ABSTRACT
Background: There is limited long-term, real-world evidence on the efficacy and safety in patients with plaque psoriasis treated with secukinumab. We present results at 136 weeks in a real-world setting with focus on special populations.
Research design and methods: Retrospective analysis of 151 patients with chronic plaque psoriasis who initiated treatment with secukinumab between September 2015 and May 2019. Secukinumab 300 mg was administered once weekly for 5 weeks followed by once monthly.
Main outcome measures: Clinical and laboratory assessments were performed up to 136 weeks.
Results: At 16 weeks, 90%, 79%, and 63% of patients achieved Psoriasis Area and Severity Index (PASI) 75, PASI 90, and PASI 100, respectively, compared with 79%, 72%, and 55% of patients after 136 weeks of therapy with secukinumab. Fifteen of the 151 patients experienced an adverse event, the most common of which was candida infection (4%). Biological treatment naïve was significantly associated with response to therapy at 1 and 2 years (P < 0.0001). There were no safety issues in patients with infection with HBV, HCV or mycobacterium tuberculosis.
Conclusions: Our results confirm the rapidity of action of secukinumab as well as its long-lasting efficacy and good safety in real-world clinical practice.
1. Introduction
Psoriasis is a chronic and debilitating autoimmune inflammatory disease; plaque psoriasis is by far the most common form, affecting up to 90% of patients [Citation1], of which around one-fifth suffer from moderate-to-severe disease [Citation2]. Moreover, psoriasis is associated with early mortality as well as an increased risk for comorbidities such as including psoriatic arthritis, cardiovascular disease, and diabetes [Citation1,Citation2]. Since the disease may also be related to anxiety, depression, and social isolation, and can negatively affect personal relationships and employment status, it has substantial psychologic and socioeconomic impact that can last a lifetime. As a result, plaque psoriasis gives rise to substantially increased use of healthcare resources and costs [Citation3].
Current treatments for moderate-to-severe psoriasis include topical therapy, phototherapy, and both non-biologic and biologic systemic agents [Citation4]. Among the available treatments, biologic agents and phototherapy are most commonly employed for moderate to severe psoriasis [Citation4]. Notwithstanding, therapies that are more efficient and cost effective are still desirable. Recently, a better understanding of the pathophysiology of plaque psoriasis has led to the identification of several promising biological targets. While the pathogenesis of plaque psoriasis is undoubtedly multifactorial, the interleukin-23/T helper 17 pathway has been shown to play a crucial role in the disease [Citation5]. In this pathway, interleukin-17A (IL-17A) is the primary effector: overexpression of this IL-17A results in hyperplasia and an overly robust inflammatory response in epiderma, leading to skin plaques and the systemic inflammation which is characteristic of psoriasis. Of note, biological therapies such as those targeting IL-17 have demonstrated to be efficacious in the treatment of moderate-to-severe plaque psoriasis, and importantly, there is increasing evidence that treatment with biologics shows a noteworthy benefit in social terms as well [Citation6]. In addition to efficacy, anti-IL-17 agents have also been shown to have a rapid onset of action that is efficacious in the long term [Citation5]. These results all highlight the utility of anti-IL-17 agents in the treatment of moderate-to-severe psoriasis as well as the key role of the IL-23/Th17 pathway in the pathogenesis of plaque psoriasis.
Secukinumab is a fully human IgG1κ anti-IL-17A monoclonal antibody that targets IL-17A [Citation7]. By selectively binding to IL-17A, secukinumab inhibits its interaction with IL-17 receptors on keratinocytes, endothelial cells, chondrocytes, and osteoblasts, thereby blocking downstream inflammatory pathways important in plaque psoriasis and other autoimmune inflammatory diseases [Citation2,Citation8,Citation9]. Secukinumab was the first in its class to be approved by the FDA for treatment of moderate to severe plaque psoriasis and has also been approved for the treatment of active psoriatic arthritis and ankylosing spondylitis. The efficacy of secukinumab in plaque psoriasis has been well demonstrated in several clinical trials and appears to be superior to other biologic agents in common use such as etanercept and ustekinumab (reviewed in [Citation10]). The phase III SCULPTURE study evaluated maintenance treatment with fixed-interval monthly secukinumab compared to as-needed treatment and showed superiority for secukinumab (300 mg: 78.2%; 150 mg: 62.1%) vs. as needed therapy (67.7%; 52.4%) in achieving Psoriasis Area and Severity Index (PASI) 75 through week 52 [Citation11], which was maintained through 3 years of treatment [Citation12]. PASI 75, 90 and 100 response rates remained sustained from year 1 (88.9%, 68.5% and 43.8%, respectively) to year 5 (88.5%, 66.4% and 41%) for patients administered fixed-interval monthly secukinumab 300 mg [Citation13]. The randomized, placebo-controlled phase III ERASURE and FIXTURE trials evaluated two fixed regimens of secukinumab [Citation14]. The proportion of patients who met PASI 75 at week 12 was higher with each secukinumab dose than with placebo or etanercept: in ERASURE, the rates of PASI 75 were 81.6% with 300 mg of secukinumab and 71.6% with 150 mg, vs. 4.5% for placebo; in FIXTURE, 77.1% of patients achieved PASI 75 with 300 mg of secukinumab and 67.0% with 150 mg, vs. 44.0% with etanercept and 4.9% with placebo (P < 0.001 for each secukinumab dose vs. comparators). Moreover, patients who were naïve to biological therapy had superior clinical responses than those with previous exposure. In all trials, secukinumab is generally reported to be well tolerated with an overall safety profile that is similar to other biologic agents used to manage psoriasis [Citation14].
