephthalate (PET) substrate with the PANI film covered onto the MoO3 nanorod framework (). In this work, comparative experiments were carried out to optimize the quality of the hybrid system and related device performance (). The optimized sensors demonstrated high selectivity and good sensitivity of 5.5 to 10 ppm TEA at room temperature. They considered that the improved response after adding MoO3 nanorods into PANI was possibly caused by two factors. On one hand, the PANI embedded on the surface areas of MoO3 robs formed a network that provided a large number of α-MoO3/PANI interfaces, which enhanced the adsorption efficiency of gas molecules. In addition, the new formed structure was conducive to the diffusion of gas molecules in the nanocomposite system. On the other hand, the more important factor for enhancing the sensing response was new-formed P-N heterojunctions, which reduced the enthalpy and activation energy of physical adsorption of gas molecules, leading to good electron-donating characteristics.
Figure 4. Schematic diagram of fabrication process of α-MoO3/PANI nanocomposites. Reprinted with permission from [Citation36]. Copyright 2017 Elsevier
![Figure 4. Schematic diagram of fabrication process of α-MoO3/PANI nanocomposites. Reprinted with permission from [Citation36]. Copyright 2017 Elsevier](/cms/asset/da88ab17-e4b3-43f6-b822-1ab8ca72edf0/tsta_a_1820845_f0004_oc.jpg)
Figure 5. SEM images of (a) MoO3 nanorods, (b) PANI powder, (c) and (d) MoO3/PANI films with different contents of MoO3 (e.g. 0.1 mg, 0.3 mg, and 1 mg); (e, f) TEM images of the α-MoO3/PANI nanocomposites.Reprinted with permission from [Citation36]. Copyright 2017 Elsevier
![Figure 5. SEM images of (a) MoO3 nanorods, (b) PANI powder, (c) and (d) MoO3/PANI films with different contents of MoO3 (e.g. 0.1 mg, 0.3 mg, and 1 mg); (e, f) TEM images of the α-MoO3/PANI nanocomposites.Reprinted with permission from [Citation36]. Copyright 2017 Elsevier](/cms/asset/ac166ef2-7e91-4aa9-8ecd-6d4ecd852dfb/tsta_a_1820845_f0005_oc.jpg)
It is worth mentioning that gas sensors based on hybrid PANI/WO3 nanocomposites on flexible PET substrates were also reported by Li et al. Flower-like [Citation38] and hollow spheres [Citation43] WO3@PANI nanocomposites were designed and applied in sensors for room-temperature NH3 detection. The highest response value was 20.1 for flower-like WO3@PANI and 25 for hollow sphere WO3@PANI to 100 ppm NH3 at room temperature.
Besides PANI, PPy is also one of the most ideal sensing candidates in the VOCs detection, since it possesses obvious advantages of good environmental stability, low operating temperature, easy synthesis, and reversible redox reaction [Citation26,Citation54–56]. Generally speaking, similar to the preparation of PANI/metal oxide nanocomposites, the typical process of preparing conductive PPy/metal oxide nanocomposites usually includes the following three steps: first, nanostructured metal oxides were prepared by chemical bath deposition and in air calcination [Citation57], in-situ growing [Citation35], hydrothermal synthesis, sol-gel method [Citation54], surfactant-assisted method, and calcination [Citation33,Citation40,Citation55], template-based hydrothermal synthesis [Citation42,Citation56], ultrasound-assisted precipitation method [Citation26] or carbon microspheres templated method [Citation56,Citation58]. Then, conducting polymer was synthesized by in-situ oxidative polymerization method [Citation29], electrochemical deposition [Citation57], chemical oxidation polymerization [Citation26], or solution method [Citation35,Citation37,Citation55]. Finally, metal oxides are combined with polymers through the interface reaction of inorganic and organic materials. In order to combine organic and inorganic materials closely to form effective PN junction and obtain high adsorption areas, a special treatment process is necessary.
Figure 6. Schematic diagram of fabrication process of the SnO2@PPy tube-in-tube structure. Reprinted with permission from [Citation37]. Copyright 2013 Royal Society of Chemistry
![Figure 6. Schematic diagram of fabrication process of the SnO2@PPy tube-in-tube structure. Reprinted with permission from [Citation37]. Copyright 2013 Royal Society of Chemistry](/cms/asset/467549fc-4121-4880-b9a9-de745f9528cf/tsta_a_1820845_f0006_oc.jpg)
For instance, Jun et al. [Citation40] prepared sensors based on tube-in-tube SnO2@PPy construction for detecting DMMP at extremely low concentrations (0.05 ppb). The tube-in-tube construction was fabricated via a single nozzle electrospinning method with two kinds of mixed solvents, i.e., N,N-dimethylformamide (DMF) and ethanol. By the control of calcination conditions and the successive evaporation of solvents with different boiling points, the tube-in-tube construction was fabricated. Then, the ferric cations (Fe3+) were modified onto the surface of SnO2, followed by a vapor deposition polymerization step in the vacuum environment to form the organic/inorganic hybrid structure (). Dongzhi Zhang et al. [Citation56] demonstrated NH3 gas sensors based on PPy/Zn2SnO4 nanocomposite with extremely high sensitivity. PPy nanospheres were combined with Zn2SnO4 hollow spheres through a layer-by-layer alternative deposition. It is worth mentioning that in addition to PANI/metal oxides and PPy/metal oxides based gas sensors, PT/metal oxides [Citation58,Citation59] and poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonic acid) (PEDOT: PSS)/metal oxide [Citation60–62] based sensors have also been fabricated and studied.
In summary, there are two main advantages of doping metal oxide into conducting polymer to form the heterostructure. On one hand, the introduction of metal oxides can adjust the electrical properties of conducting polymers and form a unique P-N junction with chemical electron conduction property. On the other hand, nanostructured metal oxides with various morphologies can be easily introduced into conductive polymers, thus greatly increasing the surface areas of the mixture. In , the notable examples of the sensors based on the hybrid system of conducting polymers and metal oxides developed in the recent decade are listed, and their main sensing properties are summarized.
