Inhibitory checkpoint proteins such as programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1) or cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) suppress anti-tumor T-cell responses. Enhancement of such checkpoint proteins is a common immune-evasive strategy in several solid tumors, including urothelial carcinoma (UC).
Immune checkpoint blockade compounds interfere with the tumor-related immune-evasive strategies. Several immunotherapeutic drugs targeting PD-1 and PD-L1 are emerging for the treatment of UC. Some of the drugs have been approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of advanced or metastatic UC [Citation1–Citation3].
A review paper very recently published in Expert Opinion On Biological Therapy gives a comprehensive update on immunotherapy options for UC [Citation4]. The update, as reported in the original paper, can be summarized as follows:
‘Immune-checkpoint inhibitors as a monotherapy are effective for first-line treatment of patients with advanced UC who are ineligible for cis-platinum and also patients who were pre-treated with platinum-based chemotherapy.’
‘The results of trials investigating the efficacy of the combination of immune-checkpoint inhibitors and chemotherapy in the first-line setting are awaited.’
‘It remains unclear whether this combination may become standard first-line treatment in advanced UC.’
Although intravesical Bacillus Calmette–Guérin (BCG) immunotherapy remains the gold standard for non-surgical management of non-muscle-invasive bladder cancer, it is reported to fail in 40% of patients [Citation5]. Encouraging results have been reported by Balar et al. on the evaluation of the antitumor activity of pembrolizumab in patients with high-risk non-muscle invasive and BCG-unresponsive bladder cancer [Citation6].
1. Pd-L1 testing by immunohistochemistry
PD-L1 testing was not originally required in UC. FDA and EMA have recently restricted the use of the anti-PD1/PD-L1 drugs Keytruda (Pembrolizumab) and Tecentriq (Atezolizumab) [Citation7]. Both drugs are only indicated as monotherapy in adult patients with locally advanced or metastatic UC, not eligible for cis-platinum-containing chemotherapy and whose tumor is PD-L1 positive by immunohistochemistry (IHC).
PD-L1 IHC testing is now required in the selection of patients with UC. There are three commercially available PD-L1 assays for this purpose (Ventana SP263, Ventana SP142, and Dako/Agilent 22C3), each of them with a corresponding manufacturer’s specific algorithm ( and ) [Citation8].
Figure 1. PD-L1 expression in invasive high-grade urothelial carcinoma. Strong and diffuse membranous expression is seen in the epithelial cells (Right) whereas it is mostly cytoplasmic in the tumor-infiltrating immune cells (left) (PD-L1 IHC 22C3 pharmDx by Dako/Agilent).
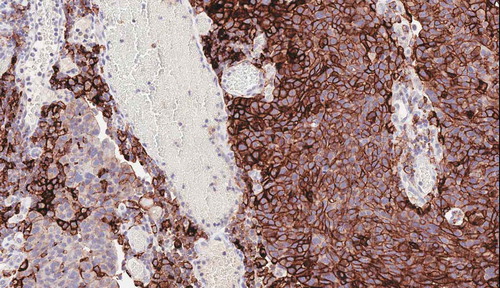
Table 1. Diagnostic PD-L1 expression assay evaluation for bladder cancer.
1.1. Harmonization of PD-L1 assessment
Pathology laboratories are interested in interchangeable tests to assess PD-L1 expression for several reasons, in particular, because Dako and Ventana platforms are not universally available and testing may have to be performed locally. Many harmonization studies on PD-L1 antibody clones have been performed in non-small-cell lung cancer, where PD-L1 testing has been clinically implemented for many years [Citation9–Citation11]. Data from such studies have shown a high level of concordance between 22C3, 28.8, and SP263 assays for tumor cell staining, with SP142 as an outlier assay.
Only few harmonization studies on UC have been reported, all basically focussing on concordance [Citation12,Citation13].