Despite the wealth of positive evidence from clinical trials on the use of secukinumab, it is important to stress that clinical evidence must be complemented with that from real-life use. While randomized controlled trials assess drug efficacy and safety, daily practice may often be different as some patients may be excluded or under-represented in clinical trials. Data from real-life thus integrates clinical trial data by including different patient populations as well as those with differing severity and multiple comorbid conditions. The efficacy and safety of secukinumab have been evaluated in short-term real-world studies [Citation15,Citation16] and is being investigated in a longer-term investigation up to 104 weeks in patients receiving secukinumab in a real-world setting with prior and concomitant use of psoriasis treatments [Citation17]. A real-world study of 330 patients was also reported throughout 52 weeks of treatment [Citation18].
The efficacy of secukinumab has been recently assessed in a multi-center, retrospective, real-life study with up to 52 weeks of observation in 107 patients, which confirmed the rapid clinical improvement of secukinumab with the duration of efficacy at 1 year [Citation19]. In particular, PASI75 was achieved by 80% of patients and PASI90 was obtained in 67.5% at week 12; 81.6% of patients who reached week 52 maintained the PASI90 response. With the objective of identifying predictors of response and document longer-term outcomes in patients with plaque psoriasis ongoing treatment with secukinumab, herein we report the results at 136 weeks of the same patient cohort, focusing on special populations given the additional long-term clinical experience we have acquired with secukinumab. We also report our experience on the efficacy of the drug in difficult-to-treat areas including the head and scalp, genital area, and palmo-plantar regions.
2. Materials and methods
2.1. Study design and patient population
The study design and characteristics have been previously described in detail [Citation19]. Briefly, retrospective data from a cohort of patients with chronic plaque psoriasis who initiated treatment with secukinumab between September 2015 and May 2019 were studied. It is important to note that patients started treatment at different times, so these data present only a ‘snapshot’ of our experience taken at the end of May 2019. The population consisted of patients attending an outpatient clinic in central Italy. Data on demographics and clinical variables were collected. For inclusion, patients had to have moderate-to-severe psoriasis and have failed to respond, had contraindications for, or did not tolerate at least one conventional treatment, including systemic therapy (methotrexate, cyclosporine, acitretin) or phototherapy (ultraviolet B, psoralen plus ultraviolet A). Only patients treated with secukinumab for at least 16 weeks were considered. All patients gave written informed consent for their participation prior to enrollment. The study was conducted in accordance with the principles of the Declaration of Helsinki.
2.2. Treatment with secukinumab
Secukinumab was administered at 300 mg subcutaneous once weekly for 5 weeks followed by once monthly injections. Patients with a baseline PASI <10.0, who presented involvement of sensitive areas such as the face, scalp, hands, or genital areas, thus affecting their QoL, were also considered eligible for treatment with secukinumab.
2.3. Outcome measures
Clinical (PASI score) and laboratory assessments were performed at baseline and up to weeks 52 as previously described [Citation19]. After week 52, evaluations were performed at weeks 88, 104, and 136. Quantiferon-TB gold tests and serology for hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV) were performed at baseline and at weeks 52 and 136. Safety and tolerability were investigated by examination of adverse events (AEs), including mild and serious AEs, clinically significant changes in laboratory values and physical examinations.
2.4. Statistical analysis
Efficacy data were analyzed by an intent-to-treat last observation carried forward imputation (ITT-LOCF) analysis in patients treated for at least 16 weeks with secukinumab. If a patient dropped out of the study, then the last available value was carried forward until the end of the treatment. Data are presented as mean ± standard deviation or as percentages. Differences in values obtained at the different time points of treatment were assessed by univariate and multivariate logistic regression analyses. In all cases, a p < 0.05 was considered statistically significant. Statistical analysis was carried using STATA 11.2 software (StataCorp LP Inc., College Station, TX, USA).