Table 1. Conducting polymer/metal oxides hybrid composites used in gas sensors
3.2. Metal-conducting polymer nanocomposites
As a kind of catalyst with high electrocatalytic activity and good stability, metal nanostructures based on such as Pd, Pt, Au, have been widely studied in gas sensing. Relative to the precious metal NPs, metal nanostructures based on Ag and Cu are also commonly used high-efficiency catalysts with low-cost and can effectively improve the thermal stability, conductivity, and adsorption performance of gas sensors. Doping metal NPs into the polymer can significantly change the electrical properties of the newly formed system, and exhibit excellent adsorption and desorption capacity of reducing gases such as H2, CO, NH3 at room temperature. It is proved that the electrical conductance of the composites is sensitive to the shape, size, and content of metal nanophase. Various species of nanostructured metals including Pd [Citation66], Au [Citation67], Ag [Citation68], Pt [Citation69], and Cu [Citation70] were used to combine with conducting polymers through different approaches such as hydrothermal synthesis [Citation19,Citation66,Citation71], in-situ chemical oxidative polymerizing [Citation68,Citation70,Citation72–76], self-assembling [Citation67,Citation69,Citation77], electrodeposition [Citation78], chloroaurate anion reduction [Citation79], template-based vapour deposition polymerization (VDP) [Citation80], and in-situ photo-polymerization [Citation81,Citation82]. At the same time, the combination of different kinds of polymers with specific NPs has also been widely studied. Take Ag NPs as an example, PANI/Ag [Citation68,Citation73,Citation81,Citation82], PPy/Ag [Citation72,Citation74], and PEDOT/Ag [Citation80] composite systems has been fabricated and applied to gas sensors.
Early in 2005, Athawale et al. [Citation66] reported a work about PANI/Pd nanocomposites synthesized by reflux method. They exposed PANI/Pd nanocomposites to different aliphatic alcohol vapor of methanol, ethanol, and isopropanol and found that the sensors based on PANI/Pd nanocomposites showed high sensitivity, well selectivity, and fast response towards methanol vapor. After then, the gas response of different kinds of metal/polymer nanocomposites has been widely studied. Among them, the influence of concentration, size, shape, and type of metal nanomaterials on device performance has become a research hotspot. Specifically, in 2009, nanocomposites of PPy/Pd were fabricated by Hong et al. [Citation19] through solution reduction of Pd ions and subsequent gas-phase polymerization of pyrrole. They found that the concentration and ratio of reactants, polymerization reaction time, and the content of polymer additives would seriously affect the morphology of the composite membrane, and finally determined the sensitivity of the nanocomposite to NH3 detection. In this work, they demonstrated that the gas-sensing properties of nanocomposites were greatly affected by the size of Pd NPs and the configuration of composite membranes. With the addition of PVP, the size of NPs can be reduced to less than 10 nm and better distribution can be achieved. The gas sensors based on the composite of PPy and small-sized Pd showed a higher response than PPy alone because the well-distributed Pd NPs provided more active surface reaction sites and affected the charge transfer between polymer and reaction gas molecules.
Figure 7. (a–c) TEM images of the hybrid PPy/Au system and (d) size distribution of Au NPs. Reprinted with permission from [Citation75]. Copyright 2013 Elsevier
![Figure 7. (a–c) TEM images of the hybrid PPy/Au system and (d) size distribution of Au NPs. Reprinted with permission from [Citation75]. Copyright 2013 Elsevier](/cms/asset/8e4e6131-8b25-46d5-a54f-0f42bd71dc23/tsta_a_1820845_f0007_b.gif)
In 2013, Zhang et al. [Citation75] developed a one-pot strategy method to prepare PPy/Au nanocomposites with uniform Au NPs dispersed on PPy. By introducing material lysine, the size of Au NPs was significantly reduced. Meanwhile, there was a good consistency distribution for the NPs in the hybrid system (). The Au NPs became very active when their sizes were smaller than 5 nm due to the quantum size effect, which is helpful for NH3 molecules to adsorb on the PPy sensing layer in the presence of atmospheric O2. The sensitivity of PPy/Au nanocomposites for room-temperature detection reached to about 1.47 for 300 ppm NH3. They contributed the obviously enhanced sensor properties to the uniform small-sized Au NPs distribution. The possible mechanism was also discussed (). Conducting polymer PPy is a kind of p-type semiconductor. In the PPy/Au hybrid system, the introduction of Au NPs will form the ‘nano Schottky effect’ at the interface, and then form a depletion layer, thus increasing the resistance of the whole system. In the presence of electron-doped NH3, the holes in the hybrid system will be consumed and the resistance will be increased.
In addition to the size of NPs, the concentration of NPs is another important research point. In 2009, Arup Choudhury [Citation68] synthesized PANI/Ag nanocomposites at the concentration of 0.5, 1.5, and 2.5 mol% Ag by in-situ chemical polymerization. It was found that the sensitivity and response/recovery time of PANI/Ag nanocomposite-based sensor was depended on the concentration of Ag NPs. Compared with pure PANI, the dielectric and conductivity of PANI/Ag nanocomposites were significantly improved, and the AC conductivity was increased by about 100 times. The sensors based on PANI/Ag nanocomposites showed faster and more reversible performance in ethanol detection. When the concentration of Ag was 2.5 mol%, the sensors showed the highest sensing capacity and the best long-term stability. After then, Park et al. [Citation80] reported the study of NH3 chemical sensors based on Ag NPs/PEDOT nanotube composites. The concentration of Ag NPs was changed by controlling the AgNO3 with 5%, 10%, 30%, and 40% (w/w). The sample Ag NPs/PEDOT nanotube with 5 wt% exhibited the highest sensitivity and the lowest detection limit to NH3. However, it was worth noting that although the higher concentration of Ag NPs can increase the surface reaction sites and improve the conductivity when the concentration was too high, the Ag NPs would agglomerate, and destroy the original morphology, which played a negative role in the gas-sensing response.
Figure 8. Possible gas detection mechanism of PPy/Au hybrids. Reprinted with permission from [Citation75]. Copyright 2013 Elsevier
![Figure 8. Possible gas detection mechanism of PPy/Au hybrids. Reprinted with permission from [Citation75]. Copyright 2013 Elsevier](/cms/asset/d07913d1-c25f-436f-84cb-bd4ae19ec86b/tsta_a_1820845_f0008_oc.jpg)
Generally, in the metal-conducting polymer nanocomposite system, metals often exist in the form of NPs, while conductive polymers often exist in the framework of thin films. However, one dimensional or quasi one-dimensional nanostructured polymer materials can also take advantage of their high surface volume ratio to optimize the application of gas sensors. A composite with a hierarchical network structure was prepared for NH3 detection on the flexible PET substrate using Ag nanowires as a framework and combining with one-dimensional-nanostructured PANI [Citation73]. Even if the concentration was as low as 5 ppm, the sensitivity was linear with the increase of NH3 concentration. Compared to the particle-like PANI films, hierarchical PANI/Ag nanocomposites based films exhibited higher selectivity to NH3. They believed that the significant increase in sensing performance was attributed to the confined nanostructures of quasi one dimensional in the network like films, the special acid-base reaction, and the enlarged specific surface areas of the layered network of PANI nanostructures.
To sum up, metal NPs can improve the sensing properties of conducting polymers, mainly due to the following reasons: firstly, the introduction of metal NPs changes the conductivity of polymers. Secondly, certain kinds of metal NPs show a chemical affinity for specific gas substances, and metal NPs as chemical receptors enhance the selectivity of sensors. Last, the effective surface area of nanocomposites interacting with target gas is increased by introducing nano metals into conductive polymers. summarizes the examples of gas sensors based on polymer/metal hybrid composites developed in recent 10 years and lists their main sensing characteristics.