Zajac et al. [Citation14] have just published a study on ‘Concordance among four commercially available, validated programmed cell death ligand-1 assays in urothelial carcinoma.’ They found that good analytical correlation was observed among the VENTANA SP263, PD-L1 IHC 22C3 pharmDx, and PD-L1 IHC 28–8 pharmDx assays for tumor cell (TC) and immune cell (IC) PD-L1 staining. However, concordance between patient PD-L1 status was only achieved for PD-L1 IHC 22C3 pharmDx vs. VENTANA SP263. Differences were observed between patient populations with UC tumors classified as PD-L1 high vs. PD-L1 low/negative using a combined positive score (CPS) ≥1, CPS ≥10, IC ≥5%, and TC/IC ≥25%. The authors concluded that The VENTANA SP263 and PD-L1 IHC 22C3 pharmDx assays are analytically similar in UC. When the different PD-L1 assays were combined with their specified clinical scoring algorithms, differences were seen in patient classification driven by substantial differences in scoring approaches. Hodgson et al. [Citation15] investigated the concordance of 3 commercial anti-PD-L1 kits (Ventana SP263, Ventana SP142, Dako 22C3) and 1 platform-independent test (Cell Signaling Technologies E1L3N) on 197 UC cases. A high level of concordant PD-L1 among 22C3, SP142, and SP263 has been reported, with 12% of the UC cases showing inconsistent results. Rijnders et al. [Citation16] compared 4 commercial anti-PD-L1 kits (Ventana SP263, Ventana SP142, Dako 22C3, and Dako 28.8) and 1 platform-independent test (Cell Signaling Technologies E1L3N) on 139 muscle-invasive UC cases. An agreement of 80–90% between the 4 commercial anti-PD-L1 kits has been addressed. Another study also investigated the comparability of four commercial anti-PD-L1 kits and reported an outlying staining behavior of the SP142 for tumor cell-based PD-L1 scores. Furthermore, this study has highlighted clinical relevance between the currently used scoring algorithms and cutoffs [Citation17].
Major limitations in such studies are the use of tissue micro-arrays (hampering the evaluation of tumor heterogeneity), the very low numbers of evaluating pathologists (limiting the inter-observer variability), the absence of assay concordance and algorithm concordance data, the lack of prospective validation and response data [Citation18].
Nonetheless, PD-L1 expression is modulated at the genetic and epigenetic level and can vary and may potentially increase during PD-1-PD-L1 blockade in some patients. Several factors are responsible for its IHC expression: the upregulation of mRNA expression can be due to an amplification of the CD274 gene encoding PD-L1, methylation of CD274 gene leads to a suppression of its transcription. The transcription is also activated in response to different signaling pathways and transcription factors such as HIF1-α, Myc, Stats, NF- κB, and AP-1, which are controlled by other interconnected pathways (PI3K/AKT/MTOR, Ras/Raf/MEK/ERK, IFN-γ/JAKs) [Citation19].
2. UC variants
The 2016 World Health Organization classification has refined criteria of pathologic features defining several variants of UC [Citation20]. Patients whose tumor is predominantly characterized by variant histologies (i.e. >50% of a certain component within the tumor specimen) usually display an aggressive clinical course as well as a poor response to conventional chemotherapy, except for the small-cell neuroendocrine carcinoma with distinct chemotherapy sensitivity [Citation21–Citation23].
Nearly two-thirds of the variant squamous cell carcinomas of UC exhibit PD-L1 protein expression, in contrast to low expression in conventional UC (20%). Such data suggest that this variant is an attractive target for PD-1/PD-L1–based immunotherapy. In addition, its association with basal-like molecular subtypes (See below in the section ‘UC subtypes’) and the high co-occurrence rate for potentially targetable oncogenic alterations (phosphoinositide-3-kinase, catalytic, alpha polypeptide, PIK3CA, and Epidermal growth factor receptor, EGFR) raise the possibility of combinatorial and personalized therapeutic approaches for such aggressive tumors in neoadjuvant, adjuvant, and/or metastatic settings [Citation24,Citation25].
In a recent study by Necchi et al. neoadjuvant pembrolizumab was used in patients with the predominant variant histology squamous cell carcinoma or with lymphoepithelioma-like (LEL) features. Six of the seven patients (86%) with squamous cell carcinoma had downstaging to pT ≤ 1, with one pT0 and two of three LEL variants had a pT0 response. On the contrary, no pathological response was observed in any of the other UC with variant histology [Citation26,Citation27].
Squamous, sarcomatoid, and plasmacytoid variants tend to have greater intra-tumoral T cell CD3 infiltration than conventional UC. Interestingly, the sarcomatoid variant has been reported to show a significantly higher PD-L1 score as compared to the nested, glandular, and conventional UC, a finding also reported in lung and kidney cancer [Citation28–Citation30].
Furthermore, the retrospective analysis conducted by Necchi et al. reported that squamous cell carcinoma harbors the highest frequency of CD274 amplification (gene encoding for PD-L1) and a significantly higher median TMB than adenocarcinoma (p < 0.001) [Citation31].