3. Results
A total of 151 patients were included in the present analysis (). Of these, 66.4% were male with a mean age of 45.3 ± 13.8 years and duration of disease of 20.5 ± 13.7 years. Moreover, 81% of patients were naïve to biological therapy, while 19% had received ≥1 previous biological agent. Regarding comorbid conditions, 31 patients had hypertension, 15 dyslipidemia, 10 had type 2 diabetes, 9 cardiovascular/cerebrovascular diseases, 5 hypothyroidism, 3 hepatic steatosis, and 1 patient had hyperthyroidism. According to Body mass index (BMI), 44.5% of patients were normal weight (BMI <25), 32.8% were overweight (25 ≤ BMI ≤ 30), and 22.6% were obese (BMI > 30).
Table 1. Demographic characteristics of the 151 patients included in the analysis.
3.1. Effectiveness
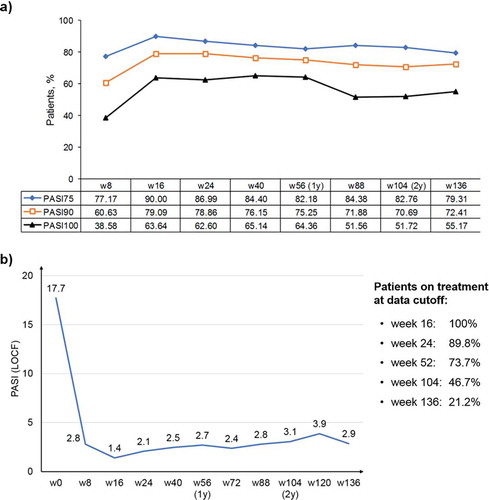
Changes in PASI 75, PASI 90, and PASI 100 are shown in ). As seen, the greatest improvements in all three indices were seen within 16 weeks of treatment and were maintained throughout the 136-week observation period. In particular, 90%, 79%, and 64% of patients achieved PASI 75, PASI 90, and PASI 100 at 16 weeks, respectively, compared with 79%, 72%, and 55% of patients after 136 weeks of therapy with secukinumab. Three patients discontinued treatment for inefficacy after 16 weeks of treatment as they did not reach PASI 75 (primary inefficacy); 17 patients (11.3%) discontinued therapy due to loss of PASI 75 (secondary inefficacy). Changes in absolute PASI are shown in ), which shows the rapid and sustained effects of secukinumab.
Figure 1. PASI improvement after initiation of secukinumab therapy, which is maintained throughout the 136-week observation period.
LOCF: last observation carried forward imputation; PASI: Psoriasis Area and Severity Index; w: week; y: year. (a) Changes in PASI 75, PASI 90, and PASI 100 during 136 weeks of treatment. Data are presented as percentages.(b) Changes in absolute PASI scores over 136 weeks of treatment. Average PASI values are reported for each time point. The sidebox reports the percentage of patients who reached that particular time point at the moment of the analysis.
Univariate logistic regression analysis showed that at 1-year treatment naïve for biological therapies, comorbidities, age and BMI were associated with PASI 75, PASI 90, and PASI 100 responses (–). HLA-C*06:02 remained significantly associated with PASI 75, PASI 90, and PASI 100 responses for up to 1 year. However, after 2 years of treatment, only treatment naïve for biological therapies remained significant for PASI 75, PASI 90, and PASI 100. In multivariate logistic regression analysis, treatment naïve to biological therapy was the best predictor of outcomes at both 1 and 2 years, and was the only variable that remained significant for PASI 75, PASI 90, and PASI 100 (). Comorbidities, age, and BMI were not significant predictors of PASI response in multivariate analysis.
Table 2. Univariate logistic regression analysis of variables associated with PASI75 response.
Table 3. Univariate logistic regression analysis of variables associated with PASI90 response.
Table 4. Univariate logistic regression analysis of variables associated with PASI100 response.
Table 5. Multivariate logistic regression analysis (stepwise analysis) of predictors of PASI response after treatment with secukinumab at 1 and 2 years.
3.2. Safety and tolerability
In all, 10% (15/151) of patients experienced an adverse event, the most common of which was candida infection (4%). Six patients had mucocutaneous Candida infection, three were of whom treated effectively with antifungal therapy. However, antifungal therapy was ineffective in the remaining three patients, and secukinumab was discontinued for this reason. The events resolved after about 1 month following discontinuation of treatment. Twelve patients discontinued secukinumab for adverse events. These included four patients due to the appearance of eczematous lesions (histological diagnosis of spongiotic dermatitis), three patients due to candida infection resistant to antifungal therapy, two patients for recurrent upper airway infections, and one patient each for increased transaminases (>3 times ULN), mild neutropenia and thrombocytopenia at the end of drug induction (which did not resolve within 12 weeks of therapy), and loss of appetite and sudden weight loss at the end of drug induction. All adverse events were resolved with treatment interruption.
