ABSTRACT
Introduction: Treatment of rheumatoid arthritis (RA) has been revolutionized by the introduction of biologic disease-modifying antirheumatic drugs, such as tumor necrosis factor (TNF) inhibitors. With patent expiry approaching for many expensive biologic molecules, such as etanercept, more affordable biosimilar drugs are being developed. LBEC0101 is an etanercept biosimilar approved in Japan and South Korea for all etanercept indications including RA.
Areas covered: We discuss the pharmacological characteristics, pharmacokinetics, efficacy, and safety of LBEC0101 compared with the etanercept reference product (ETN-RP). Preclinical studies showed that the binding affinity to TNFα and biological activity of LBEC0101 were similar to those of the ETN-RP. The pharmacokinetic profile of LBEC0101 was also similar to that of the ETN-RP. A Phase III, randomized, double-blind, 54-week study showed that the efficacy of LBEC0101 was equivalent to that of the ETN-RP in RA patients. An extension study showed that efficacy was sustained long-term in patients receiving LBEC0101 and in those switching from the ETN-RP to LBEC0101. The safety profile of LBEC0101 was also confirmed to be comparable with the ETN-RP.
Expert opinion: LBEC0101 has shown equivalent pharmacokinetics and efficacy and comparable safety to the ETN-RP, and the lower cost of LBEC0101 provides a good cost–benefit ratio.
1. Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease characterized by inflammation [Citation1,Citation2]. Left untreated, RA can lead to progressive joint damage and deformity [Citation3]. The World Health Organization estimates that the prevalence of RA is between 0.3% and 1%, and that within 10 years of onset, at least half of the affected patients in developed countries are unable to work full-time because of the debilitating symptoms [Citation4].
Tumor necrosis factor (TNF) is an inflammatory cytokine that plays a pivotal role in the pathogenesis of RA [Citation5]. The introduction of biologic disease-modifying antirheumatic drugs (bDMARDs), such as TNF inhibitors, has revolutionized the treatment of RA; low disease activity and even clinical remission are now achievable targets of therapy [Citation6]. Current guidelines for the treatment of RA recommend the early use of TNF inhibitors in patients who do not achieve remission with methotrexate alone [Citation7–10]. Approved TNF inhibitors include etanercept, infliximab, adalimumab, certolizumab pegol, and golimumab [Citation11]. Etanercept was the first TNF inhibitor approved to treat RA in the US (1998), Europe (2000), Korea (2003) and Japan (2005) [Citation12–14]. However, while TNF inhibitor treatment is highly effective, the expensive medical cost exerts a financial burden on patients and healthcare systems [Citation15–17].
Recently, the approaching patent expiration for originator biologic molecules has led to an interest in the development of molecules with biological similarity to originator biologics, which are known as biosimilars [Citation18]. Biosimilars are developed to have no clinically meaningful differences between the biosimilar and the originator product in terms of efficacy, safety, purity, and potency [Citation19]. Regulatory guidelines for biosimilar products are broadly similar in different regions and contain recommendations and requirements for preclinical data, clinical trial data, and pharmacovigilance [Citation19–21]. Biosimilar drugs can be approved for use in all indications held by the originator biologic by extrapolation of efficacy and safety data from at least one indication of the originator and with adequate scientific justification, that is, without direct comparative clinical studies in all indications [Citation22]. Regarding switching from an originator product to a biosimilar, numerous published biosimilar studies have reported no safety or efficacy concerns even though not all regulatory guidelines require collection of such data [Citation23–27].
The current outlook for TNF inhibitor biosimilars is good. Recent reviews have concluded that currently available data support the equivalence of TNF inhibitor biosimilars SB4 (etanercept biosimilar) and ABP501 (adalimumab biosimilar) to their originators with regards to safety, efficacy, immunogenicity, and tolerability [Citation28,Citation29]. For infliximab biosimilar development, it was recently reported that switching from infliximab to biosimilar infliximab had no detrimental effects on long-term (18 months) efficacy and safety in patients with ankylosing spondylitis [Citation30].
