ABSTRACT
Background and objective: Biosimilars are approved biologics that are comparable to an originator product with respect to quality, safety and efficacy. Herein, the authors describe the functional and non-clinical studies designed to determine the biosimilarity of GP2015 and originator etanercept (Enbrel®).
Methods: The development of an Enbrel biosimilar (GP2015) involved extensive characterization of the originator. A step-wise target-directed and iterative technical development program involving state-of-the-art functional characterization studies and non-clinical evaluations (pharmacokinetics, pharmacodynamics and safety/toxicology) was applied with the aim of confirming that GP2015 is comparable to originator (Enbrel) at the non-clinical level.
Results: In in vitro tests, GP2015 and Enbrel had comparable binding affinities to TNF-α, C1q complement and a complete panel of Fc-Receptors. Comprehensive functional characterization testing confirmed the comparability of GP2015 with Enbrel in terms of its ability to bind to and neutralize TNF-α, which reflects the primary mechanism of action of etanercept. Non-clinical data confirmed that the proposed biosimilar to Enbrel, GP2015, is comparable with regards to its pharmacokinetic properties and pharmacodynamic activity, and efficacy as well as safety/toxicity.
Conclusion: The proposed Enbrel biosimilar, GP2015, was shown to be comparable to its originator product in studies designed to confirm biosimilarity.
1. Introduction
For chronic inflammatory diseases such as rheumatoid arthritis and psoriasis, the introduction of biological disease-modifying therapy has transformed the therapeutic landscape [Citation1–Citation3]. Etanercept is an example of a biologic disease-modifying antirheumatic drug that can alter the course of the disease by halting or slowing the inflammatory process. It has become one of the preferred options for this disease management approach [Citation2,Citation4]. Etanercept is a recombinant DNA dimeric fusion protein that consists of the extracellular ligand-binding domain of human soluble tumor necrosis factor receptor 2 (TNF-R2/p75) and the Fc portion of the human IgG-1 antibody. It is used in the treatment of moderate-to-severe rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, axial spondyloarthritis (European Union [EU] only), non-radiographic axial spondyloarthritis (EU only), severe plaque psoriasis, and pediatric plaque psoriasis (EU only). Etanercept exerts its pharmacological activity by inhibiting the activation of TNF-R1 and TNF-R2 expressing cells by antagonizing soluble TNF-α [Citation5–Citation8]. In addition to TNF-α, etanercept also binds lymphotoxin-α (LT-α; formerly called TNF-β) which is structurally similar to soluble TNF and binds specifically to the same TNF receptors, although the significance of this antagonistic effect to its overall mechanism of action and clinical profile is less well characterized [Citation5]. Etanercept binds with lower affinity to tmTNF compared with anti-TNF-α monoclonal antibodies (mAbs) and additionally, it only binds at a 1:1 stoichiometry, which is unlike the therapeutic mAbs that are capable of cross-linking of tmTNF in TNF-producing cells. Etanercept has been shown to be less able to induce complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) compared with anti-TNF-α mAbs [Citation5,Citation9,Citation10].
Biologics such as etanercept have significantly improved long-term clinical outcomes in various inflammatory diseases, but they have also markedly increased the cost of treatment and the financial burden on health-care providers [Citation11]. Biosimilars are copies of currently approved biological agents, and they are required to undergo a comprehensive development and rigorous approval process in highly regulated markets to confirm their comparability. The goal in developing biosimilar versions of biologics is to reduce the cost of drug treatment, to increase access to effective biological therapies for a wider group of patients who will benefit from receiving them, and to foster competition and sustain innovation [Citation11,Citation12].
Biosimilars are approved on the basis of comparable quality, pharmacokinetics (PK), pharmacodynamic activity, safety, and efficacy to originator products. The main goal during development is to ensure that the structural and quality attributes of the proposed biosimilar are comparable to those of the originator product, with no clinically meaningful differences [Citation12]. This can be achieved using a target-directed development approach, based on characterization of multiple batches of the originator product to develop an in-depth understanding of its characteristics, including an understanding of its inherent variability. The variability and criticality (in terms of successful biosimilar development) of a broad range of quality attributes of the originator then defines the so-called biosimilar goal posts, i.e. a range of characteristics of the originator product that the proposed biosimilar has to fit within. During the biosimilar development of GP2015, more than 80 quality attributes ranging from protein structure and modifications, impurities to biological activity of originator etanercept were characterized using an array of state-of-the-art sensitive analytical techniques. The proposed biosimilar was then systematically engineered to match the originator product across cell lines and bioprocesses, and confirmed on a batch-to-batch basis [Citation13,Citation14]. Before entering clinical trials, biosimilarity is established based on physicochemical and functional characterization as well as in vitro and/or in vivo studies. This stepwise comparability exercise between the biosimilar and the originator product is also reflected in the regulatory guidance recommendations for the licensing of biosimilars published by the World Health Organization [Citation15] as well as by both the European Medicines Agency (EMA) and the United States (US) Food and Drug Administration (FDA) [Citation16–Citation20]. Authorized biosimilars are required to be as safe and efficacious as originator biologicals [Citation2]. It should be noted that originator biologicals are subject to manufacturing changes that must be reported to the regulatory agencies (EMA, FDA, etc.). In the case of originator etanercept (Enbrel®, Amgen) approximately 20 such changes have been reported to and approved by the EMA, FDA, and other agencies, and they have had no adverse impact on the safety or efficacy of the originator product [Citation3]. The scientific principles of a change in the manufacturing process of an originator biological and the development of a biosimilar share much in common [Citation3,Citation21].