3.3. Patients with HBV and HCV infection
In our experience, secukinumab is safe in patients with a history of HBV infection and active HBV infection. In three patients with previous HBV infection (HBcAb +, HBsAg-, HBV-negative DNA), there was no reactivation of the virus (monitored by liver enzymes and HBV-DNA). In two patients with active HBV infection (HBsAg +, HBcAb +, HBV DNA +), antiretroviral therapy was performed before starting treatment with secukinumab. Complete clearance of cutaneous symptoms was obtained in 16 weeks of secukinumab therapy and the viral load was no longer detectable during treatment. One patient had chronic HBV and HCV co-infection; in this case, likewise, there were no safety or tolerability issues with secukinumab.
3.4. Mycobacterium tuberculosis
Six patients were positive for the Quantiferon test at baseline. In four of these patients who were positive at screening (with latent tuberculosis), secukinumab therapy was undertaken simultaneously with antituberculous prophylaxis. In one patient with a previous history of TB, based on the recommendations of the infectivologist consultation, secukinumab therapy was undertaken as per the Summary of Product Characteristics. In the remaining patient, antituberculous prophylaxis was not undertaken. No evidence of disease reactivation was observed during anti-tuberculosis prophylaxis, and there were no safety concerns.
3.5. Down’s syndrome
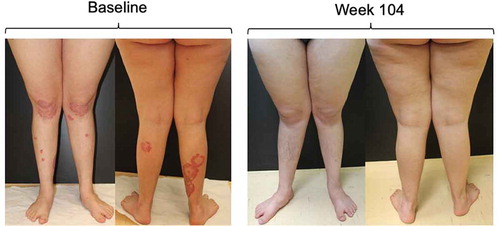
One patient, a 26-year-old woman, was affected by Down syndrome and chronic plaque psoriasis, who had been previously treated with topical agents and methotrexate, but with little benefit. Due to significant cardiovascular comorbidities (surgically corrected tetralogy of Fallot, patent foramen ovale with mild valvular insufficiency, heart failure class II NYHA), in September 2014 therapy with ustekinumab, 45 mg was initiated with good disease control initially. However, the persistence of plaques in the lower limbs generated discomfort in the patient in 2017; the patient refused the application of topical therapy by her mother and was highly irritable and restless. For this reason, therapy with secukinumab was started in November 2017. The plaques had completely cleared within 8 weeks, and at week 104 secukinumab still had excellent efficacy in maintaining plaque clearance (). No safety concerns emerged during treatment with secukinumab in this patient.
3.6. Difficult-to-treat localizations
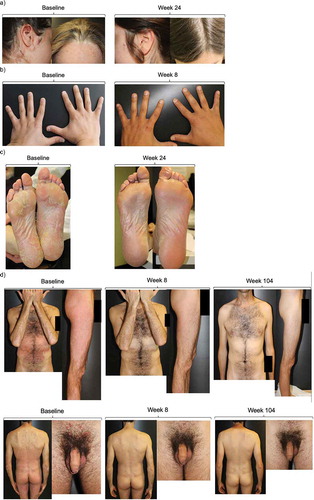
Outcomes of several patients with plaque psoriasis in difficult-to-treat locations are documented below. Images showing plaques on the scalp and head in a female patient are shown in ). Administration of secukinumab was associated with near clearance of plaques within 8 weeks. The patient remains clear of plaques after 1 year of treatment, at the time of writing. Nail psoriasis was also resolved in another patient within 8 weeks of secukinumab therapy, as well-plantar plaques in a patient after 24 weeks (,)). In a male patient with psoriasis in the torso, legs, suprapubic area and penis foreskin with little response to acitretin and cyclosporine, secukinumab treatment led to complete clearance of plaques within 8 weeks ()). The patient remains clear of plaques 2 years later.
Figure 3. Plaque psoriasis in difficult-to-treat locations before and after initiation of secukinumab therapy.
(a) Patient with head and scalp plaques prior to initiating secukinumab and after 24 weeks of therapy. (b) Patient with nail psoriasis prior to initiating secukinumab and after 8 weeks of therapy. (c) Patient with plantar plaques prior to initiating secukinumab and after 24 weeks of therapy. (d) Patient with torso, leg, and, genital localizations at baseline and after 8 and 104 weeks of treatment.
4. Discussion
In this real-life prospective study in 151 patients, outcomes at 136 weeks showed that secukinumab has a long-lasting duration, similar to those at shorter treatment times. Secukinumab does not appear to lose its effectiveness over time, and PASI indices remained high and stable over 136 weeks of therapy. At short treatment time, biological treatment naïve, normal body weight and no/few comorbidities were found to be the best predictors of PASI response. However, at multivariate analysis, only biological treatment naïve remained significantly associated with the highest level of response to therapy.