Substantial cost savings attributable to biosimilars have been projected. A model published in 2018 that estimated potential future savings from biosimilars in the United States projected that in the 10-year period from 2017 to 2026, biosimilars will reduce direct spending on biologic drugs by US$54 billion [Citation16]. A budget impact model conducted in the UK and published in 2019 predicted that cost savings will lead to infliximab and etanercept being replaced by respective biosimilars by 2020 [Citation31]. Due to their generally lower acquisition costs compared with the originator biologics, biosimilars have the potential to introduce price competition into the market, resulting in reduced costs for patient treatment [Citation15,Citation32]. It is likely that the introduction of biosimilars into clinical practice will significantly lessen the financial burden on healthcare institutions for RA treatment worldwide.
2. Overview of the biosimilars market
Anti-TNF agents sales globally were reported to be US$35.73 billion in 2015 and increased to US$39.84 billion in 2017 [Citation33]. However, biosimilar TNF inhibitors have been available since 2012 [Citation34], and the market share for biosimilars is predicted to increase as patents expire, allowing new products to enter the market, and as confidence and acceptance grow from doctors and patients [Citation35]. Indeed, global revenues for the etanercept originator product show a declining trend over the past few years: US$9.2 billion in 2016, US$8.3 billion in 2017, and US$7.4 billion in 2018 [Citation33]. Meanwhile, sales of an etanercept biosimilar, SB4, have increased: US$100.6 million in 2016, US$370.8 million in 2017, and US$485.2 million in 2018 [Citation36].
3. Introduction to LBEC0101
LBEC0101 has been developed as a biosimilar to the etanercept reference product (ETN-RP) [Citation37], which is a dimeric fusion protein that consists of the extracellular ligand binding portion of the 75 kDa TNF receptor linked to the Fc portion of human immunoglobulin G1 [Citation12]. LBEC0101 binds competitively to TNF, which is overexpressed in RA [Citation5]. This prevents TNF from binding to its cell-surface receptors, and thereby inhibits its biological activity [Citation38]. The high similarity in the structural and functional properties and biological activities between LBEC0101 and the ETN-RP was demonstrated by in vitro and in vivo studies, including a TNFα binding affinity study [Citation39]. LBEC0101 was the first etanercept biosimilar approved for the treatment of RA and juvenile idiopathic arthritis in Japan in 2018 [Citation40]. In South Korea, LBEC0101 has been approved for RA, psoriatic arthritis, axial spondyloarthritis, and psoriasis in adults, and juvenile idiopathic arthritis in pediatric patients [Citation41].
LBEC0101 is available in a pre-filled syringe, auto-injector syringe pen, and vial. Pre-filled syringes are available in two strengths (25 mg and 50 mg). The auto-injector syringe pen is also available in two strengths, 25 mg and 50 mg, both of which are approved in Japan; only the 50-mg dose is available in Korea. Vials, which are only available in Japan, are available in two strengths (10 mg and 25 mg).
4. Pharmacodynamics and pharmacokinetics
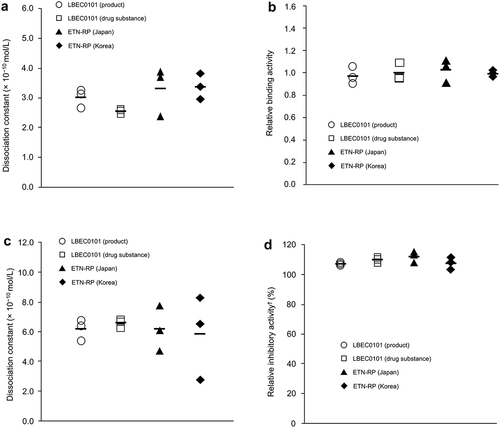
In vitro studies show that pharmacologic characteristics, such as the binding affinity of LBEC0101 to soluble TNFα, transmembrane TNFα, and soluble lymphotoxin-α, and the neutralizing effect of TNFα biological activity, are similar to those of the ETN-RP () [Citation37].