3. UC subtypes and PD-L1 expression
3.1. UC molecular subtypes
UCs could be basically assigned to two molecular subtypes, such as luminal and basal [Citation32]. Both subtypes may have different sensitivities to current chemotherapy and/or immunotherapy. Exploratory analyses in many trials correlated retrospectively The Cancer Genome Atlas (TCGA) urothelial cancer subtype with the response to PD-1/PD-L1-based immunotherapy [Citation33].
The technology needed for comprehensive molecular analyses, such as high-throughput genome analysis, is expensive. It is not applicable for routine diagnostics. It has been suggested that the immunohistochemical expression of only two markers, luminal (Cytokeratin 20) and basal (Cytokeratin 5/6) should suffice to identify the molecular subtypes of UC in an accurate manner, thus representing a molecular subtyping potentially applicable in the daily practice in all pathology laboratories [Citation34,Citation35].
According to results presented in the IMvigor 210, a phase II trial of Atezolizumab in platinum-treated metastatic or locally advanced UC [Citation36], as well as in other retrospective studies, PD-L1 expression on tumor infiltrating immune cells appeared to be highly expressed in the basal vs. the luminal subtype (i.e. 60% vs. 23%). High PD-L1 expression in tumor cells was reported in the basal subtype (i.e. 39% in basal vs. 4% in luminal). This did not correlate with an objective response rate [Citation37,Citation38].
Similar results have been obtained in the latest taxonomy (i.e. BOLD classification) published by Tan et al. [Citation39] Of the six molecular subtypes with different overall survival and molecular patterns, high infiltration of immune cells was present in the mesenchymal-like (MES) and in the squamous cell carcinoma subtypes (SCC). Both molecular subtypes belong to the basal type. They are represented by cluster III and IV of the TGCA classification [Citation33]. Pathway analyses revealed that PD-1 and CTLA-4 expression were enriched in the MES and SCC subtypes. The immune cell infiltrates between subtypes was different. The luminal-like has greater T-cell CD8+ infiltration, while MES and SCC subtypes have higher infiltration of tumor-associated macrophage M2 [Citation39].
There are disadvantages with molecular subtyping, in particular with TCGA subtyping: This technique is difficult to standardize, patients treated with immunotherapy are often restricted to small cohorts and the status of the immune microenvironment is not assessed [Citation18].
3.2. Tumor-infiltrating immune cells and transforming growth factor β
It has been shown that a gene set associated with CD8 + T-effector cells is highly correlated with immune cell infiltrate, with complete response to atezolizumab. In the IMvigor210 study [Citation36], a higher response rate was seen in the luminal (cluster II – TGCA [Citation33], similar to HER2 (human epidermal growth factor receptor 2)-like in the BOLD classification [Citation39]) subtype, characterized by the presence of activated T effector cells. Basal clusters III/IV also showed high PD-L1 immune-cell expression as well as CD8 + effector genes, including a high PD-L1 expression on tumor cells. One of the explanations why basal clusters presented with response rates different from luminal clusters might be the presence of immunosuppressive factors. This prevents the activation of the PD-L1/PD-1 pathway by inhibiting effective T-cell activation.
One of these immunosuppressive factors linked with a lack of response is a signature of transforming growth factor β (TGF-β) signaling in fibroblasts, a cytokine connected with several pro-tumorigenic effects such as promoting immunosuppression, angiogenesis, fibroblast activation and metastasis. Mariathasan et al. [Citation40] showed that the inhibition of TGF-β receptor signaling by anti-TGF-β targeted antibodies increased the ability of Atezolizumab, an anti–PD-L1 targeted agent, to increase anti-tumor immunity by increasing the amounts of CD8 + T-cells in the tumor bed and by reprogramming peritumoral stromal fibroblasts.
4. Tumor mutational burden
An important role in the efficiency of immunotherapy is tumor-specific antigens, i.e. neoantigens. They distinguish tumor cells from normal cells and can stimulate a tumor-specific immune response. The tumors which belong the luminal cluster II-HER2-like have a high neoantigen load and tumor mutational burden (TMB), this being linked with a durable immune checkpoint inhibition response. At the same time, HER2-like demonstrates lower hypoxia signaling, elevated DNA-replication/cell-cycle signaling, high PPARG (Peroxisome proliferator-activated receptor gamma) and MRE11 (Double-strand break repair protein MRE11) expression, all linked to good outcome when bladder-preserving trimodal treatment (maximal transurethral resection, radiotherapy, and chemotherapy or immunotherapy) is applied [Citation41].