Table 2. Conducting polymer/metal nanostructures hybrid composites used in gas sensors
3.3. Carbon nanotube/graphene-conducting polymer nanocomposites
3.3.1. Carbon nanotube-conducting polymer nanocomposites
Carbon nanotubes composed entirely of carbon atoms are very suitable for chemical sensing because of their unique wildly adjustable conductivity, excellent mechanical properties, and high environmental stability [Citation84–89]. However, in gas-sensing applications, the material shows poor sensitivity and selectivity, which usually needs surface modification. Therefore, it seems to be a win-win choice to combine carbon nanotubes with polymer gas-sensing materials, which usually exhibit relatively low chemical stability and conductivity but good sensitivity and selectivity.
In 2011, PANI/single-walled carbon nanotube (SWCNT) composites-based N2H2 sensors were fabricated by Ding et al. and the internal response mechanism was discussed [Citation85]. They demonstrated that the electron transfer process was easier due to the effective interaction between the SWCNT core and the PANI shell (). When exposed to the oxidizing atmosphere, electrons transferred from the SWCNT core to PANI shell, which effectively improved the oxidizing energy barrier between emeraldine/pernigraniline forms. When exposed to the reducing environment upon N2H4, the SWCNT core could promote the transition between emeraldine and leucoemeraldine formed of PANI, which made the sensing of PANI/CNTs composite reversible.
Figure 9. (a) Cyclic voltammograms of pristine SWNTs, PANI, and SWNT/PANI nanocomposites; (b) Three oxidation states of PANI. Reprinted with permission from [Citation85]. Copyright 2010 John Wiley and Sons
![Figure 9. (a) Cyclic voltammograms of pristine SWNTs, PANI, and SWNT/PANI nanocomposites; (b) Three oxidation states of PANI. Reprinted with permission from [Citation85]. Copyright 2010 John Wiley and Sons](/cms/asset/ad370f35-c47c-4615-be99-a7ffde5c6669/tsta_a_1820845_f0009_b.gif)
The combination of CNTs and polymer materials and the formation of good morphology and distribution are the key to affect the sensing performance. Therefore, the influence of the bonding approach on the quality and sensing properties of composites is also a challenging task. For instance, Liao et al. [Citation86] used N-phenyl-p-phenylenediamine as an initiator to assist the polymerization process of PANI/SWCNT composite nanofibers. The nanocomposites showed widely tunable conductivities from 10−4 to 102 S/cm. Chemosensors based on PANI/1.0 wt% SWCNT composite nanofibers showed a much more rapid response of 120 s to 100 ppb HCl and NH3 vapors compared to pure PANI nanofibers which response time was 1000 s. In 2017, tetra-b-carboxyphthalocyanine cobalt(II) (TcPcCo) was employed to fabricate ultra-fast PANI/multi-walled CNT (MWCNT) nanocomposites based NH3 gas sensor [Citation87]. In this work, TcPcCo not only acted like a sensitive accelerator for gas sensors but also promoted the polymerization of MWCNTs and PANI to improve the conductivity of PANI in the form of dopant. Due to the synergistic effects of TcPcCo, PANI, and the MWCNTs, the sensors based on MWCNT/PANI hybrids showed an extremely rapid response/recovery time of 5.0/12.0 s to 100 ppm NH3. The highest sensitivity was 140.99% to 100 ppm NH3 and the lowest detection limit was 36 ppb.
Compared with polymer gas-sensing materials, CNTs exhibit another unique advantage of higher light transmittance. Therefore, it is also meaningful to fabricate gas sensors based on CNTs/polymer hybrid systems on flexible transparent substrates for some specific applications. Wan et al. [Citation88] prepared a chemical gas sensor with transparent and flexible characteristics using a transparent functional multiwalled CNT (FMWCNT) network-based conductive film combined with nanostructured PANI nanorods. The nanocomposite thin film was fabricated through in-situ chemical-oxidizing polymerization method of aniline in a MWCNT liquid suspension (). The flexible and transparent chemical gas sensor based on PANI/MWCNT nanocomposite film showed a high transmittance of 85% at 550 nm. Meanwhile, the sensor possessed excellent sensitivity at room temperature. After 500 bend/stretch cycles, no significant degradation was observed in performance, showing good flexibility and portable wearable properties (). They believed that CNTs improved the efficiency of electron transport and aggregation. At the same time, the hierarchical structures of the composites increased the surface area to volume ratio, thereby improving sensing performance and making sensors more reliable.
Figure 10. Schematic diagram of fabrication process of the hierarchically PANI/FMWCNT nanocomposites. Reprinted with permission from [Citation88]. Copyright 2015 John Wiley and Sons
![Figure 10. Schematic diagram of fabrication process of the hierarchically PANI/FMWCNT nanocomposites. Reprinted with permission from [Citation88]. Copyright 2015 John Wiley and Sons](/cms/asset/68dcb763-0a90-4904-a8bd-7b7d8e69dd33/tsta_a_1820845_f0010_oc.jpg)
Figure 11. (a), (b) Gas-sensing sensitivity of sensors based on PANI/FMWCNT nanocomposite network; (c) selectivity and (d) flexibility of the sensors based on PANI/FMWCNT nanocomposite network. Reprinted with permission from [Citation88]. Copyright 2015 John Wiley and Sons
![Figure 11. (a), (b) Gas-sensing sensitivity of sensors based on PANI/FMWCNT nanocomposite network; (c) selectivity and (d) flexibility of the sensors based on PANI/FMWCNT nanocomposite network. Reprinted with permission from [Citation88]. Copyright 2015 John Wiley and Sons](/cms/asset/986e5b16-2bdc-4dbc-bcb3-9707ac66529f/tsta_a_1820845_f0011_oc.jpg)
3.3.2. Graphene-conducting polymer nanocomposites
Graphene, well known as a two-dimensional sp2 bonded honeycomb carbon sheet with single atomic layer thickness, is widely applied in chemical sensing, field-effect transistors, transparent conductive electrodes due to its interesting quantum Hall effect, excellent mechanical strength, high thermal conductivity, and large specific surface area. Graphene sensors exhibit good sensitivity to H2, NO2, NH3, H2O, CO, and other gases, and has become one of the most popular candidate sensors in the field of gas sensing. The excellent gas sensitivity of graphene is mainly due to the following two aspects: firstly, the two-dimensional honeycomb structure of graphene exposes all carbon atoms to the environment, providing a large surface area for the reaction with gas molecules. Secondly, the high-quality graphene lattice and its two-dimensional properties make it has lower electrical noise and easier to shield charge fluctuations than a one-dimensional system. Therefore, scientists have made a lot of exploration on the preparation methods of polymer/graphene nanocomposites and the related gas-sensing properties.