Figure 1. LBEC0101 drug substance and ETN-RP binding affinities to (a) sTNFα, (b) mTNFα, (c) sLTα, and (d) TNFα neutralization
The pharmacokinetics of LBEC0101 were compared with those of the ETN-RP in healthy male participants in a randomized, double-blind, single-dose, 2 × 2 crossover design Phase I study (NCT01725620) [Citation38]. LBEC0101 showed a similar pharmacokinetic profile to the ETN-RP after a single subcutaneous injection in 43 evaluable subjects. Geometric mean ratios (90% confidence intervals [CI]) for LBEC0101 relative to the ETN-RP were 1.02 (0.92–1.13) for maximum concentration and 0.96 (0.87–1.05) for area under the curve from time zero to infinity (within a conventional bioequivalence criterion of 0.80–1.25) ().
Table 1. Summary of pharmacokinetic parameters of LBEC0101 and etanercept reference product after a single 25-mg subcutaneous injection in healthy males
In a pharmacokinetic analysis of a Phase III efficacy study to evaluate the similarities between LBEC0101 and the ETN-RP in terms of efficacy and safety in patients with active RA inadequately responding to methotrexate, mean trough concentration values between week 12 and week 52 were similar for the LBEC0101 group (2579.1 ng/mL to 4143.4 ng/mL) and the ETN-RP group (2166.8 ng/mL to 3586.5 ng/mL) [Citation39].
5. Clinical efficacy
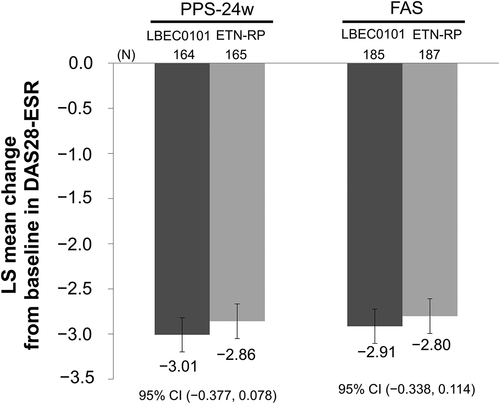
The efficacy of LBEC0101 compared with the ETN-RP was investigated in a 54-week Phase III study (N = 374; LBEC0101 [n = 187], ETN-RP [n = 187]) in patients with active RA despite methotrexate treatment (NCT02357069) [Citation39]. In this multicenter (30 sites in Korea and 48 sites in Japan), randomized, double-blind, parallel-group study, the clinical efficacy of LBEC0101 was equivalent to that of ETN-RP. The primary efficacy endpoint was the change from baseline in the disease activity score in 28 joints based on erythrocyte sedimentation rate [DAS28-ESR] at week 24, and was −3.01 (95% CI −3.198 to −2.820) in the LBEC0101 group and −2.86 (95% CI −3.051 to −2.667) in the ETN-RP group (). The estimated between-group difference was −0.15, and its 95% CI was −0.377 to 0.078, which was within the prespecified equivalence margin of −0.6 to 0.6. The results of the secondary endpoint (American College of Rheumatology 20% response rates [ACR20] at week 24) were also similar between the groups (LBEC0101 93.3% versus ETN-RP 86.7%); ACR20 values continued to be similar at week 52 (LBEC0101 92.0% versus ETN-RP 88.4%). The ACR50 and ACR70 response rates showed a similar trend to ACR20. ACR50 values were 74.4% for LBEC0101 versus 63.0% for the ETN-RP at week 24, and 74.7% for LBEC0101 versus 65.8% for the ETN-RP at week 52. ACR70 values were 50.6% for LBEC0101 versus 39.4% for the ETN-RP at week 24, and 58.0% for LBEC0101 versus 50.0% for the ETN-RP at week 52.