GP2015 is a proposed etanercept biosimilar to originator etanercept which is marketed as Enbrel (INN: etanercept; p75TNF-R IgG1 Fc-fusion protein) in the US and EU. Comprehensive analytical and physicochemical comparisons of GP2015, EU-authorized Enbrel and US-licensed Enbrel demonstrated a high level of structural similarity between the various products, including identical amino acid sequences, indistinguishable protein folding, and highly-comparable glycosylation and product-related impurities [Citation22]. Core quality attributes evaluated as part of the overall biosimilarity exercise included primary structure (amino acid sequence), higher order structure (protein folding: secondary and tertiary structures), protein modifications (N-glycosylation, O-glycosylation, sialic acids, oxidation, deamidation, charge variants, glycation, N- and C-terminal heterogeneity and disulfide bridging), and impurities (aggregates and fragments, DNA, protein A and host cell proteins). Herein, we report the extensive functional characterization of GP2015 versus multiple samples of originator Enbrel sourced from the US and EU (henceforth called Enbrel). The potential impact of differences in quality attributes between GP2015 and originator Enbrel was further evaluated in a comprehensive nonclinical development program which comprised comparative animal studies to compare PK and pharmacodynamic properties as well as toxicity. Additionally, a new formulation for GP2015 was developed and tested as part of this biosimilarity exercise. The overall aim of this stepwise comparability exercise was to confirm the biosimilarity of GP2015 and commercially available Enbrel prior to starting clinical trials.
2. Methods
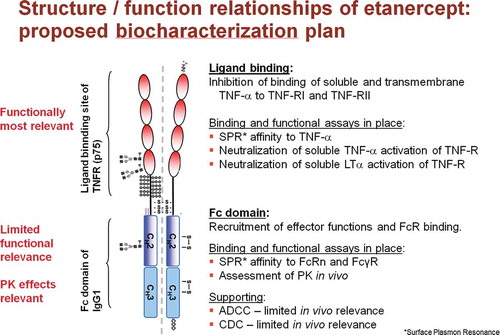
Etanercept exerts its primary pharmacological activity by antagonizing TNF-α, and thereby the activation of TNF-R1 and TNF-R2 expressing cells. Based on this mechanism of action of the etanercept molecule, and as part of a comprehensive functional characterization program, both binding assays and cell-based assays were performed to investigate the quality attributes of GP2015 versus those of Enbrel ().
Figure 1. Panel of assays to measure (in vitro) ligand binding and effector function of Enbrel /GP2015 in relation to the structure of the etanercept molecule.
Studies involving animals to compare the PK, pharmacodynamic, and toxicological properties of GP2015 and Enbrel were conducted in compliance with local and international animal welfare regulations and were all approved by the local ethical committee.
2.1. Binding studies
The real-time binding of GP2015 and Enbrel to recombinant TNF-α and a panel of human Fcγ receptors and FcRn was analyzed using a surface plasmon resonance (SPR) assay [Biacore™ T200 (GE Healthcare)].
2.1.1. Binding to TNF
To assess the binding of etanercept to TNF-α, a competitive binding assay based on SPR was developed. Briefly, a Biacore™ CM5 (GE Healthcare) sensor chip was coated with an anti-TNF antibody by amine coupling (Amine coupling kit, GE Healthcare). Graded amounts of etanercept samples were added to a 200-nM solution of recombinant human TNF-α, followed by an overnight incubation at 2–8°C to allow complex formation. Free TNF-α, which was not complexed to etanercept, was subsequently quantified by injecting the sample across the anti-TNF flow cell of the sensor chip. The relative potency of test samples was determined by parallel line evaluation of the dose–response curves according to European Pharmacopoeia recommendations.
2.1.2. Binding to human C1q
Binding to human C1q was determined by ELISA. Briefly, graded amounts of etanercept were coated on ELISA plates. A fixed concentration of human C1q was added, and bound C1q quantified using a peroxidase-coupled anti-C1q antibody. The binding ability of C1q to test samples was determined by comparison to a reference standard, and the relative potency calculated according to Ph. Eur. recommendations.
2.1.3. Binding to human Fcγ receptors
Recombinant Fcγ receptors were immobilized covalently on CM5 sensor chips using standard amine coupling chemistry (Amine coupling kit, GE Healthcare). To serve as an online reference, one flow cell on each sensor chip used was activated with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochlorid (EDC)/N-hydroxysuccinimide (NHS) and deactivated with 1 M ethanolamine, pH 8.5, according to the manufacturer’s recommendations.
For the high-affinity receptor (FcγRIa), etanercept samples were diluted to concentrations between 0.2 and 100 nM in HBS-EP (GE Healthcare) and applied to the FcγRIa-derivatized flow cell. All samples were injected twice, and injection order was randomized. To allow the compensation of systemic effects by double referencing, two injections of HBS-EP running buffer were performed along with the samples. Kinetic rate constants were derived from the zero-adjusted, double-referenced sensorgrams by global fitting, using local Rmax to account for the observed slight loss of surface activity over time, and not allowing adjustments for bulk changes in refractive index.
For low-affinity receptors (FcγRIIa, FcγRIIb, FcγRIIIaF158, FcγRIIIaV158, and FcγRIIIb), GP2015 samples were diluted to concentrations between 0.5 and 50 µM in HBS-EP and applied to the Fc receptor-derivatized flow cell. All samples were injected twice, and injection order was randomized. To allow the compensation of systemic effects by double referencing, two injections of HBS-EP running buffer were performed along with the samples. Equilibrium dissociation constants (KD) were derived from plots showing the concentration-dependent steady-state binding of GP2015 to Fcγ receptors by nonlinear curve fitting to a 1:1 interaction model.
2.1.4. Binding to human FcRn
Recombinant human FcRn was biotinylated enzymatically, using a C-terminal Avi-tag sequence. Biotinylation was performed using recombinant biotin protein ligase (Avidity) according to the manufacturer’s recommendations. To bind biotinylated FcRn to the sensor chip surface, it was diluted in 10 mM sodium acetate, pH 4.5, and applied to a previously untreated flow cell of a SA sensor chip. Etanercept samples were diluted in HBS-EP adjusted to pH 6.4 with HCl, and applied to the FcRn-derivatized sensor chip surface at concentrations between 8 nM and 10 µM. KD was determined as described above.