For example, in early 2010, Al-Mashat et al. [Citation90] reported gas sensors based on PANI/graphene nanocomposites via a chemical synthetic method for H2 detection for the first time. In this work, PANI nanofibers were formed on the surface of graphene by ultrasonic treatment of graphene in a mixed solution of aniline monomer and ammonium persulfate. They found that the PANI/graphene nanocomposite-based gas sensors possessed a sensitivity of 16.57% to 1% H2 gas, which was much higher than that of pure graphene sheets and PANI nanofibers. For NH3 detection, Wu et al. [Citation91] fabricated PANI/graphene composites synthesized by chemical oxidative polymerization. The results indicated that PANI/graphene-based sensors exhibited a sensitivity of about 5 times higher than that of pure PANI, showing an approximately linear relationship within NH3 concentration ranging from 1 to 6400 ppm. The minimum detection limit of PANI/graphene sensors was about 1 ppm, approximately one-tenth of the detection limit of pure PANI. The preparation cost of graphene is very high, especially in large-area manufacturing. Therefore, reduced graphene oxide (rGO) with high surface area and relatively low cost becomes a good alternative material. Guo et al. [Citation92] modified PANI NPs on rGO and finally integrated them into PANI nanofibers to form a hierarchical network film for NH3 detection. The optimized sensors showed a detectable concentration range from 100 ppb to 100 ppm, with a response time of 36 s and a recovery time of 18 s. Meanwhile, the network film exhibited an excellent transparency (90.3% at 550 nm) and reliable flexibility with no significant lack in performance after 1000 bend/extension cycles.
Generally speaking, nanostructured carbon possesses the advantages of a well-defined huge area to volume ratio and wide conductivity range. The carbon framework can be altered with functional groups, and the nanostructured carbon itself can also be modified on the conductive polymer as functional groups. lists the notable examples of conducting polymer/CNTs and polymer/graphene nanocomposite-based sensors in recent 10 years and lists their main sensing performance.
Table 3. Conducting polymer/CNT and polymer/graphene nanostructures hybrid composites used in gas sensors
3.4. Polymer-based ternary nanocomposites
As mentioned above, the binary hybrid system composed of metal oxides, metal NPs, CNTs, or graphene with conductive polymers shows a good synergistic effect, which is conducive to optimizing the sensing characteristics. In order to further enhance the sensing performance, the research of ternary hybrid system has attracted more and more attention in recent years. Various gas sensors based on ternary nanocomposite have been synthesized recently for gas-sensing research, mainly include metal particles-metal oxide-conducting polymers [Citation106–109], metal particles-carbon nanotubes-conducting polymers [Citation110], metal particles-graphene-conducting polymers [Citation111,Citation112], metal oxide-graphene-conducting polymers [Citation113–117], metal oxide-metal oxide-conducting polymers [Citation118], metal oxide-metal oxide-metal oxide-conducting polymers [Citation119].
In 2017, Liu et al. [Citation107] applied an in-situ self-assembly method to fabricate Au-TiO2-PANI ternary nanocomposite thin film-based NH3 gas sensors (). They tested response characteristics to NH3 concentrations ranging from 10 to 50 ppm at room temperature. The results showed that compared with the PANI-TiO2 binary film, the sensors based on PANI-TiO2-Au ternary composites performed a higher response value of 48.6% to 123% and shorter response time of 52 to 122 s, as well as better selectivity, and reversibility, which could be attributed to the synergy of nano junctions and the combined effect of Au nanorods catalysis.
Figure 12. Schematic diagram of fabrication process of the PANI-TiO2-Au ternary nanocomposites. Reprinted with permission from [Citation107]. Copyright 2017 Elsevier
![Figure 12. Schematic diagram of fabrication process of the PANI-TiO2-Au ternary nanocomposites. Reprinted with permission from [Citation107]. Copyright 2017 Elsevier](/cms/asset/964a84b4-c76b-4620-8c8f-36e321e6347b/tsta_a_1820845_f0012_oc.jpg)
Figure 13. Fabrication process of the (CPPy)/CNTs/Pd nanocomposites. Reprinted with permission from [Citation110]. Copyright 2015 Royal Society of Chemistry
![Figure 13. Fabrication process of the (CPPy)/CNTs/Pd nanocomposites. Reprinted with permission from [Citation110]. Copyright 2015 Royal Society of Chemistry](/cms/asset/4c5b4116-bb99-4019-b6ef-2114c8407546/tsta_a_1820845_f0013_oc.jpg)
Due to the success of CNTs and metal nanocomposites applied in gas sensing, researchers began to couple the metal NPs and CNTs with conductive polymers to obtain new composite sensing materials with better gas-sensing properties. For example, Park et al. [Citation110] introduced a novel and simple method for preparing carboxylated polypyrrole (CPPy)/CNTs/Pd nanocomposites for NH3 detection (). Through accurate material proportion and experimental condition optimization, the sensors possessed the minimum detection limit as low as 1 ppm to H2 and exhibited excellent reproducibility and reversibility.
In 2019, Zhang et al. [Citation117] synthesized a kind of ZnO/graphene quantum dots (GQDs)/PANI nanocomposites through in-situ polymerization. When exposed to acetone atmosphere at room temperature, ZnO/GQDs/PANI nanocomposite sensors exhibited high sensitivity about 2% to 500 ppb acetone, short response/recovery time of 15/27 s, reliable repeatability, outstanding selectivity, as well as remarkable long-term stability. It should be noted that the ternary material mixture system based on the conductive polymer is not a simple random combination. In order to realize the synergistic reinforcement effect of various materials, process compatibility, morphology, and structure, composition ratio and function distribution should be considered comprehensively. lists the notable examples of conducting ternary nanocomposite-based sensors developed in the recent decade and summarizes their main sensing performance.
Table 4. Conducting polymer/multicomponent nanostructures hybrid composites used in gas sensors
4. Conclusions and outlook
Outstanding achievements have been achieved in conducting polymer-based gas sensors in the past decades of years. However, to realize future commercialization, future efforts to increase the gas-sensing performance including high sensitivity, long-term stability, high selectivity, and high reversibility are still necessary. To date, in order to improve gas-sensing responses, a great number of doping inorganic nanomaterials and doping techniques has been developed to construct P-N or Schottky heterojunctions, improve electrical conductivity and modulate film morphology. In this review, we summarized recent advances in conducting polymer-inorganic nanocomposite-based gas sensors with excellent gas-sensing performances. Up to now, inorganic nanomaterials including metal oxides, metal, carbon nanotube, graphene, and binary nanocomposites (such as metal/metal oxide, metal oxide/carbon) to form conducting polymer-inorganic nanocomposites have been reported, which have been found to be an excellent platform for high-performance room-temperature gas sensing. This review clearly demonstrates that the sensing characteristics of the nanocomposite depend both on the composition and structural characteristics of individual constituents and the synergistic effects between them.