Figure 2. DAS28-ESR change from baseline (LBEC0101 versus the ETN-RP)
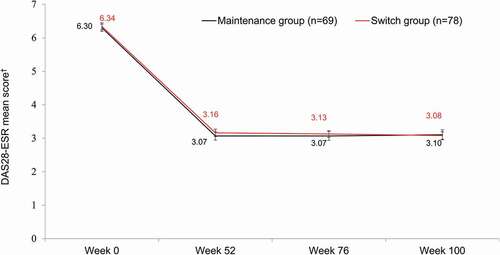
In an extension study, conducted in Korea only, patients who were treated with the ETN-RP during the randomized controlled Phase III study switched to LBEC0101 (switch group, n = 78), while those treated with LBEC0101 continued to receive LBEC0101 (maintenance group, n = 70) (NCT02715908) [Citation42]. The efficacy of LBEC0101 was assessed up to week 100 in the extension study. Administration of LBEC0101 showed sustained efficacy in patients who continued therapy and also in those who switched from the ETN-RP to LBEC0101. DAS28-ESR mean scores were maintained in both groups from week 52 to week 100 (3.07–3.10 in the maintenance group versus 3.16–3.08 in the switch group) (). At week 100, ACR response rates were similar in the maintenance and switch groups: 79.7% versus 83.3%, respectively, for ACR20 response; 65.2% versus 66.7% for ACR50 response; and 44.9% versus 42.3% for ACR70 response.
Figure 3. DAS28-ESR over time to week 100 in the maintenance and switch groups
In the Phase III study, the mean (± standard deviation) methotrexate dose at baseline was 11.3 (±2.9) mg/week for the LBEC0101 group, and 11.1 (±3.0) mg/week for the ETN-RP group [Citation39], which were lower than the baseline methotrexate doses reported in clinical studies with other etanercept biosimilars (15.6 [±4.52] mg/week for the SB4 group and 15.5 [±4.60] mg/week for the ETN-RP group in the global study with SB4 [Citation43]; and 16.0 [±4.9] mg/week for the GP2015 group and 17.1 [±4.6] mg/week for the ETN-RP group in the EQUIRA study [Citation44]). A subgroup analysis performed according to methotrexate dose (≤10 mg/week, >10 mg/week) showed that efficacy and safety results were comparable between the LBEC0101 and ETN-RP groups regardless of methotrexate dose [Citation39].
6. Safety and tolerability
In the Phase III study, LBEC0101 was well tolerated with a comparable safety profile to the ETN-RP [Citation39]. The incidence of adverse events (AEs) was comparable: 92.0% for LBEC0101 and 92.5% for the ETN-RP (). The incidence of adverse drug reactions (ADRs) (51.3% for LBEC0101 and 62.0% for the ETN-RP), serious AEs (16.6% for LBEC0101 and 10.7% for the ETN-RP), and serious ADRs (7.0% in both groups) were also similar between groups (). The incidence of injection site reactions was significantly lower in patients treated with LBEC0101 compared with those treated with the ETN-RP. Injection site erythema, pruritus, and swelling were reported in 25.1%, 20.3%, and 7.0% of patients, respectively, in the ETN-RP group compared with only 5.3%, 3.7%, and 2.1% of patients, respectively, in the LBEC0101 group. The respective treatment differences in the incidence of injection site erythema, pruritus, and swelling between the groups were −19.8% (95% CI: −26.8, −12.8), −16.6% (95% CI: −23.0, −10.2), and −4.8% (95% CI: −9.0, −0.6). Anti-drug antibodies (ADAs) were detected in fewer patients receiving LBEC0101 than the ETN-RP. At baseline, two patients (1.1%) in the ETN-RP group were ADA-positive and none in the LBEC0101 group; after treatment, 18 patients (9.6%) in the ETN-RP group developed ADAs compared with only three patients (1.6%) in the LBEC0101 group, and the treatment difference between the groups was −8.0% (95% CI: −12.6, −3.4). None of the patients were positive for neutralizing antibody. The reason for the differences in incidence of injection site reactions and ADAs between the groups is not yet known. The difference observed in ADA incidence is likely not attributable to the assay system as both systems (using labeled ETN-RP and LBEC0101) produced similar results [Citation39]. Interestingly, previous studies comparing etanercept biosimilars, SB4 and GP2015, with the ETN-RP have also reported unexplained lower incidences of injection site reactions and ADAs with the biosimilars compared to the ETN-RP [Citation43,Citation45,Citation46]. It has been proposed that differences in the composition and container closure system between SB4 and ENT-RP – for instance, the lack of latex in the needle shield in SB4 – may account for the lower frequency of injection site reactions reported for patients treated with SB4 [Citation28,Citation46]. Indeed, immunogenicity may be affected by factors such as aggregates and impurities in the product, and the container closure system [Citation39], but further investigation is required to fully explain the differences in incidences of injection site reactions and ADAs between LBEC0101 and the ETN-RP.