2.2. Functional neutralization of TNF-α and LT-α
2.2.1. Reporter-gene assay
Binding and functional neutralization of TNF-α and LT-α by GP2015 and Enbrel was measured using HEK293 cells stably transfected with an NFκB-luciferase reporter gene. This assay reflects the primary mode of action (MoA) of etanercept [Citation23,Citation24]. In a microtiter plate, 20,000 cells per well were stimulated with 4 ng/ml TNF-α or LT-α in the presence of graded amounts of GP2015 or Enbrel. After overnight incubation, cells were lysed and luciferase activity was quantified in the lysates using a luminogenic substrate. The potency of GP2015 or Enbrel was determined by comparison to a reference standard and relative potency was calculated using a parallel line assay according to the European Pharmacopoeia.
2.2.2. Apoptosis inhibition
The ability of GP2015 or Enbrel to inhibit TNF-induced apoptosis was determined as follows: U937 cells were seeded on a microtiter plate at a density of 10,000 cells per well. Cells were induced to undergo apoptosis by addition of 40 ng/ml TNF-α and 50 ng/ml cycloheximide. GP2015 was titrated to the system. Cells were incubated at 37°C for 4 h, and activity of effector caspases-3 and -7 was quantified using the luminogenic caspase substrate CaspaseGlo. Relative potency of test samples was determined by parallel line evaluation of the dose–response curves according to European Pharmacopoeia recommendations.
2.3. Cytotoxicity
While ADCC and CDC are not considered to contribute to the MoA of etanercept these assays are included as part of the comprehensive pharmacological assessment to fully characterize Enbrel and GP2015. TNF antagonists may induce cytotoxic effects in transmembrane TNF-bearing cells via Fc-dependent mechanisms including CDC and ADCC [Citation5].
2.3.1. CDC activity
Approximately 5000 target cells (Jurkat T cells stably transfected with a non-cleavable variant of human pro-TNF-α) were incubated with 33% human serum and graded amounts of GP2015 or Enbrel. These cells constitutively express human TNF-α as a transmembrane protein on their surface. After 2 h incubation at 37°C, viable cells were quantified by chemiluminescence using CellTiterGlo reagent. Relative potency of test samples was determined by parallel line evaluation of the dose–response curves according to European Pharmacopoeia recommendations.
2.3.2. ADCC activity
Approximately 5000 target cells (HEK293 cells stably transfected with a non-cleavable variant of human pro-TNF-α) were labeled with the cytoplasmic fluorescence dye calcein, and subsequently co-incubated with 50,000 effector cells (NK3.3 immortalized natural killer cell line; kindly provided by Dr Jacki Kornbluth, St Louis University). GP2015 was added at graded amounts from 0.005 to 10 µg/ml. After 50–70 min incubation at 37°C, cells and debris were sedimented by centrifugation, and the calcein released from the dying target cells into the supernatant was quantified fluorometrically.
Relative dynamic range was defined as the dynamic range (Δ between highest and lowest point of the dose–response curve) of a sample divided by the dynamic range of the corresponding reference standard dose–response curve.
2.4. PK studies
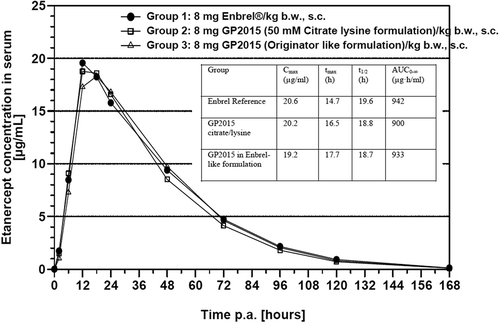
A pilot study was performed to investigate alternative buffer solutions to formulate GP2015. This single dose PK study was performed in 6 groups of 10 rabbits that each received etanercept 8 mg/kg subcutaneously (s.c.) either as GP2015 (5 different formulations: see in Section 3.4) or as Enbrel in a buffer comprising 25 mM phosphate/25 mM arginine HCl) which served as controls. The results of this study were used to decide on the final formulation of GP2015 to be used for further testing in clinical studies and in the final product composition.
Table 1. The experimental groups in the human TNF transgenic mouse model Tg197 [Citation24] used to compare the therapeutic efficacy of GP2015 and Enbrel at a pharmacologically sensitive dose of 10 mg/kg.
Table 2. GP2015/Enbrel neutralization of TNF-α and LT-α in an RGA, binding to TNF-α and Fc receptors (SPR; Biacore assay).
Table 3. Ratios of mean PK parameters in male rabbits for five GP2015 formulations relative to Enbrel/EU [25 mM phosphate/25 mM arginine HCl buffer].
The PK properties of GP2015 and the reference product Enbrel were subsequently compared in confirmatory single dose studies in rabbits and a multiple dose study in cynomolgus monkeys (as part of a toxicology study; see Section 2.6) using representative material intended for clinical use. In the rabbit PK study, three groups of rabbits (20 per group) received etanercept 8 mg/kg s.c. as GP2015 in the selected citrate/lysine formulation, or GP2015 in an Enbrel-like buffer (25 mM phosphate/25 mM arginine HCl), or commercially available Enbrel. The study duration was 2 weeks and etanercept serum concentrations over time were monitored, and an assessment of local tolerability and any clinical signs/symptoms was made. The primary end points for this study were maximum serum concentration (Cmax), the time to reach Cmax (tmax), the area under the plasma concentration–time curve (AUC), and elimination half-life (t1/2).