There are disadvantages with TMB. For instance, it is difficult to standardize between sequencing assays, the relationship between TMB and neoantigen burden is still poorly defined and it does not assess the status of immune microenvironment [Citation18] ().
Figure 2. Complex interplay of chemokines and cytokines classify the inflammatory state of the tumor microenvironment. Interferon-g (IFN-g) released by activated T cells and NK cells activates STAT1, IDO-1 (indolamine oxygenase I) and CMKLR1 in dendritic cells and macrophages (1). STAT-1 mediated signaling and additional pathways produce the chemokines CCL5 and CXCL9 (2). This recruits additional T cells into the tumor microenviroment through CXCR6. IFN-g stimulates the expression of HLA molecules and proteasome components including PSMB10 (3). Finally, IFN-g upregulates a number of immune checkpoint molecules including PD-L1, PD-L2, TIGIT, LAG-3, and B7-H3 on T cells (4). Reproduced under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) – Figure 4a Ref [Citation18]. J Immunother Cancer. 2017; 5:94.
![Figure 2. Complex interplay of chemokines and cytokines classify the inflammatory state of the tumor microenvironment. Interferon-g (IFN-g) released by activated T cells and NK cells activates STAT1, IDO-1 (indolamine oxygenase I) and CMKLR1 in dendritic cells and macrophages (1). STAT-1 mediated signaling and additional pathways produce the chemokines CCL5 and CXCL9 (2). This recruits additional T cells into the tumor microenviroment through CXCR6. IFN-g stimulates the expression of HLA molecules and proteasome components including PSMB10 (3). Finally, IFN-g upregulates a number of immune checkpoint molecules including PD-L1, PD-L2, TIGIT, LAG-3, and B7-H3 on T cells (4). Reproduced under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) – Figure 4a Ref [Citation18]. J Immunother Cancer. 2017; 5:94.](/cms/asset/97656089-481c-42a5-acef-31dc8c8ad15b/iebt_a_1733965_f0002_oc.jpg)
5. Immune gene expression profiling
A difficulty with PD-L1 status as a predictive biomarker is represented by the fact that subjective scoring in IHC tissue sections gives information regarding only a single factor in the microenvironment of the tumor. An advantage of immune gene expression profiling is that RNA can be evaluated and quantified from multiple cell types within a single specimen, and therefore be more representative for the entire tumor microenvironment. In addition to that, immune expression profiling has the potential to precisely quantify the inflammatory status of a certain tumor by evaluating cytokines, chemokines, as well as cell-surface proteins. They can better approximate a ‘hot tumor’ than PD-L1 expression alone [Citation42,Citation43]. There are a few investigations who have dealt with success with immune gene expression profiling by using tissues from different tumor types [Citation44].
A subset of 18 specific genes, selected from 680 different genes analyzed with a Nanostring technique, is evaluated prospectively in 3 Phase III trials with pembrolizumab (NCT02628067 [Citation45], NCT02559687 [Citation46], and NCT02564263 [Citation47]). The utility of Nanostring-based gene expression signatures to predict response to immunotherapy depends on the results of such studies. If successful, the information should provide treatment decisions in UC.
There are some disadvantages with immune gene expression profiling. In particular, the lack in standardization of the commercially available gene panels [Citation18].
6. Future directions
Although PD-L1 IHC testing is required in selected UC patient populations, it is becoming clear that the predictive value of PD-L1 alone for immune checkpoint blockade selection in advanced UC might not be enough. In the last decades, translational research using human samples and mouse models has investigated multiple mechanisms that are involved in the antitumor immune response. In bladder cancer, histotype variants and molecular subtypes are associated with different sensitivities to immunotherapy/chemotherapy. On the other side, the host-specific parameters, as a type of immune infiltrate, activation and infiltration levels, immune gene expression profiling, are important factors that need to be assessed. The future prospective is to incorporate all these factors in a predictive model of response based on currently available clinical and translational data, that can be applied in the clinical practice [Citation48].
7. Conclusions
Numerous clinical and molecular biomarkers that might predict immune response are under examination, including PD-L1 expression in tumor cells and immune cells, tumor mutational burden, molecular subtypes defined by gene expression profiling signatures, and the host immune system activation. All these potential biomarkers are subjects to technical issue such as reproducibility, different score algorithms, accuracy, test reliability, dynamic changes, tumor heterogeneity, sampling variability, and lack of standardization. Though, prospective validation and clinical utilization of putative biomarkers face challenges.