Table 2. Summary of AEs during the Phase III double-blind study and the extension study
In the subsequent Phase III extension study, there were similar incidences of AEs, serious AEs, ADRs, and serious ADRs in patients after continued therapy or after switching from the ETN-RP to LBEC0101 () [Citation42]. There were no immunogenicity concerns after switching from the ETN-RP to a biosimilar or in patients continuing with LBEC0101. Injection site reactions were lower than reported in the preceding double-blind Phase III study, possibly because of the finer 29G needle used in the extension study [Citation42] compared with the 27G needle used in the Phase III study [Citation39,Citation47,Citation48]. Overall, the safety profile for LBEC0101 was maintained from the Phase III double-blind study, for both maintenance and switch groups, with no notable differences between groups and no new safety concerns.
7. Regulatory affairs
As of August 2019, LBEC0101 () is sold in South Korea and Japan (both approved in 2018) for RA in adults and juvenile idiopathic arthritis in pediatric patients [Citation40], and additionally in South Korea for psoriatic arthritis, axial spondyloarthritis, and psoriasis in adults [Citation41].
8. Conclusion
LBEC0101, an approved etanercept biosimilar, has shown equivalent pharmacokinetics, clinical efficacy, safety, and tolerability to the ETN-RP.
9. Expert opinion
9.1. What, if any, improvement does the drug hold over other therapies?
While LBEC0101 and the ETN-RP appear equivalent in terms of efficacy, as with other biosimilars, the lower cost of LBEC0101 is an advantage compared with the ETN-RP. In the context of other etanercept biosimilars, LBEC0101 was found to have a similar pattern of efficacy and a comparable safety profile. In the double-blind Phase III study, 93.3% and 86.7% ACR20 responses were reported for LBEC0101 and ETN-RP groups, respectively, at week 24 [Citation39]. The global study with the SB4 biosimilar reported respective ACR20 responses of 78.1% and 80.3% for SB4 and ETN-RP groups [Citation42], and the EQUIRA study reported respective ACR20 responses of 88.0% and 92.9% for GP2015 and ETN-RP groups [Citation44]; all at week 24. Lower incidence rates of injection site reactions were reported for etanercept biosimilars compared to the ETN-RP. In the Phase III study with LBEC0101, respective incidences of 10.2% and 34.2% for injection site reactions were reported for the LBEC0101 and ETN-RP groups, with even lower incidences reported for LBEC0101 in the Phase III extension study (LBEC0101 maintenance group, 1.4%; LBEC0101 switch group, 6.4%) [Citation39,Citation42]. In the global clinical study with SB4, the incident rate was 3.7% vs 17.2% for SB4 and ETN-RP groups, respectively [Citation43], and the EQUIRA study reported a respective 7.0% vs 18.4% incident rate for GP2015 and ETN-RP groups [Citation44].
9.2. What, if any, impact is this drug likely to have on current treatment strategies?
As LBEC0101 is a biosimilar, there will likely be no effect on current treatment strategies. However, as with other biosimilars, LBEC0101 will be a lower-cost alternative option for patients who cannot afford ETN-RP.
The easy-to-use pen device used for LBEC0101 may be a preferred option for patients who have difficulty in using the original etanercept pen or who want to avoid injection site reactions, as the smaller needle size (29G) may contribute to fewer injection site reactions [Citation47–50]. Of note, the incidence of injection site reactions in the LBEC0101 extension study was lower than that in the preceding Phase III study [Citation39,Citation42].