2.5. In vivo efficacy in a human Tg197 TNF transgenic mouse model of polyarthritis
The Tg197 human TNF transgenic mouse model of polyarthritis is a well-established model in which the pathogenesis and treatment of rheumatoid disease in humans may be investigated [Citation25]. A pilot dose-finding study was performed to ascertain a dosage of Enbrel that produced a sensitive sub-therapeutic clinical response in terms of clinical symptoms, and which could be used in a follow up study to detect potential differences between Enbrel and GP2015.
Based on the findings of the dose-ranging study a dosage of Enbrel 10 mg/kg by i.p. injection produced an intermediate response and was used in a comparative study with GP2015. Details of the experimental groups tested in this study including dosage regimens, dosing frequency, numbers of mice treated, and duration of follow-up are shown in . Treatment was initiated after the onset of arthritis (sixth week of age) and was continued over 4 weeks with interim assessments [Citation25]. The effects of the two products were monitored by weekly assessments of body weight and clinical symptoms. Arthritis symptoms were evaluated macroscopically using a scoring system that has been described previously [Citation26]. Scores ranged from 0.0 [no signs of arthritis] to 3.0 [heavy arthritis with ankylosis detected on flexion, severely impaired movement, and the mouse moribund]. At the end of follow-up (3 days after a single administration; 3 days after five administrations; and 3 days after nine administrations), the mice were sacrificed, and serum samples and ankle joints collected. The ankle joints were fixed in formalin, decalcified, and stained before sections were taken for histopathological assessment and scoring [0.0 no detectable pathology to 4.0 extensive cartilage destruction and bone erosion].
2.6. Comparative toxicity study
Toxicity including local tolerance and immunogenicity as well as toxicokinetics of GP2015 was compared to Enbrel in a 4-week repeat-dose study in cynomolgus monkeys. In this study GP2015 and Enbrel were administered at a dosage of 15 mg/kg body weight once every 3 days for 28 days by the s.c. route. In addition, a vehicle control group was included in the study and these animals received the formulation buffer used for GP2015. Each of the 3 groups comprised 3 male and 3 female monkeys.
Blood samples to determine PK parameters were taken from all animals pre-dose and at regular intervals on days 1 and 7; pre-dose on days 13, 16, 19, 22, and 25; and pre-dose and at regular intervals after dosing on day 28. Evaluation of toxicity was based on daily assessments (unless stated otherwise) of clinical observations, bodyweight, food consumption, clinical pathology (hematology, blood chemistry, and urinalysis: pretreatment and week 4), body temperature (pretreatment and 23–25 h after dosing on days 5 and 26), blood pressure (pretreatment and 23–25 h after dosing on days 5 and 26), electrocardiography (pretreatment and 23–25 h after dosing on days 5 and 26), ophthalmoscopy (pretreatment and week 4), organ weights (necroscopy), and anatomical pathology evaluations (microscopic and macroscopic: necroscopy).
3. Results
3.1. Binding studies
The binding of GP2015 (89–101%) and Enbrel (85–101%) to TNF-α in a SPR-based assay demonstrated complete overlap (). This highlights the comparability of the two products with respect to the pivotal step in the pharmacology of etanercept. Furthermore, the intrinsic binding affinities of the two etanercept products to human C1q and a range of TNF Fc receptors are also shown in , and again they exhibit a high level of comparability.
3.2. Functional neutralization
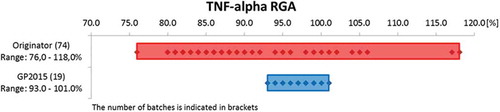
With respect to the primary MoA of etanercept, neutralization of TNF-α, and consistent with comparable binding affinities, there was complete overlap of the values reported for GP2015 and Enbrel in the Luciferase reporter-gene assay (RGA) (). shows that the range of values for TNF-α neutralization across multiple batches of GP2015 was much narrower than that for originator Enbrel. In addition, there was also comparable inhibition of apoptosis induced by TNF-α and complete overlap of values for the neutralization of LT-α ().
Figure 2. Dose-dependent inhibition of TNF-α function by GP2015 and Enbrel in a cellular NFκB-dependent luciferase reporter gene assay.
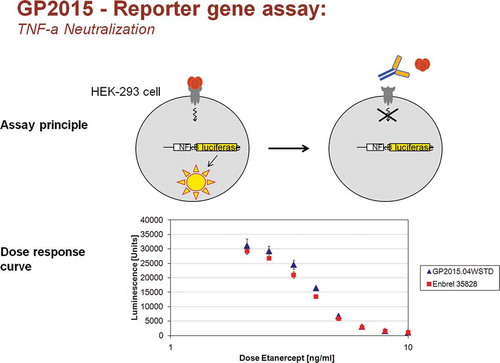
Figure 3. Results for TNF-α neutralization for different batches of Enbrel (Originator) and GP2015 (Biosimilar) in the reporter-gene assay.
These nonclinical data confirm the comparability of the proposed biosimilar GP2015 with its reference product Enbrel at the functional level.
3.3. Fc-mediated cytotoxicity
The results of cell-based assays to measure CDC and ADCC activity are summarized in . Enbrel exhibited higher activity than GP2015 in the ADCC assay (196–217% vs. 79–121%), whereas values for CDC activity were slightly lower for Enbrel compared with GP2015 (59–101% vs. 93–132%). However, since CDC and ADCC are not implicated in the efficacy and safety of etanercept, these differences are deemed to be of no clinical relevance [Citation5].
3.4. PK properties
Results for the pilot study in rabbits are presented in and they highlight the suitability of the 50 mM citrate/25 mM lysine buffer for the GP2015 formulation.