Although conducting polymer-inorganic nanocomposites have been adopted as excellent room-temperature gas-sensing elements in the literature, there are still more challenges to the understanding of the nature behind and further sensing performance enhancement toward practical applications. First, advanced analytical methods and theoretical modeling are necessary to analyze the intrinsic functions of inorganic nanomaterials responsible for gas-sensing enhancement effects, which could guide us to explore new nanocomposite systems toward practical applications. Second, from the point of view of synthesis methods, inorganic nanomaterials tend to aggregate in conducting polymer precursors and solvents, and nanocomposite films should be adhered well to substrates and electrodes. Exploring new synthesis methods of inorganic nanomaterials and nanocomposite films including modifying the surface of inorganic nanomaterials, adopting high-aspect-ratio inorganic nanomaterials, substrate surface functional group modification, etc., should be the next direction for researchers. Third, selectivity is still a major concern for practical applications of conducting polymer-inorganic nanocomposites. The development of target-analyte-specific nanocomposites with high sensitivity and long-term stability is urgently required. The interfaces between organic host materials and inorganic guest nanomaterials are the key toward high selectivity and sensitivity. Therefore, the interface needs to be more clearly clarified and understood by means of advanced interface analyze techniques. Forth, the robustness to the humidity of the gas sensor is important for practical applications because the environment is always complex with high humidity. Interestingly, PANI exhibits better sensing response and recovery properties at higher humidity, which makes PANI-based nanocomposites promising for high-humidity application cases. The sensing response enhancement is always contributed to the protonation doping effects of PANI by water molecules. However, the inherent mechanisms need to be systematically investigated, which will promote the applications of polymer-inorganic nanocomposites based gas sensor in practical high-humidity environments. We hope that this review will inspire the creation of new nanocomposite systems, synthesis methods, sensing mechanisms, and interface physics, and promote further development of high-performance room-temperature gas sensors.
Disclosure statement
There are no conflicts of interest to declare.
Additional information
Funding
References
- Ibanez JG, Rincon ME, Gutierrez-Granados S, et al. Conducting polymers in the fields of energy, environmental remediation, and chemical-chiral sensors. Chem Rev. 2018;118:4731–4816.
- Wu W. Stretchable electronics: functional materials, fabrication strategies and applications. Sci Technol Adv Mater. 2019;20:187–224.
- Park SJ, Park CS, Yoon H. Chemo-electrical gas sensors based on conducting polymer hybrids. Polymers (Basel). 2017;9:155.
- Huang J, Virji S, Weiller BH, et al. Nanostructured polyaniline sensors. Chem Eur J. 2004;10:1314–1319.
- Zhang Y, Kim JJ, Chen D, et al. Electrospun polyaniline fibers as highly sensitive room temperature chemiresistive sensors for ammonia and nitrogen dioxide gases. Adv Funct Mater. 2014;24:4005–4014.
- Kwon OS, Park E, Kweon OY, et al. Novel flexible chemical gas sensor based on poly(3,4-ethylenedioxythiophene) nanotube membrane. Talanta. 2010;82:1338–1343.
- Yang G, Zhang M, Dong D, et al. TiO2 based sensor with butterfly wing configurations for fast acetone detection at room temperature. J Mater Chem C. 2019;7:11118–11125.
- He L, Liu Y, Liu J, et al. Core-shell noble-metal@metal-organic-framework nanoparticles with highly selective sensing property. Angew Chem Int Ed Engl. 2013;52:3741–3745.
- Zhou Y, Azumi R. Carbon nanotube based transparent conductive films: progress, challenges, and perspectives. Sci Technol Adv Mater. 2016;17:493–516.
- Giampiccolo A, Tobaldi DM, Leonardi SG, et al. Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2. Appl Catal B-Environ. 2019;243:183–194.
- Pirsa S, Alizadeh N. Design and fabrication of gas sensor based on nanostructure conductive polypyrrole for determination of volatile organic solvents. Sensor Actuat B-Chem. 2010;147:461–466.
- Li X, Xu J, Jiang Y, et al. Toward agricultural ammonia volatilization monitoring: A flexible polyaniline/Ti3C2T hybrid sensitive films based gas sensor. Sensor Actuat B-Chem. 2020;316:128144.
- Jian Y, Hu W, Zhao Z, et al. Gas sensors based on chemi-resistive hybrid functional nanomaterials. Nano-Micro Lett. 2020;12:71.
- Hangarter CM, Chartuprayoon N, Hernández SC, et al. Hybridized conducting polymer chemiresistive nano-sensors. Nano Today. 2013;8:39–55.
- Zhang Y, Zhang J, Jiang Y, et al. Ultrasensitive flexible NH3 gas sensor based on polyaniline/SrGe4O9 nanocomposite with ppt-level detection ability at room temperature. Sensor Actuat B-Chem. 2020;319:128293.
- Li S, Liu A, Yang Z, et al. Room temperature gas sensor based on tin dioxide@ polyaniline nanocomposite assembled on flexible substrate: ppb-level detection of NH3. Sensor Actuat B-Chem. 2019;299:126970.
- Lu CF, Liao SF, Wang KH, et al. Rapid template-free synthesis of nanostructured conducting polymer films by tuning their morphology using hyperbranched polymer additives. Nanoscale. 2019;11:20977–20986.
- Yang Y, Li S, Yang W, et al. In situ polymerization deposition of porous conducting polymer on reduced graphene oxide for gas sensor. ACS Appl Mater Inter. 2014;6:13807–13814.
- Hong L, Li Y, Yang M. Fabrication and ammonia gas sensing of palladium/polypyrrole nanocomposite. Sensor Actuat B-Chem. 2010;145:25–31.
- Wang X, Gong L, Zhang D, et al. Room temperature ammonia gas sensor based on polyaniline/copper ferrite binary nanocomposites. Sensor Actuat B-Chem. 2020;322:128615.
- Kaushik A, Kumar R, Arya SK, et al. Organic-inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem Rev. 2015;115:4571–4606.
- Chang Q, Zhao K, Chen X, et al. Preparation of gold/polyaniline/multiwall carbon nanotube nanocomposites and application in ammonia gas detection. J Mater Sci. 2008;43:5861–5866.
- Do J-S, Chang W-B. Amperometric nitrogen dioxide gas sensor based on PAn/Au/Nafion® prepared by constant current and cyclic voltammetry methods. Sensor Actuat B-Chem. 2004;101:97–106.