9.3. How likely are physicians to prescribe the drug?
While the indication of LBEC0101 is the same as that of the ETN-RP, the lower cost along with the equivalent efficacy and comparable safety and tolerability profiles of LBEC0101 mean that physicians may prescribe it to patients who cannot afford the ETN-RP.
9.4. What data are still needed?
Patients and physicians may find it desirable to have additional clinical data on interchangeability and extrapolated indications. While data related to a single switch from the ETN-RP to LBEC0101 was obtained in the extension study, interchangeability data (i.e. repeatedly switching between LBEC0101 and the ETN-RP) may be useful for consideration of patient safety in clinical settings. Moreover, while the efficacy and safety of LBEC0101 was confirmed in RA patients, no data have been obtained for extrapolated indications such as JIA, and future studies in these extrapolated indications would be helpful to physicians wanting to use LBEC0101 instead of the ETN-RP.
9.5. Where is the drug likely to be in five years’ time?
As of August 2019, LBEC0101 is approved only in South Korea and Japan; it is hoped that in the near future it will be launched in additional countries, expanding patient access to a lower-cost etanercept treatment.
Box 1. Drug summary box
Article Highlights
LBEC0101, an etanercept biosimilar, has shown similar pharmacokinetics, clinical efficacy, safety, and tolerability to etanercept in patients with rheumatoid arthritis.
Patients treated with LBEC0101 reported fewer injection site reactions and fewer incidences of anti-drug antibody compared with etanercept.
LBEC0101 has a cost–benefit advantage over etanercept because of its lower cost.
LBEC0101 is currently sold in South Korea and Japan for the treatment of rheumatoid arthritis in adults and juvenile idiopathic arthritis in pediatric patients; and additionally in South Korea for psoriatic arthritis, axial spondyloarthritis, and psoriasis in adults.
Additional studies on interchangeability data, and safety and efficacy data for extrapolated indications (other than rheumatoid arthritis) are warranted for expanded use of LBEC0101 in clinical settings.
Declaration of interest
All authors have received non-financial support from LG Chem, Ltd. (formerly, LG Life Sciences, Ltd.) and YW Song has received a grant from the Ministry of Science, ICT and Future Planning (NRF-2015M3A9B6052011, 2019M3A9A8065574) related to the submitted work. All authors have received grants for the published double-blind Phase III study [39] and the subsequent extension study of LBEC0101 [42] from LG Chem, Ltd., outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Acknowledgments
We thank Sheena Hunt (PhD), Sally-Ann Mitchell (PhD) and Sarah Bubeck (PhD) of Edanz Medical Writing, for providing medical writing support, which was funded by LG Chem, Ltd. (formerly, LG Life Sciences, Ltd).
Additional information
Funding
References
- Croia C, Bursi R, Sutera D, et al. One year in review 2019: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):347–357.
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108.
- Grassi W, De Angelis R, Lamanna G, et al. The clinical features of rheumatoid arthritis. Eur J Radiol. 1998;27(Suppl 1):S18–24.
- World Health Organization. Chronic rheumatic conditions [Internet]. 2019 [cited 2019 Aug 8]. Available from: https://www.who.int/chp/topics/rheumatic/en/
- Toussirot E, Wendling D. The use of TNF-alpha blocking agents in rheumatoid arthritis: an overview. Expert Opin Pharmacother. 2004;5(3):581–594.
- Rein P, Mueller RB. Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther. 2017;4(2):247–261.
- Chinese Rheumatology Association. 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Zhonghua Nei Ke Za Zhi. 2018;57(4):242–251. Chinese.
- Kameda H, Fujii T, Nakajima A, et al. Japan college of rheumatology guideline for the use of methotrexate in patients with rheumatoid arthritis. Mod Rheumatol. 2019;29(1):31–40.
- American College of Rheumatology. Updated guideline for the management of rheumatoid arthritis 2018 [Internet]. 2018 [cited 2019 Aug 8]. Available from: https://www.rheumatology.org/Portals/0/Files/Rheumatoid-Arthritis-Guideline-Project-Plan.pdf
- Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977.