The main PK study in rabbits showed almost complete overlap of the serum concentration–time curves for the 3 groups (). For AUC, Cmax, tmax, and elimination t1/2, the values were within the standard bioequivalence margins of 80–125% [Citation27]. Furthermore, when GP2015 was formulated in Enbrel-like buffer (25 mM phosphate/25 mM arginine HCl) the PK profiles were also comparable, reinforcing the biosimilarity of GP2015 to Enbrel. No signs of local intolerance were observed in these studies in rabbits and no other obvious adverse effects with regard to clinical observations or body weight were recorded (data not shown).
3.5. In vivo efficacy in a human Tg197 TNF transgenic mouse model of polyarthritis
The results of the pilot study showed that Enbrel produced dose-dependent inhibition of Tg197 arthritic pathology compared to the age-related aggravation of Tg197 pathology found in saline control groups. Overall, a dosage of 10 mg/kg i.p. was shown to be the optimal sensitive dose level that would most likely reveal any potential differences in efficacy between GP2015 and Enbrel.
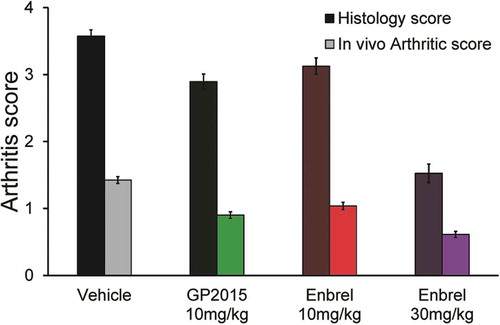
In the comparative study, using this model of arthritis, the effects of Enbrel and GP2015 were indistinguishable (). Age-related arthritis progression [as assessed by arthritic scores (morphological and functional changes on both ankle joints)] was confirmed in this model. Distinct pathological severity changes started from week 6 in the control group (when treatment was initiated) and progressed to advanced arthritis by week 10 in the group that received only vehicle. During the course of the study, there were no marked differences between GP2015 and Enbrel (both 10 mg/kg i.p.) in terms of changes in arthritic scores (). At the 10-mg/kg dosage, both regimens were inferior to Enbrel 30 mg/kg i.p. and superior to control (vehicle) to a similar extent. Histopathological findings paralleled changes in arthritis scores and, overall, GP2015 10 mg/kg produced similar improvements in histopathological scores as Enbrel 10 mg/kg (). The results of this study demonstrated that the effects of GP2015 and Enbrel (both 10 mg/kg i.p.) were indistinguishable in inhibiting Tg197 arthritic pathology and the underlying histopathology. These results support the view that the small changes observed for CDC and ADCC observed during functional testing are of no consequence to the efficacy of GP2015 in this well-established animal model of polyarthritis.
Figure 5. Arthritic score assessment in the human TNF transgenic mouse model Tg197 [Citation25]. At a pharmacologically sensitive dose of 10 mg/kg, GP2015 and Enbrel were indistinguishable in inhibiting arthritic disease symptoms when compared to vehicle buffer-treated control group.
![Figure 5. Arthritic score assessment in the human TNF transgenic mouse model Tg197 [Citation25]. At a pharmacologically sensitive dose of 10 mg/kg, GP2015 and Enbrel were indistinguishable in inhibiting arthritic disease symptoms when compared to vehicle buffer-treated control group.](/cms/asset/c629e858-141f-4d2b-b03c-336d0603c634/iebt_a_1217329_f0005_oc.jpg)
Figure 6. The histopathology and in-life mean arthritic score at week 10 in experimental Tg197 mice. By week 10 the mean disease severity scores of the biweekly treated groups (from week 6 onwards), were as follows: (G5) Enbrel at 10 mg/kg = 3.13 (HS) and 1.04 (AS), (G6) GP2015 at 10 mg/kg = 2.89 (HS) and 0.9 (AS), (G7) vehicle GP2015 buffer = 3.58 (HS) and 1.43 (AS), (G8) Enbrel at 30 mg/kg = 1.53 (HS) and 0.61 (AS) Control mice at week 6 had a score of 2.81 (HS) and 1.19 (AS). Bars indicate standard errors of the mean.
4. Toxicity/Safety profile
The toxicity/safety profile of GP2015 was assessed in the cynomolgus monkey which has been shown to be a relevant animal model for etanercept. The same model was used in the safety assessment of the originator Enbrel [Citation28,Citation29] Overall, administration of GP2015 and Enbrel 15 mg/kg s.c. to cynomolgus monkeys once every 3 days for 28 days showed comparable toxicological profiles.
There were no treatment-related effects on bodyweight, food consumption, urinalysis, heart rate, and blood pressure, and no deaths occurred during the toxicity study. The major toxicity for both GP2015 and Enbrel were injection-site reactions observed between days 26 and 32. These were transient local skin reactions at the injection sites in one GP2015-treated male and two Enbrel/EU-treated males, which generally coincided with the description of a rash. In these three animals, there was evidence of a regenerative anemia [decreased hemoglobin (−17–28% vs. placebo), red blood cells (−14–27% vs. placebo) and platelet count (−31–50% vs. placebo)]. In addition, there was a reduction in neutrophils (−77–94% vs. placebo). Individual animals had elevated numbers of monocytes, lymphocytes, basophils, and large unstained cells. Treatment-related histopathological changes were present in the skin at four different injection sites. They were characterized by perivascular mononuclear cell infiltration, primarily within the dermis, but also in the subcutis and muscle (minimal to mild) of two males treated with GP2015, two males treated with Enbrel, all females treated with GP2015 and two females treated with Enbrel. These skin/injection site lesions generally correlated with those animals demonstrating decreased exposure and production of antidrug antibodies (ADAs), and are therefore considered to be related to immunogenicity. In addition, there was an overall increase in inflammatory lesions in the skin of treated animals compared with control animals, including hyperkeratosis, dermatitis, myositis, and cellulitis, which were considered to be nonspecific inflammatory responses to administration of Enbrel and GP2015.