- Shirsat MD, Bangar MA, Deshusses MA, et al. Polyaniline nanowires-gold nanoparticles hybrid network based chemiresistive hydrogen sulfide sensor. Appl Phys Lett. 2009;94:083502.
- Tai H, Jiang Y, Xie G, et al. Fabrication and gas sensitivity of polyaniline–titanium dioxide nanocomposite thin film. Sensor Actuat B-Chem. 2007;125:644–650.
- Barkade SS, Pinjari DV, Singh AK, et al. Ultrasound assisted miniemulsion polymerization for preparation of polypyrrole–zinc oxide (PPy/ZnO) functional latex for liquefied petroleum gas sensing. Ind Eng Chem Res. 2013;52:7704–7712.
- Jun Seop L. Multidimensional polypyrrole/iron oxyhydroxide hybrid nanoparticles for chemical nerve gas agent sensing application. ACS Nano. 2013;11:10139—10147.
- Wang L, Huang H, Xiao S, et al. Enhanced sensitivity and stability of room-temperature NH3 sensors using core-shell CeO2 nanoparticles@cross-linked PANI with p-n heterojunctions. ACS Appl Mater Inter. 2014;6:14131–14140.
- Sun J, Shu X, Tian Y, et al. Preparation of polypyrrole@WO3 hybrids with p-n heterojunction and sensing performance to triethylamine at room temperature. Sensor Actuat B-Chem. 2017;238:510–517.
- Gong J, Li Y, Hu Z, et al. Ultrasensitive NH3 gas sensor from polyaniline nanograin enchased TiO2 fibers. J Phys Chem C. 2010;114:9970–9974.
- Li Y, Ban H, Yang M. Highly sensitive NH3 gas sensors based on novel polypyrrole-coated SnO2 nanosheet nanocomposites. Sensor Actuat B-Chem. 2016;224:449–457.
- Li Y, Zhao H, Ban H, et al. Composites of Fe2O3 nanosheets with polyaniline: preparation, gas sensing properties and sensing mechanism. Sensor Actuat B-Chem. 2017;245:34–43.
- Beniwal A, Sunny. Electrospun SnO2/PPy nanocomposite for ultra-low ammonia concentration detection at room temperature. Sensor Actuat B-Chem. 2019;296:126660.
- Talwar V, Singh O, Singh RC. ZnO assisted polyaniline nanofibers and its application as ammonia gas sensor. Sensor Actuat B-Chem. 2014;191:276–282.
- Li Y, Jiao M, Yang M. In-situ grown nanostructured ZnO via a green approach and gas sensing properties of polypyrrole/ZnO nanohybrids. Sensor Actuat B-Chem. 2017;238:596–604.
- Bai S, Zhao Y, Sun J, et al. Preparation of conducting films based on α-MoO3/PANI hybrids and their sensing properties to triethylamine at room temperature. Sensor Actuat B-Chem. 2017;239:131–138.
- Jun J, Lee JS, Shin DH, et al. Fabrication of a one-dimensional tube-in-tube polypyrrole/tin oxide structure for highly sensitive DMMP sensor applications. J Mater Chem A. 2017;5:17335–17340.
- Li S, Lin P, Zhao L, et al. The room temperature gas sensor based on Polyaniline@flower-like WO3 nanocomposites and flexible PET substrate for NH3 detection. Sensor Actuat B-Chem. 2018;259:505–513.
- Kulkarni SB, Navale YH, Navale ST, et al. Enhanced ammonia sensing characteristics of tungsten oxide decorated polyaniline hybrid nanocomposites. Org Electron. 2017;45:65–73.
- Jamalabadi H, Mani-Varnosfaderani A, Alizadeh N. Detection of alkyl amine vapors using PPy-ZnO hybrid nanocomposite sensor array and artificial neural network. Sensor Actuat A-Phys. 2018;280:228–237.
- Kulkarni SB, Navale YH, Navale ST, et al. Hybrid polyaniline-WO3 flexible sensor: A room temperature competence towards NH3 gas. Sensor Actuat B-Chem. 2019;288:279–288.
- Zhang J, Wang S, Xu M, et al. Polypyrrole-coated SnO hollow spheres and their application for ammonia sensor. J Phys Chem C. 2009;113:1662–1665.
- Li S, Liu A, Yang Z, et al. Design and preparation of the WO3 hollow spheres@ PANI conducting films for room temperature flexible NH3 sensing device. Sensor Actuat B-Chem. 2019;289:252–259.
- Deshpande NG, Gudage YG, Sharma R, et al. Studies on tin oxide-intercalated polyaniline nanocomposite for ammonia gas sensing applications. Sensor Actuat B-Chem. 2009;138:76–84.
- Bai S, Tian Y, Cui M, et al. Polyaniline@SnO2 heterojunction loading on flexible PET thin film for detection of NH3 at room temperature. Sensor Actuat B-Chem. 2016;226:540–547.
- Betty CA, Choudhury S, Arora S. Tin oxide–polyaniline heterostructure sensors for highly sensitive and selective detection of toxic gases at room temperature. Sensor Actuat B-Chem. 2015;220:288–294.
- Xu H, Ju D, Li W, et al. Low-working-temperature, fast-response-speed NO2 sensor with nanoporous-SnO2/polyaniline double-layered film. Sensor Actuat B-Chem. 2016;224:654–660.
- Jian KS, Chang CJ, Wu JJ, et al. High response CO sensor based on a polyaniline/SnO2 nanocomposite. Polymers (Basel). 2019;11:184.
- Sen T, Shimpi NG, Mishra S, et al. Polyaniline/γ-Fe2O3 nanocomposite for room temperature LPG sensing. Sensor Actuat B-Chem. 2014;190:120–126.
- Liu C, Tai H, Zhang P, et al. A high-performance flexible gas sensor based on self-assembled PANI-CeO2 nanocomposite thin film for trace-level NH3 detection at room temperature. Sensor Actuat B-Chem. 2018;261:587–597.
- Das M, Sarkar D. One-pot synthesis of zinc oxide - polyaniline nanocomposite for fabrication of efficient room temperature ammonia gas sensor. Ceram Int. 2017;43:11123–11131.
- Li Y, Jiao M, Zhao H, et al. High performance gas sensors based on in-situ fabricated ZnO/polyaniline nanocomposite: the effect of morphology on the sensing properties. Sensor Actuat B-Chem. 2018;264:285–295.
- Kotresh S, Ravikiran YT, SC VK, et al. Solution based–spin cast processed LPG sensor at room temperature. Sensor Actuat A-Phys. 2017;263:687–692.
- Mane AT, Navale ST, Sen S, et al. Nitrogen dioxide (NO2) sensing performance of p-polypyrrole/n-tungsten oxide hybrid nanocomposites at room temperature. Org Electron. 2015;16:195–204.