- Food & Drug Administration. Information on tumor necrosis factor (TNF) blockers (marketed as remicade, enbrel, humira, cimzia, and simponi) [Internet]. 2015 [cited 2019 Aug 8]. Available from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-tumor-necrosis-factor-tnf-blockers-marketed-remicade-enbrel-humira-cimzia-and-simponi
- Hassett B, Singh E, Mahgoub E, et al. Manufacturing history of etanercept (Enbrel®): consistency of product quality through major process revisions. MAbs. 2018;10(1):159–165.
- Son JH, Cha SW. Anti-TNF-alpha therapy for ankylosing spondylitis. Clin Orthop Surg. 2010;2(1):28–33.
- Miyasaka N, Takeuchi T, Eguchi K. Guidelines for the proper use of etanercept in Japan. Mod Rheumatol. 2006;16(2):63–67.
- Manova M, Savova A, Vasileva M, et al. Comparative price analysis of biological products for treatment of rheumatoid arthritis. Front Pharmacol. 2018;9:1070.
- Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3.
- Schulze-Koops H, Skapenko A. Biosimilars in rheumatology: a review of the evidence and their place in the treatment algorithm. Rheumatology (Oxford). 2017;56(Suppl4):iv30–iv48.
- Remuzat C, Dorey J, Cristeau O, et al. Key drivers for market penetration of biosimilars in Europe. J Mark Access Health Policy. 2017;5(1):1272308.
- Declerck P, Danesi R, Petersel D, et al. The language of biosimilars: clarification, definitions, and regulatory aspects. Drugs. 2017;77(6):671–677.
- Kuribayashi R, Sawanobori K. Current Japanese regulatory systems for generics and biosimilars. J Pharm Sci. 2018;107(3):785–787.
- Mysler E, Pineda C, Horiuchi T, et al. Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol Int. 2016;36(5):613–625.
- Tesser JR, Furst DE, Jacobs I. Biosimilars and the extrapolation of indications for inflammatory conditions. Biologics. 2017;11:5–11.
- Glintborg B, Sorensen IJ, Loft AG, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76(8):1426–1431.
- Goll GL, Jorgensen KK, Sexton J, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial. J Intern Med. 2019;285(6):653–669.
- Jaworski J, Matucci-Cerinic M, Schulze-Koops H, et al. Switch from reference etanercept to SDZ ETN, an etanercept biosimilar, does not impact efficacy, safety, and immunogenicity of etanercept in patients with moderate-to-severe rheumatoid arthritis: 48-week results from the phase III, randomized, double-blind EQUIRA study. Arthritis Res Ther. 2019;21(1):130.
- Park W, Suh CH, Shim SC, et al. Efficacy and safety of switching from innovator rituximab to biosimilar CT-P10 compared with continued treatment with CT-P10: results of a 56-week open-label study in patients with rheumatoid arthritis. BioDrugs. 2017;31(4):369–377.
- Smolen JS, Choe JY, Prodanovic N, et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: results of a randomised, double-blind, phase III transition study. Ann Rheum Dis. 2018;77(2):234–240.
- Pelechas E, Drosos AA. Etanercept biosimilar SB-4. Expert Opin Biol Ther. 2019;19(3):173–179.
- Pelechas E, Voulgari PV, Drosos AA. ABP 501 for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2018;18(3):317–322.
- Kaltsonoudis E, Pelechas E, Voulgari PV, et al. Maintained clinical remission in ankylosing spondylitis patients switched from reference infliximab to its biosimilar: an 18-month comparative open-label study. J Clin Med. 2019;8(7):pii:E956.
- Aladul MI, Fitzpatrick RW, Chapman SR. The effect of new biosimilars in rheumatology and gastroenterology specialities on UK healthcare budgets: results of a budget impact analysis. Res Social Adm Pharm. 2019;15(3):310–317.