The main findings relating to PK parameters during this study are presented in . Following administration of GP2015 and Enbrel 15 mg/kg s.c. once every 3 days to male and female cynomolgus monkeys, serum etanercept concentrations increased steadily. Mean maximal levels (62.2–83.9 µg/ml) were reached after a mean of 22.0–29.3 h after first administration on day 1. There was some accumulation on day 7 after repeat dosing (mean accumulation ratio RAAUC 1.33–1.38 for GP2015 and 1.56–1.93 for Enbrel). However, by day 28, Cmax, AUC, and elimination t1/2 were all markedly reduced which is consistent with increased ADA clearance. However, the development of ADAs was only confirmed in four monkeys (two in each group). On days 1 and 7, there were no appreciable gender-related PK differences and overall exposure to GP2015 and Enbrel was similar and Frel ranged from 81% to 101% in male and 88–136% in female monkeys.
Table 4. Mean ± standard deviation toxicokinetic parameters following subcutaneous administration of GP2015 or Enbrel 15 mg/kg subcutaneously (once every 3 days for 4 weeks) to groups of cynomolgus monkeys (n = 3 in each group).
5. Discussion
5.1. Biosimilars: background
The introduction of biosimilars, which in-line with regulatory requirements have no clinically meaningful differences compared with the originator product in terms of quality, safety, and efficacy, has the potential to expand access to these important medicines. Biosimilars are systematically designed to be as close to the originator product as the originator product is to itself, taking into consideration the variability observed from batch-to-batch manufacturing changes over time. To help achieve this high level of comparability, state-of-the-art analytical technologies allow a thorough physicochemical characterization of biologics. When combined with detailed functional characterization and an increasing understanding of the structure–function relationships, meaningful prediction of the biological function, and clinical performance of the biosimilar can be achieved [Citation30–Citation32]. This evidence, along with nonclinical and clinical studies to confirm biosimilarity, provides the foundation for the confirmation of comparability between the biologic and its proposed biosimilar. The totality of this approach is a prerequisite for regulatory approval and has led to the concept of ‘totality-of-evidence’ [Citation12]. Comparing the biosimilar to different batches of the reference product over an extended period is designed to ensure that the two products are comparable in terms of purity, potency, and safety, including immunogenicity.
5.2. GP2015: binding and functional characterization
Comprehensive functional characterization testing confirmed the comparability of GP2015 with Enbrel in terms of its ability to bind to and neutralize TNF-α which reflects the primary mechanism of action of etanercept. With regards to TNF-α neutralization, there was complete overlap of the values reported for GP2015 and Enbrel in the RGA and also in an assay based on inhibition of apoptosis induced by TNF-R expressing cells. Furthermore, it was shown to be comparable in terms of neutralizing LT-α, and binding to C1q and all human Fcγ receptor subtypes (FcγRIa, FcγRIIa, FcγRIIb, FcγRIIIa, FcγRIIIb, and FcRn). With respect to the functional integrity of the Fc domain, as measured by determining the affinity of etanercept to human C1q and Fc receptors including FcRn, GP2015 and Enbrel batches were analytically indistinguishable. This similarity in target binding is a critical prerequisite for comparable functional biological activity for biosimilar GP2015 compared with originator Enbrel.
5.3. GP2015: Fc-mediated cytotoxicity
Differences between GP2015 and Enbrel in ADCC and/or CDC activity are not thought to be clinically meaningful. Briefly, Fc-mediated effector functions do not contribute to the efficacy of TNF-α antagonists in any of the indications for which Enbrel is currently licensed. In addition, experimental conditions used to assess the ADCC activity of etanercept were geared toward an extremely high sensitivity of the analytical system to detect potential changes. In an experimental setting closer to expected physiological conditions, neither Enbrel nor GP2015 were able to induce measurable ADCC-mediated target cell depletion (data not presented). The reduced or lack of ability by etanercept to induce either ADCC or CDC upon engagement with tmTNF is extensively covered in the literature. It has been reported to be a direct consequence of differences with regards to its avidity and ability to induce cross-linking of tmTNF when compared to anti-TNF IgGs. Whenever etanercept has been found to induce ADCC or CDC in the literature it has only occurred under nonphysiological conditions, and it is dependent on the overexpression of tmTNF on the target cells [Citation6,Citation10,Citation33]. Furthermore, it is acknowledged in the European Public Assessment Report (EPAR) documentation for Benepali® (etanercept biosimilar) that ADCC and CDC are not mechanisms of action of etanercept. Hence, we can conclude that differences observed between GP2015 and Enbrel for ADCC and CDC are of no clinical significance in respect to either safety or efficacy.
5.4. GP2015: formulation/buffer and PK
The formulation of biosimilar medicines is an important consideration. Consequently, it was necessary to develop a stable formulation for GP2015 which was able to preserve the physicochemical and biological characteristics of the proposed biosimilar, whilst maintaining a similar PK profile and equivalent level of clinical performance in terms of efficacy and safety. In a pilot study, based upon PK comparability data and stability assessments, a formulation comprising 50 mM citrate/25 mM lysine provided the optimal results, and was used in subsequent PK and pharmacodynamic studies for GP2015. This citrate/lysine buffer differs from the buffer used for Enbrel (25 mM phosphate/25 mM arginine HCl).
The PK profile of GP2015 was evaluated in a single dose study in rabbits and a repeat-dose study in cynomolgus monkeys. In the rabbit study, there was almost complete overlap of the serum concentration–time curves for GP2015 and Enbrel. For Cmax, tmax, AUC, and elimination t1/2, the values were all well within the 80–125% range which is the standard set for bioequivalence. Furthermore, when GP2015 was formulated in an Enbrel-like buffer the PK profiles were also comparable, reinforcing the biosimilarity of GP2015 to Enbrel and the suitability of the citrate/lysine buffer for continued development. The PK profiles of GP2015 and Enbrel were also shown to be comparable in a toxicity study in cynomolgus monkeys. Serum etanercept concentrations increased steadily and similar mean maximal levels were reached for GP2015 and Enbrel on days 1 and 7. There were no appreciable gender-related PK differences and overall exposure to GP2015 and Enbrel was similar; Frel ranged from 81% to 101% in male and 88% to 136% in female monkeys highlighting the biosimilarity of GP2015 and Enbrel. The bioequivalence (Cmax and AUC) of GP2015 and etanercept originator product, with no relevant differences in tolerability/safety, was recently confirmed in a randomized, single-dose, crossover study in 54 healthy male subjects [Citation34].