- Jamalabadi H, Alizadeh N. Enhanced low-temperature response of PPy-WO3 hybrid nanocomposite based gas sensor deposited by electrospinning method for selective and sensitive acetone detection. IEEE Sens J. 2017;17:2322–2328.
- Zhang D, Wu Z, Zong X, et al. Fabrication of polypyrrole/Zn2SnO4 nanofilm for ultra-highly sensitive ammonia sensing application. Sensor Actuat B-Chem. 2018;274:575–586.
- Bulakhe RN, Patil SV, Deshmukh PR, et al. Fabrication and performance of polypyrrole (Ppy)/TiO2 heterojunction for room temperature operated LPG sensor. Sensor Actuat B-Chem. 2013;181:417–423.
- Xu M, Zhang J, Wang S, et al. Gas sensing properties of SnO2 hollow spheres/polythiophene inorganic–organic hybrids. Sensor Actuat B-Chem. 2010;146:8–13.
- Bai S, Zhang K, Sun J, et al. Polythiophene-WO3 hybrid architectures for low-temperature H2S detection. Sensor Actuat B-Chem. 2014;197:142–148.
- Taccola S, Greco F, Zucca A, et al. Characterization of free-standing PEDOT:PSS/iron oxide nanoparticle composite thin films and application as conformable humidity sensors. ACS Appl Mater Inter. 2013;5:6324–6332.
- Zampetti E, Pantalei S, Muzyczuk A, et al. A high sensitive NO2 gas sensor based on PEDOT–PSS/TiO2 nanofibres. Sensor Actuat B-Chem. 2013;176:390–398.
- Lin Y, Huang L, Chen L, et al. Fully gravure-printed NO2 gas sensor on a polyimide foil using WO3-PEDOT:PSS nanocomposites and Ag electrodes. Sensor Actuat B-Chem. 2015;216:176–183.
- Kim SG, Jun J, Lee JS, et al. A highly sensitive wireless nitrogen dioxide gas sensor based on an organic conductive nanocomposite paste. J Mater Chem A. 2019;7:8451–8459.
- Su P-G, Peng Y-T. Fabrication of a room-temperature H2S gas sensor based on PPy/WO3 nanocomposite films by in-situ photopolymerization. Sensor Actuat B-Chem. 2014;193:637–643.
- Mane AT, Navale ST, Patil VB. Room temperature NO2 gas sensing properties of DBSA doped PPy–WO3 hybrid nanocomposite sensor. Org Electron. 2015;19:15–25.
- Athawale AA, Bhagwat SV, Katre PP. Nanocomposite of Pd–polyaniline as a selective methanol sensor. Sensor Actuat B-Chem. 2006;114:263–267.
- Jiang S, Chen J, Tang J, et al. Au nanoparticles-functionalized two-dimensional patterned conducting PANI nanobowl monolayer for gas sensor. Sensor Actuat B-Chem. 2009;140:520–524.
- Choudhury A. Polyaniline/silver nanocomposites: dielectric properties and ethanol vapour sensitivity. Sensor Actuat B-Chem. 2009;138:318–325.
- Su P-G, Shiu -C-C. Flexible H2 sensor fabricated by layer-by-layer self-assembly of thin films of polypyrrole and modified in situ with Pt nanoparticles. Sensor Actuat B-Chem. 2011;157:275–281.
- Patil UV, Ramgir NS, Karmakar N, et al. Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films. Appl Surf Sci. 2015;339:69–74.
- Cho S, Lee JS, Jun J, et al. High-sensitivity hydrogen gas sensors based on Pd-decorated nanoporous poly(aniline-co-aniline-2-sulfonic acid): poly(4-styrenesulfonicacid). J Mater Chem A. 2014;2:1955–1966.
- Yang X, Li L, Yan F. Polypyrrole/silver composite nanotubes for gas sensors. Sensor Actuat B-Chem. 2010;145:495–500.
- Bai S, Sun C, Wan P, et al. Transparent conducting films of hierarchically nanostructured polyaniline networks on flexible substrates for high-performance gas sensors. Small. 2015;11:306–310.
- Karmakar N, Fernandes R, Jain S, et al. Room temperature NO2 gas sensing properties of p-toluenesulfonic acid doped silver-polypyrrole nanocomposite. Sensor Actuat B-Chem. 2017;242:118–126.
- Zhang J, Liu X, Wu S, et al. One-pot fabrication of uniform polypyrrole/Au nanocomposites and investigation for gas sensing. Sensor Actuat B-Chem. 2013;186:695–700.
- Gaikwad N, Bhanoth S, More PV, et al. Chemically designed Pt/PPy nano-composite for effective LPG gas sensor. Nanoscale. 2014;6:2746–2751.
- Zhao D, Li L, Niu W, et al. Highly conductive polythiophene films doped with chloroauric acid for dual-mode sensing of volatile organic amines and thiols. Sensor Actuat B-Chem. 2017;243:380–387.
- Tuan CV, Tuan MA, Hieu NV, et al. Electrochemical synthesis of polyaniline nanowires on Pt interdigitated microelectrode for room temperature NH3 gas sensor application. Curr Appl Phys. 2012;12:1011–1016.
- Liu C, Hayashi K, Toko K. Au nanoparticles decorated polyaniline nanofiber sensor for detecting volatile sulfur compounds in expired breath. Sensor Actuat B-Chem. 2012;161:504–509.
- Park E, Kwon OS, Park SJ, et al. One-pot synthesis of silver nanoparticles decorated poly(3,4-ethylenedioxythiophene) nanotubes for chemical sensor application. J Mater Chem. 2012;22:1521–1526.
- Li Z-F, Blum FD, Bertino MF, et al. Understanding the response of nanostructured polyaniline gas sensors. Sensor Actuat B-Chem. 2013;183:419–427.
- Mekki A, Joshi N, Singh A, et al. H2S sensing using in situ photo-polymerized polyaniline–silver nanocomposite films on flexible substrates. Org Electron. 2014;15:71–81.
- Hien HT, Giang HT, Hieu NV, et al. Elaboration of Pd-nanoparticle decorated polyaniline films for room temperature NH3 gas sensors. Sensor Actuat B-Chem. 2017;249:348–356.
- Wang F, H W G, Swager TM. Carbon nanotube/polythiophene chemiresistive sensors for chemical warfare agents. J Am Chem Soc. 2008;130:5392–5393.
- Ding M, Tang Y, Gou P, et al. Chemical sensing with polyaniline coated single-walled carbon nanotubes. Adv Mater. 2011;23:536–540.
- Liao Y, Zhang C, Zhang Y, et al. Carbon nanotube/polyaniline composite nanofibers: facile synthesis and chemosensors. Nano Lett. 2011;11:954–959.
- Wu H, Chen Z, Zhang J, et al. Phthalocyanine-mediated non-covalent coupling of carbon nanotubes with polyaniline for ultrafast NH3 gas sensors. J Mater Chem A. 2017;5:24493–24501.