These results are in good agreement with previous real-life studies with secukinumab. In the previous study by Galluzzo et al. in 107 patients with plaque psoriasis with up to 52 weeks of observation, at study end, 92.1%, 81.6%, and 78.9% of patients achieved PASI 75, PASI 90, and PASI 100, respectively [Citation19]. PASI 90 was achieved by 42.0% of patients who were naïve to biologics and by 17.0% of patients with prior exposure to biologics (OR 0.24; p = 0.001); PASI100 was reached by 25.5% of naïve patients and 9.8% with prior exposure to biologics (OR 0.28; p = 0.015). To better understand the response in patients with prior systemic treatment, the GAIN study (NCT02474069) is currently assessing if, in PASI ≥ 75 to PASI < 90 responders at week 16, an every 2 -week or every 4-week dose is more favorable in achieving PASI 90.
In the real-world retrospective study by Chiricozzi et al. in 330 patients, patients who were naïve to biologics also showed a greater likelihood of achieve PASI score of ≤1, ≤2, ≤3, and ≤5 at week 12, compared to bio-experienced patients, and the greater efficacy in bio-naïve patients was confirmed at weeks 24 and 52 [Citation18]. The study by Notario et al. reported that after 52 weeks of treatment with secukinumab, 69% and 46% of 136 patients achieved PASI 75 and PASI 90, respectively [Citation20]. These percentages are somewhat lower than observed herein and other studies, although it was also noted that effectiveness in clinical practice may be lower in patients with high BMI and who had been previously treated with other biologic agents [Citation20]. Rompoti recently reported outcomes of 83 patients followed for 104 weeks in real-world practice, wherein drug survival was 74.5% at study end [Citation21]. Similarly, another real-world analysis reported that the drug survival of patients with psoriasis and treated with secukinumab was 83% at 12 months and 78.8% at 18 months [Citation22]. To our knowledge, 136 weeks of follow-up in a real-world analysis are the longest reported to date.
All these studies, including the present, help to define the clinical profile of best responders to secukinumab. While it appears clear that treatment-naïve patients have a better long-term response, several predictors of response during shorter treatment times have been found. A real-world study from Taiwan found that PASI response rates were lower in patients with prior failure of a biologic agent, although with overall response rates that were broadly in line with the present and aforementioned studies [Citation23]. Obesity has been reported to be associated with a better response at shorter treatment times but was not significantly associated with maintenance of PASI response at 1 or 2 years. As mentioned, the real-life study in Spain also reported that BMI ≥30 was related to poorer response, but the study was limited to 52 weeks of observation [Citation20]. Results from the open-label OPTIMIZE study reported that patients ≥90 kg had greater PASI 90 responses with a q2w (57%) compared to a q4w regimen (40%), even if the difference was not statistically significant [Citation24]. The CAIN457A2324 study (NCT03504852) is specifically addressing the effects of secukinumab high dose vs. standard dose in heavy bodyweight subjects (≥90 kg) with moderate to severe plaque psoriasis.
The 24-week, phase IIIb SUPREME study reported that secukinumab has good efficacy independently of HLA-C*06:02 status in patients with moderate-to-severe plaque-type psoriasis [Citation25], a finding that was confirmed in the 72-week extension phase of the trial [Citation26]. This is in contrast to the results of our real-life study, which found that HLA-C*06:02 was a statistically significant marker of rapid achievement of PASI 75, PASI 90, and PASI 100 at most time points for up to 1 year and also for PASI 90 at 72 weeks based on univariate logistic regression analysis. HLA-C*06:02 has been related to more severe and early-onset psoriasis [Citation27], and has also been suggested to be a predictive biomarker of treatment response to biologics in patients with psoriasis in a large observational study studying responses to adalimumab and ustekinumab [Citation28]. That study concluded that HLA-C*06:02 status might be of benefit in selecting the most appropriate treatment for severe psoriasis. Our results provide further support for the use of HLA-C*06:02 as a predictive marker of rapidly achieved and maintained PASI responses. However, it is clear that future study is needed to fully understand the utility of HLA-C*06:02 in predicting response to biological agents, and secukinumab in particular.
The presence of comorbidities is another factor that we found was associated with poorer outcomes in the short term, but which was not confirmed in the long term. In a pooled analysis of Phase 3 studies, it was reported that the efficacy in elderly subjects (who had higher baseline frequencies of cardiovascular and metabolic disorders) was comparable to that in younger subjects throughout 52 weeks of treatment [Citation29]. In Galluzzo real-life study, there was a negative association between the number of comorbidities and achievement of PASI100 at various time points (OR 0.42, 0.45, 0.34, and 0.34; p = 0.031, 0.011, 0.003, and 0.027 at weeks 4, 12, 24, and 36, respectively) [Citation19]. However, in that analysis, comorbidities were not significantly associated with skin clearance at multivariate analysis, as was seen herein. Moreover, the real-life, ongoing PROSPECT study in almost 2000 subjects did not report any association with response and comorbidities at 24 weeks of analysis [Citation30]. In other real-life studies, the possibility that comorbidities are related to better response has not been documented but may be worthy of further study. Based on its favorable safety and efficacy profile, secukinumab is now emerging as the preferred agent to manage patients with psoriasis and comorbidities such as psoriatic arthritis, obesity, and lupus, as well as latent tuberculosis [Citation31,Citation32].