5.5. GP2015: antiarthritis activity
A pharmacodynamic comparability study in a human TNF-α transgenic (Tg197) mouse model of polyarthritis confirmed the comparability of GP2015 and Enbrel at the therapeutically sensitive dose of 10 mg/kg. Tg197 TNF transgenic mice overexpress human TNF-α and develop chronic arthritis with histopathological features such as bone erosions and cartilage destruction, and clinical symptoms which are similar to those observed in human rheumatoid arthritis [Citation25]. In this model, both regimens arrested age-related progression of rheumatoid arthritis, with an effect mid-way between the advanced arthritis found in vehicle-treated mice and the disease regression produced by saturating high-dose Enbrel (30 mg/kg). While these findings do not abrogate the need for clinical data, they are strong support for the comparability of GP2015 and Enbrel in a well-established animal model of chronic arthritis.
5.6. GP2015: toxicology and safety
In terms of toxicity, s.c. administration of GP2015 and Enbrel to male and female cynomolgus monkeys, once every 3 days for 4 weeks, showed comparable toxicological profiles following the development of an immunogenicity reaction. Histopathological changes at the injection site, characterized by minimal to mild peri-vascular mononuclear cell infiltration, generally correlated with those animals demonstrating decreased exposure and production of ADAs and was therefore considered to be related to immunogenicity. Additional evidence of immunogenicity was observed in one GP2015-treated and two Enbrel-treated monkeys. These animals presented with an injection site rash/erythema, abdominal/inner thigh ecchymosis, regenerative anemia as a consequence of red blood cell hemolysis and thrombocytopenia at approximately day 28; the symptoms resolved within 3 days. There were no other treatment-related toxicity effects in this study or in studies involving rabbits.
Adverse cutaneous effects have been reported with increasing frequency in patients with rheumatic diseases treated with TNF-α antagonists such as etanercept [Citation35–Citation37]. Injection site reactions are common and usually self-limiting. Recall site reactions where the four rotated injection sites simultaneously develop a hypersensitivity reaction have also been reported for etanercept and in all cases, the rash has responded to antihistamines and therapy was continued. Overall, GP2015 appeared to be well tolerated in the various animal studies and there were no unexpected safety findings.
5.7. Strengths and limitations
The strength of this biosimilarity exercise is based upon the number of investigations undertaken using state-of-the-art analytical procedures and well-established tests to determine functional performance. Furthermore, nonclinical comparability was confirmed in animal models extensively used in this pharmacological setting. Further confirmatory data are being generated in clinical studies in psoriasis and rheumatoid arthritis which provide the statistical power not afforded by nonclinical in vivo animal studies.
6. Conclusions
In summary, this analytical and nonclinical comparability exercise confirmed that the proposed biosimilar GP2015 and the reference product (Enbrel) are comparable with regard to functional (target binding and anti-TNF-α biological activity), PK, and toxicological profiles. Furthermore, the pharmacodynamic biosimilarity of GP2015 and Enbrel was confirmed in a well-established animal model of rheumatoid arthritis (TNF-α transgenic [Tg197] mouse). These findings form the basis for the so-called totality of the evidence approach to reduce any residual uncertainty about the ‘sameness’ of the proposed biosimilar GP2015 to Enbrel, and they also provide the necessary evidence to conduct a tailored clinical comparability program to finally prove biosimilarity. Indeed, the comparable efficacy and safety (including immunogenicity) of GP2015 and Enbrel was recently demonstrated in patients with psoriasis, a highly sensitive indication for the evaluation of TNF-α antagonists [Citation22].
Declaration of interest
The authors are all paid employees of either Sandoz Biopharmaceuticals/Hexal AG, Holzkirchen, Germany, Hexal AG Oberhaching or Novartis Pharma AG, Basel, Switzerland. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Writing assistance was utilized in the production of this manuscript and funded by Sandoz Biopharmaceuticals/Hexal AG, and Novartis Pharma AG.
Acknowledgments
Editorial assistance was kindly provided by Dr Peter Weber and Dr Steve Clissold of ContentEdNet, Germany. The authors acknowledge the role of BioMedCode relating to studies involving the human Tg197 TNF transgenic mouse model of polyarthritis in identifying sensitive study designs to optimally address efficacy comparability in this well-establish and characterized disease model.
Additional information
Funding
References
- Alten R, Cronstein BN. Clinical trial development for biosimilars. Semin Arthritis Rheum. 2015;44:S2–S8. doi:10.1016/j.semarthrit.2015.04.002.
- Monti S, Montecucco C, Bugatti S, et al. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open. 2015;1(Suppl 1):e000057. doi:10.1136/rmdopen-2015-000057.
- Schneider CK. Biosimilars in rheumatology: the wind of change. Ann Rheum Dis. 2013;72:315–318. doi:10.1136/annrheumdis-2012-202941.
- Avci AB, Feist E, Burmester G-R. Biologicals in rheumatoid arthritis: current and future. RMD Open. 2015;1(1):e000127. doi:10.1136/rmdopen-2015-000127.
- Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi:10.1016/j.pharmthera.2007.10.001.
- Arora T, Padaki R, Liu L, et al. Differences in binding and effector functions between classes of TNF antagonists. Cytokine. 2009;45:124–131. doi:10.1016/j.cyto.2008.11.008.