- Wan P, Wen X, Sun C, et al. Flexible transparent films Based on nanocomposite networks of polyaniline and carbon nanotubes for high-performance gas sensing. Small. 2015;11:5409–5415.
- Shen S, Fan Z, Deng J, et al. An LC passive wireless gas sensor based on PANI/CNT composite. Sensors (Basel). 2018;18:3022.
- Al-Mashat L, Shin K, Kalantar-Zadeh K, et al. Graphene/polyaniline nanocomposite for hydrogen sensing. J Phys Chem C. 2010;114:16168–16173.
- Wu Z, Chen X, Zhu S, et al. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sensor Actuat B-Chem. 2013;178:485–493.
- Guo Y, Wang T, Chen F, et al. Hierarchical graphene-polyaniline nanocomposite films for high-performance flexible electronic gas sensors. Nanoscale. 2016;8:12073–12080.
- Sharma S, Hussain S, Singh S, et al. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sensor Actuat B-Chem. 2014;194:213–219.
- Abdulla S, Mathew TL, Pullithadathil B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sensor Actuat B-Chem. 2015;221:1523–1534.
- Bora A, Mohan K, Pegu D, et al. A room temperature methanol vapor sensor based on highly conducting carboxylated multi-walled carbon nanotube/polyaniline nanotube composite. Sensor Actuat B-Chem. 2017;253:977–986.
- Hong SY, Oh JH, Park H, et al. Polyurethane foam coated with a multi-walled carbon nanotube/polyaniline nanocomposite for a skin-like stretchable array of multi-functional sensors. NPG Asia Mater. 2017;9:e448–e448.
- Liu B, Liu X, Yuan Z, et al. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sensor Actuat B-Chem. 2019;295:86–92.
- Kim M-S, Kim S, Kong HJ, et al. Tunable electrical-Sensing performance of random-alternating layered graphene/polyaniline Nanoarchitectures. J Phys Chem C. 2016;120:18289–18295.
- Cho S, Lee JS, Jun J, et al. Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high-molecular weight PSS dopant and their application in H2S detection. Nanoscale. 2014;6:15181–15195.
- Zhang L, Li C, Liu A, et al. Electrosynthesis of graphene oxide/polypyrene composite films and their applications for sensing organic vapors. J Mater Chem. 2012;22:8438.
- Huang X, Hu N, Gao R, et al. Reduced graphene oxide–polyaniline hybrid: preparation, characterization and its applications for ammonia gas sensing. J Mater Chem. 2012;22:22488.
- Bai S, Zhao Y, Sun J, et al. Ultrasensitive room temperature NH3 sensor based on a graphene-polyaniline hybrid loaded on PET thin film. Chem Commun (Camb). 2015;51:7524–7527.
- Hakimi M, Salehi A, Boroumand FA. Fabrication and characterization of an ammonia gas sensor based on PEDOT-PSS with N-doped graphene quantum dots dopant. IEEE Sens J. 2016;16:6149–6154.
- Hakimi M, Salehi A, Boroumand FA, et al. Fabrication of a room temperature ammonia gas sensor based on polyaniline with N-doped graphene quantum dots. IEEE Sens J. 2018;18:2245–2252.
- Gavgani JN, Hasani A, Nouri M, et al. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sensor Actuat B-Chem. 2016;229:239–248.
- Jiang T, Wang Z, Li Z, et al. Synergic effect within n-type inorganic–p-type organic nano-hybrids in gas sensors. J Mater Chem C. 2013;1:3017.
- Liu C, Tai H, Zhang P, et al. Enhanced ammonia-sensing properties of PANI-TiO2-Au ternary self-assembly nanocomposite thin film at room temperature. Sensor Actuat B-Chem. 2017;246:85–95.
- Shu J, Qiu Z, Lv S, et al. Cu2+-Doped SnO2 nanograin/polypyrrole nanospheres with synergic enhanced properties for ultrasensitive room-temperature H2S gas sensing. Anal Chem. 2017;89:11135–11142.
- Li S, Diao Y, Yang Z, et al. Enhanced room temperature gas sensor based on Au-loaded mesoporous In2O3 nanospheres@polyaniline core-shell nanohybrid assembled on flexible PET substrate for NH3 detection. Sensor Actuat B-Chem. 2018;276:526–533.
- Park SJ, Kwon OS, Jang J. A high-performance hydrogen gas sensor using ultrathin polypyrrole-coated CNT nanohybrids. Chem Commun (Camb). 2013;49:4673–4675.
- Vellaichamy B, Ponniah SK, Prakash P. An in-situ synthesis of novel Au@NG-PPy nanocomposite for enhanced electrocatalytic activity toward selective and sensitive sensing of catechol in natural samples. Sensor Actuat B-Chem. 2017;253:392–399.
- Yin Y, Zhang H, Huang P, et al. Inducement of nanoscale Cu–BTC on nanocomposite of PPy–rGO and its performance in ammonia sensing. Mater Res Bull. 2018;99:152–160.
- Xiang C, Jiang D, Zou Y, et al. Ammonia sensor based on polypyrrole–graphene nanocomposite decorated with titania nanoparticles. Ceram Int. 2015;41:6432–6438.
- Andre RS, Shimizu FM, Miyazaki CM, et al. Hybrid layer-by-layer (LbL) films of polyaniline, graphene oxide and zinc oxide to detect ammonia. Sensor Actuat B-Chem. 2017;238:795–801.
- Kulkarni S, Patil P, Mujumdar A, et al. Synthesis and evaluation of gas sensing properties of PANI, PANI/SnO2 and PANI/SnO2/rGO nanocomposites at room temperature. Inorg Chem Commun. 2018;96:90–96.
- Zhang D, Wu Z, Zong X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sensor Actuat B-Chem. 2019;289:32–41.
- Zhang D, Wu Z, Zong X. Metal-organic frameworks-derived zinc oxide nanopolyhedra/S, N: graphene quantum dots/polyaniline ternary nanohybrid for high-performance acetone sensing. Sensor Actuat B-Chem. 2019;288:232–242.
- Quan L, Sun J, Bai S, et al. A flexible sensor based on polyaniline hybrid using ZnO as template and sensing properties to triethylamine at room temperature. Appl Surf Sci. 2017;399:583–591.
- Pang Z, Nie Q, Lv P, et al. Design of flexible PANI-coated CuO-TiO2-SiO2 heterostructure nanofibers with high ammonia sensing response values. Nanotechnology. 2017;28:225501.
- Zhang D, Wu Z, Li P, et al. Facile fabrication of polyaniline/multi-walled carbon nanotubes/molybdenum disulfide ternary nanocomposite and its high-performance ammonia-sensing at room temperature. Sensor Actuat B-Chem. 2018;258:895–905.