We considered it worthwhile documenting the good responses seen in patients with plaques in difficult-to-treat locations, such as the head and scalp, palmo-plantar regions, and genital area. Similar results were underlined in the real-life study by Rompoti et al. in which specific locations such as scalp and palmoplantar psoriasis were effectively treated by secukinumab [Citation21]. In their study, 94% of patients had scalp plaques, and it was noted that secukinumab led to rapid clearance within week 4, with results that were sustained in the majority of patients up to week 104. Those results, and the cases described herein, are similar to results in randomized trials with secukinumab, which show rapid and sustained clinical improvement [Citation10,Citation11,Citation14].
Our results also confirm the overall favorable safety profile of secukinumab at treatment times that exceed 2 years. Other real-world studies have also reported that there are no unexpected safety issues in real-world settings, and that the safety and tolerability profile matches that seen in clinical trials [Citation18–Citation21,Citation30]. Real-world data is thus reassuring in terms of the long-term safety of secukinumab. In this light, the 52-week, randomized, double-blind, placebo-controlled CARIMA study in 151 patients with moderate to severe plaque psoriasis and without clinical CV disease examined the impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters. Interestingly, at week 12, mean flow-mediated dilatation was numerically higher, but not statistically significant, in patients receiving secukinumab versus those receiving placebo [Citation33]. At week 52, however, flow-mediated dilatation was significantly higher than baseline in patients receiving 300 mg secukinumab for 52 weeks, while there were no effects on other cardiovascular markers. This suggests that secukinumab might even have a beneficial effect on cardiovascular risk through the improvement of endothelial function in patients with plaque psoriasis.
The percentages of adverse events observed herein of 10% are similar to that reported in other similar studies with shorter follow-up. The investigation by Rompoti reported adverse events in 7.2% of cases within the first 16 weeks of treatment. After that time, two patients discontinued secukinumab for adverse events, but neither of which was drug-related (cancer and heart attack) [Citation21]. In the large PROSPECT study, discontinuation of prior treatment due to adverse events was high in subjects who had previously undergone conventional systemic treatment (22.5%) but was low in those receiving prior biologic treatment (2.4%). Notario et al. reported that 26.5% of patients discontinued treatment by week 52 due to lack or loss of response (n = 29), adverse events (n = 2), or other causes (n = 5). Herein, three patients discontinued treatment for inefficacy after 16 weeks of treatment due to lack of achievement of PASI 75 and 17 patients (11.3%) discontinued therapy due to loss of PASI 75 (secondary inefficacy). Overall, the safety profile seen in real-world studies is consistent with that seen in controlled clinical trials.
Of interest, we also documented the safety of secukinumab in patients with a history of HBV infection and active HBV infection. In those with active HBV infection, according to the Summary of Product Characteristics, we first administered antiretroviral therapy prior to starting treatment with secukinumab. Six of our patients were positive for latent tuberculosis at baseline, and in four of these patients secukinumab was administered together with antituberculous prophylaxis: there was no evidence of disease reactivation during the period of prophylaxis. Thus, there were no safety or tolerability issues with secukinumab in any of the cases of HBV or TB infection. The favorable outcomes in patients with HBV infection have been previously reported [Citation19].
Our results extend previous studies by confirming the effectiveness of secukinumab in the long term. One open question is whether early intensive systemic treatment might treatment-free remission by modifying the long-term natural course of the disease. This possibility is being investigated in the STEPIn trial [Citation34]. The study is comparing secukinumab 300 mg with nb-UVB, and patients will be monitored for disease activity up to week 208. Immunological changes and pathogenic memory T cells in skin biopsies will also be assessed to better understand the potential for disease modification by biological agents.
Limited information is available at present on the cost-effectiveness and impact of secukinumab on patient-reported outcomes, although it has been reported that secukinumab is associated with improvements in the quality of life [Citation21]. Registry studies are underway to address this question and will help better define its value in real-world settings [Citation30,Citation35].