- Kaymakcalan Z. Beam C and Salfeld J. Murine model for assessing adalimumab, infliximab and etanercept to prevent polyarthritis. Ann Rheum Dis. 2003;62(Suppl 1):136–137.
- Taylor PC. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr Opin Pharmacol. 2010;10:308–315. doi:10.1016/j.coph.2010.01.005.
- Pattacini L, Boiardi L, Casali B, et al. Differential effects of anti-TNF-alpha drugs on fibroblast-like synoviocyte apoptosis. Rheumatology. 2010;49:480–489. doi:10.1093/rheumatology/kep358.
- Benucci M, Saviola G, Manfredi M, et al. Tumor necrosis factors blocking agents: analogies and differences. Acta Biomed. 2012;83:72–80.
- Cornes P. The economic pressures for biosimilar drug use in cancer medicine. Targ Oncol. 2012;7(Suppl S1):S57–S67. doi:10.1007/s11523-011-0196-3.
- Socinski MA, Curigliano G, Jacobs I, et al. Clinical considerations for the development of biosimilars in oncology. MAbs. 2015;7:286–293. doi:10.1080/19420862.2015.1008346.
- McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91:405–417. doi:10.1038/clpt.2011.343.
- McCamish M, Woollett G. The rise of the biosimilar. Expert Rev Clin Pharmacol. 2012;5:597–599. doi:10.1586/ecp.12.60.
- World Health Organization (WHO) Expert Committee on Biological Standardization. Guidelines on evaluation of similar biotherapeutic products (SBPs). Geneva [ updated 2009 Oct 19–23; cited 2016 Jan]. Available from: http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.
- European Medicines Agency. EMA/CHMP/437/04 guideline on similar biological medicinal products [ updated 2005 Oct; cited Jan 2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf.
- European Medicines Agency. EMEA/CHMP/BMWP/42832/2005 guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues [ updated 2006 Feb; cited 2016 Jan]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdf.
- European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance – non-clinical and clinical issues. Committee for Medicinal Products for Human Use (CHMP). 2015 [cited 2015 Jul 30]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf.
- US Department of Health and Human Services. Guidance for industry quality considerations in demonstrating biosimilarity to a reference protein product [ updated 2012 Feb; cited 2016 Jan]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf.
- US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). 2015 [cited 2016 Jan]. Available from: www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf.
- McCamish M, Woollett G. The continuum of comparability extends to biosimilarity: how much is enough and what clinical data are necessary? Clin Pharmacol Ther. 2013;93:315–317. doi:10.1038/clpt.2013.17.
- McCamish M, DaSilva A, Mayer RE, et al. A totality-of-the-evidence approach to the development of GP2015, a proposed etanercept biosimilar. 17th Annual Congress of the European League against Rheumatism; 2016 Jun 8–11; London, UK; Abstract 0321.
- Dhillon S, Lyseng-Williamson KA, Scott LJ. Etanercept: a review of its use in the management of rheumatoid arthritis. Drugs. 2007;67:1211–1241.
- Campa M, Ryan C, Menter A. An overview of developing TNF-α targeted therapy for the treatment of psoriasis. Expert Opin Investig Drugs. 2015;24:1343–1354. doi:10.1517/13543784.2015.1076793.
- Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031.
- Douni E, Sfikakis PP, Haralambous S, et al. Attenuation of inflammatory polyarthritis in TNF transgenic mice by diacerein: comparative analysis with dexamethasone, methotrexate and anti-TNF protocols. Arthritis Res Ther. 2004;6:R65–R72. doi:10.1186/ar1028.
- European Medicines Agency. Guideline on the investigation of bioequivalence. Committee for Medicinal Products for Human Use (CHMP). 2010 [cited 2016 Mar]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf.
- Enbrel BLA 98-0286. [updated 1998 Nov 2; cited 2015 Mar 17]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/.
- Enbrel: EPAR – Product Information. Summary of product characteristics [updated 2014 Nov 6; cited 2015 Jan 15]. Available from: http://www.ema.europa.eu/ema/.
- Berkowitz SA, Engen JR, Mazzeo JR, et al. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov. 2012;11:527–540. doi:10.1038/nrd3746.
- Beck A, Diemer H, Ayoub D, et al. Analytical characterization of biosimilar antibodies and Fc-fusion proteins. Trends Anal Chem. 2013;48:81–95. doi:10.1016/j.trac.2013.02.014.
- Holzmann J, Balser S, Windisch J. Totality of the evidence at work: the first U.S. biosimilar. Expert Opin Biol Ther. 2016;16:137–142. doi:10.1517/14712598.2016.1128410.
- Horiuchi T, Mitoma H, Harashima S, et al. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology. 2010;49:1215–1228. doi:10.1093/rheumatology/keq031.
- Afonso M, Heinrich SS, Poetzl J, et al. Pharmacokinetics and safety of GP2015, a proposed etanercept biosimilar, and etanercept originator product in healthy male subjects: a randomised two-way crossover study. 17th Annual Congress of the European League against Rheumatism; 2016 Jun 8–11; London, UK; Abstract 0145.
- Lee H-H, Song I-H, Friedrich M, et al. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-alpha antagonists. Br J Dermatol. 2007;156:486–491. doi:10.1111/j.1365-2133.2007.07682.x.
- Borrás-Blasco J, Navarro-Ruiz A, Borrás C, et al. Adverse cutaneous reactions induced by TNF-alpha antagonist therapy. South Med J. 2009;102:1133–1140. doi:10.1097/SMJ.0b013e3181bb554c.
- Rajakulendran S, Deighton C. Adverse dermatological reactions in rheumatoid arthritis patients treated with etanercept, an anti-TNFalpha drug. Curr Drug Saf. 2006;1:259–264.