- Quintiles IMS. The impact of biosimilar competition in Europe [Internet]. 2017 [cited 2019 Aug 8]. Available from: https://www.medicinesforeurope.com/wp-content/uploads/2017/05/IMS-Biosimilar-2017_V9.pdf
- LaMerie. Blockbuster biologics 2018: sales of recombinant therapeutic antibodies & proteins [Internet]. 2018. [cited 2019 Aug 8]. Available from: https://lamerie.com/report/blockbuster-biologics-2018-sales-of-recombinant-therapeutic-antibodies-proteins/
- Cohen S, Kay J. Biosimilars: implications for rheumatoid arthritis therapy. Curr Opin Rheumatol. 2017;29(3):260–268.
- Kaida-Yip F, Deshpande K, Saran T, et al. Biosimilars: review of current applications, obstacles, and their future in medicine. World J Clin Cases. 2018;6(8):161–166.
- Biogen. 2018 annual report [Internet]. 2019 [cited 2019 Aug 8]. Available from: https://biogen.gcs-web.com/static-files/30ea6b55-6a57-48ae-840d-96ac58ddf031
- Kyono H, Haraguchi K, Kojima S, et al. Quality characteristics and nonclinical/clinical profiles of Etanercept BS SC [MA]. Folia Pharmacol Jpn. 2018;151(6):261–272. Japanese.
- Lee H, Chung H, Lee S, et al. LBEC0101, A proposed etanercept biosimilar: pharmacokinetics, immunogenicity, and tolerability profiles compared with a reference biologic product in healthy male subjects. BioDrugs. 2017;31(4):349–355.
- Matsuno H, Tomomitsu M, Hagino A, et al. Phase III, multicentre, double-blind, randomised, parallel-group study to evaluate the similarities between LBEC0101 and etanercept reference product in terms of efficacy and safety in patients with active rheumatoid arthritis inadequately responding to methotrexate. Ann Rheum Dis. 2018;77(4):488–494.
- Mochida Pharmaceutical Co. L. Mochida obtains marketing approval for etanercept biosimilar in Japan [Internet]. 2018 [cited 2019 Aug 8]. Available from: http://www.mochida.co.jp/english/news/docs/2018/180119_etanerceptBS.pdf
- Center for Biosimilars. Eye on pharma: LG chem’s etanercept biosimilar gains Korean approval [Internet]. 2019 [cited 2019 July 9]. Available from: https://www.centerforbiosimilars.com/news/eye-on-pharma-lg-chems-etanercept-biosimilar-gains-korean-approval
- Park MC, Matsuno H, Kim J, et al. Long-term efficacy, safety and immunogenicity in patients with rheumatoid arthritis continuing on an etanercept biosimilar (LBEC0101) or switching from reference etanercept to LBEC0101: an open-label extension of a phase III multicentre, randomised, double-blind, parallel-group study. Arthritis Res Ther. 2019;21(1):122.
- Emery P, Vencovsky J, Sylwestrzak A, et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):51–57.
- Matucci-Cerinic M, Allanore Y, Kavanaugh A, et al. Efficacy, safety and immunogenicity of GP2015, an etanercept biosimilar, compared with the reference etanercept in patients with moderate-to-severe rheumatoid arthritis: 24-week results from the comparative phase III, randomised, double-blind EQUIRA study. RMD Open. 2018;4:e000757.
- Griffiths CEM, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176(4):928–938.
- Girolomoni G, Feldman SR, Emery P, et al. Comparison of injection-site reactions between the etanercept biosimilar SB4 and the reference etanercept in patients with rheumatoid arthritis from a phase III study. Br J Dermatol. 2018;178(3):e215–e216.
- Jaber A, Bozzato GB, Vedrine L, et al. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38.
- Glenski S, Conner J. 29 gauge needles improve patient satisfaction over 27 gauge needles for daily glatiramer acetate injections. Drug Healthc Patient Saf. 2009;1:81–86.
- Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23(1–2):37–43.
- Siegmund T, Blankenfeld H, Schumm-Draeger PM. Comparison of usability and patient preference for insulin pen needles produced with different production techniques: “thin-wall” needles compared to “regular-wall” needles: an open-label study. Diabetes Technol Ther. 2009;11(8):523–528.