This study has some limitations, such as the somewhat small cohort, especially in the long term after 2 years of treatment. However, our results broadly confirm the efficacy and safety of secukinumab in real-world clinical practice. Results demonstrating better outcomes in biologic treatment-naïve patients suggest that earlier systemic treatment with a biologic agent may be warranted. In this regard, the concept of lesional memory, or immunologic scar, is rapidly taking shape. Clark et al. found that several inflammatory cytokines such as IL-17, IL-22, and IFN-γ were upregulated in biopsies of clinically healed lesions [Citation36]. Later, Park et al. discussed the emerging role of tissue-resident memory T-cells, which undoubtedly play a pivotal integral role in the chronic relapsing course of psoriasis [Citation37]. It would appear that a systemic continuum, coined ‘the psoriatic march,’ begins early and continues indeterminately. Adaptive immunity, which is characteristic of stable disease and innate immunity, and dominant in active and reactive disease, initiates systemic inflammation and increases the risk the well-known metabolic, cardiovascular, arthritic, renal, and psychiatric comorbidities associated with psoriasis. Indeed, it has been hypothesized that treatment with systemic agents including biologics is undertaken only when topical agents fail to control the disease, even in those with moderate to severe disease. However, there is evidence that in other immune-mediated inflammatory diseases such as rheumatoid arthritis and Crohn’s disease targeted systemic treatment may improve long-term outcomes when administered early in the disease process [Citation38]. Therefore, early intervention in the treatment pathway, with the aim of complete clearance of lesions, may help to improve control of symptoms and could possibly modify the course of disease and minimize the burden for patients, and reducing the risk of comorbidities such as obesity, diabetes, and nonalcoholic fatty liver disease. To better understand this, dedicated early-intervention studies with long-term follow-up are needed.
5. Conclusions
Our results confirm the efficacy and safety of secukinumab in real-world practice. It is important to underline that the drug leads to rapid clinical improvement: 60.6% of patients achieved PASI 90 within week 8, and 40% had completely clear skin. Moreover, no safety concerns were present in a patient with Down's syndrome and severe cardiovascular comorbidities, or in those with HCV/HBV or latent TB. It was also highly effective in several difficult-to-treat localizations of plaque psoriasis. Our results on biologic treatment-naïve patients further suggest that earlier systemic treatment with a biologic agent may be warranted, even if this must be better studied in dedicated trials.
Author Contributions
M Galluzzo and M Talamonti conceived the study. All authors collected the data, analyzed the data, prepared the manuscript and agree to be accountable for all aspects of the work.
Declaration of interest
L Bianchi has served as a speaker and as a consultant for Abbvie, Novartis, Janssen-Cilag, Pfizer, UCB, and Leo-Pharma outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One of the reviewers of this paper has received research funds from Abbvie, Boehringer Ingelheim, Celgene, Eli Lilly, Incyte, Janssen/Johnson & Johnson, Leo Pharmaceuticals, Medimmune/Astra Zeneca, Novartis, Pfizer, Sciderm, Valeant, and ViDac. This reviewer also acts as a consultant for Allergan, Aqua, Boehringer-Ingelheim, LEO Pharma, Menlo, Mitsubishi, Promius and Theravance. Two additional peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Acknowledgments
Editorial assistance for the manuscript was provided by Dr. Patrick Moore, on behalf of Health Publishing & Services srl. This unconditional support was funded by Novartis Farma, Italy.
References
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016 Dec;9(9):504–513.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008 May;58(5):826–850.
- Schaefer CP, Cappelleri JC, Cheng R, et al. Health care resource use, productivity, and costs among patients with moderate to severe plaque psoriasis in the United States. J Am Acad Dermatol. 2015 Oct;73(4):585–593 e3.
- Albaghdadi A. Current and under development treatment modalities of psoriasis: a review. Endocr Metab Immune Disord Drug Targets. 2017;17(3):189–199.
- Ly K, Smith MP, Thibodeaux Q, et al. Anti IL-17 in psoriasis. Expert Rev Clin Immunol. 2019 Nov;15(11):1185–1194.
- Polistena B, Calzavara-Pinton P, Altomare G, et al. The impact of biologic therapy in chronic plaque psoriasis from a societal perspective: an analysis based on Italian actual clinical practice. J Eur Acad Dermatol Venereol. 2015 Dec;29(12):2411–2416.
- Patel DD, Lee DM, Kolbinger F, et al. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013 Apr;72(Suppl 2):ii116–23.
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012 Oct;11(10):763–776.
- Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015 Feb;75(3):329–338.
- Frieder J, Kivelevitch D, Menter A. Secukinumab: a review of the anti-IL-17A biologic for the treatment of psoriasis. Ther Adv Chronic Dis. 2018 Jan;9(1):5–21.
- Mrowietz U, Leonardi CL, Girolomoni G, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015 Jul;73(1):27–36 e1.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017 Oct;177(4):1033–1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018 Sep;32(9):1507–1514.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014 Jul 24;371(4):326–338.
- Georgakopoulos JR, Ighani A, Zhou LL, et al. Efficacy and safety of secukinumab in treating moderate to severe plaque psoriasis in two real-world Canadian dermatology clinics: a multicenter retrospective study. J Eur Acad Dermatol Venereol. 2018 Jan;32(1):e32–e34.
- Schwensen JF, Clemmensen A, Sand C, et al. Effectiveness and safety of secukinumab in 69 patients with moderate to severe plaque psoriasis: a retrospective multicenter study. Dermatol Ther. 2017 Nov;30(6):e12550.
- Korber A, Thaci D, von Kiedrowski R, et al. Secukinumab treatment of moderate to severe plaque psoriasis in routine clinical care: real-life data of prior and concomitant use of psoriasis treatments from the PROSPECT study. J Eur Acad Dermatol Venereol. 2018 Mar;32(3):411–419.
- Chiricozzi A, Balato A, Conrad C, et al. Secukinumab demonstrates improvements in absolute and relative psoriasis area severity indices in moderate-to-severe plaque psoriasis: results from a European, multicentric, retrospective, real-world study. J Dermatolog Treat. 2019;2:1–8.
- Galluzzo M, Talamonti M, De Simone C, et al. Secukinumab in moderate-to-severe plaque psoriasis: a multi-center, retrospective, real-life study up to 52 weeks observation. Expert Opin Biol Ther. 2018 Jul;18(7):727–735.
- Notario J, Deza G, Vilarrasa E, et al. Treatment of patients with plaque psoriasis with secukinumab in a real-life setting: a 52-week, multicenter, retrospective study in Spain. J Dermatolog Treat. 2019 Aug;30(5):424–429.
- Rompoti N, Katsimbri P, Kokkalis G, et al. Real world data from the use of secukinumab in the treatment of moderate-to-severe psoriasis, including scalp and palmoplantar psoriasis: a 104-week clinical study. Dermatol Ther. 2019 Sep;32(5):e13006.
- Torres T, Balato A, Conrad C, et al. Secukinumab drug survival in patients with psoriasis: a multicenter, real-world, retrospective study. J Am Acad Dermatol. 2019 Jul;81(1):273–275.
- Ger TY, Huang YH, Hui RC, et al. Effectiveness and safety of secukinumab for psoriasis in real-world practice: analysis of subgroups stratified by prior biologic failure or reimbursement. Ther Adv Chronic Dis. 2019;10:2040622319843756.
- Reich K, Puig L, Szepietowski JC, et al. Secukinumab dosing optimization in patients with moderate-to-severe plaque psoriasis: results from the randomized, open-label OPTIMISE study. Br J Dermatol. 2019 May 17. doi: 10.1111/bjd.18143. [Epub ahead of print].
- Costanzo A, Bianchi L, Flori ML, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: SUPREME study. Br J Dermatol. 2018 Nov;179(5):1072–1080.
- Papini M, Cusano F, Romanelli M, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: results from extension phase of the SUPREME study. Br J Dermatol. 2019 Aug;181(2):413–414.
- Gudjonsson JE, Karason A, Antonsdottir AA, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with Psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002 Feb;118(2):362–365.
- Dand N, Duckworth M, Baudry D, et al. HLA-C*06:02 genotype is a predictive biomarker of biologic treatment response in psoriasis. J Allergy Clin Immunol. 2019 Jun;143(6):2120–2130.
- Korber A, Papavassilis C, Bhosekar V, et al. Efficacy and safety of secukinumab in elderly subjects with moderate to severe plaque psoriasis: a pooled analysis of phase III studies. Drugs Aging. 2018 Feb;35(2):135–144.
- Thaci D, Korber A, von Kiedrowski R, et al. Secukinumab is effective in treatment of moderate-to-severe plaque psoriasis: real-life effectiveness and safety from the PROSPECT study. J Eur Acad Dermatol Venereol. 2019 Sep 21. doi: 10.1111/jdv.15962. [Epub ahead of print].
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019 Jan;80(1):27–40.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019 Jan;80(1):43–53.
- von Stebut E, Reich K, Thaci D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019 May;139(5):1054–1062.
- Iversen L, Eidsmo L, Austad J, et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification - rationale and design of the randomized, multicenter STEPIn study. J Eur Acad Dermatol Venereol. 2018 Nov;32(11):1930–1939.
- Papp KA, Gooderham M, Beecker J, et al. Rationale, objectives and design of PURE, a prospective registry of patients with moderate to severe chronic plaque psoriasis in Canada and Latin America. BMC Dermatol. 2019 Jun 21;19(1):9.
- Clark RA. Gone but not forgotten: lesional memory in psoriatic skin. J Invest Dermatol. 2011 Feb;131(2):283–285.
- Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015 Jul;21(7):688–697.
- Girolomoni G, Griffiths CE, Krueger J, et al. Early intervention in psoriasis and immune-mediated inflammatory diseases: a hypothesis paper. J Dermatolog Treat. 2015 Apr;26(2):103–112.