As reported by Lovitch and Rodig [Citation49], ‘As immunotherapy gains in complexity and is used in combination with agents that target oncogenic, intracellular signaling pathways, diagnostic pathologists will play an increasingly important part in identifying and quantifying cellular and molecular biomarkers in tissue samples that reflect the nature and magnitude of the antitumor immune response.’
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Mehta K, Patel K, Parikh RA. Immunotherapy in genitourinary malignancies. J Hematol Oncol. 2017;10(1):95.
- Gevaert T, Montironi R, Lopez-Beltran A, et al. Genito-urinary genomics and emerging biomarkers for immunomodulatory cancer treatment. Semin Cancer Biol. 2018;52(Pt 2):216–227.
- Three drugs approved for urothelial carcinoma by FDA | cancer discovery. [cited 2018 Apr 18]. Available from: http://cancerdiscovery.aacrjournals.org/content/7/7/659
- Bilgin B, Sendur MA, Hizal M, et al. An update on immunotherapy options for urothelial cancer. Expert Opin Biol Ther. 2019;19:1265–1274.
- Zlotta AR, Fleshner NE, Jewett MA. The management of BCG failure in non-muscle-invasive bladder cancer: an update. Can Urol Assoc J. 2009;3(6 Suppl 4):S199–205.
- Balar AV, Kulkarni GS, Uchio EM, et al. Keynote 057: phase II trial of Pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC)unresponsive to bacillus calmette-guérin (BCG). J Clin Oncol. 2019;37(7_suppl): 350–350. DOI:10.1200/JCO.2019.37.7_suppl.350
- FDA limits the use of Tecentriq and Keytruda for some urothelial cancer patients. Available from: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm612484.htm
- Gevaert T, Eckstein M, Montironi R, et al. Re: Maud Rijnders, Astrid A.M. van der Veldt, Tahlita C.M. Zuiverloon, et al. PD-L1 antibody comparison in urothelial carcinoma. Eur Urol. in press. https://doi.10.1016/j.eururo.2018.11.002. Eur Urol. 2019. DOI:10.1016/j.eururo.2019.01.038
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–222.
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13(9):1302–1311.
- Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol. 2018;29(4):953–958.
- Tretiakova M, Fulton R, Kocherginsky M, et al. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: comparison of four commonly used antibodies and RNA expression. Mod Pathol. 2018;31(4):623–632.
- Zavalishina L, Tsimafeyeu I, Povilaitite P, et al. RUSSCO-RSP comparative study of immunohistochemistry diagnostic assays for PD-L1 expression in urothelial bladder cancer. Virchows Arch. 2018;473(6):719–724.
- Zajac M, Scott M, Ratcliffe M, et al. Concordance among four commercially available, validated programmed cell death ligand-1 assays in urothelial carcinoma. Diagn Pathol. 2019;14(1):99.
- Hodgson A, Slodkowska E, Jungbluth A, et al. PD-L1 immunohistochemistry assay concordance in urothelial carcinoma of the bladder and hypopharyngeal squamous cell carcinoma. Am J Surg Pathol. 2018;42(8):1059–1066.
- Rijnders M, van der Veldt AAM, Zuiverloon TCM, et al. PD-L1 antibody comparison in urothelial carcinoma. Eur Urol. 2019;75(3):538–540.
- Eckstein M, Erben P, Kriegmair MC, et al. Performance of the Food and Drug Administration/EMA-approved programmed cell death ligand-1 assays in urothelial carcinoma with emphasis on therapy stratification for first-line use of atezolizumab and pembrolizumab. Eur J Cancer. 2019;106:234–243.
- Aggen DH, Drake CG. Biomarkers for immunotherapy in bladder cancer: a moving target. J Immunother Cancer. 2017;5(1):94.
- Wang Y, Wang H, Yao H, et al. Regulation of PD-L1: emerging routes for targeting tumor immune evasion. Front Pharmacol. 2018;9:536.
- Moch H, Humphrey PA, Ulbright TM, et al. WHO classification of tumours of the urinary system and male genital organs. 4th ed. Lyon: IARC; 2016.
- Lopez-Beltran A, Cheng L, Raspollini MR, et al. Variants of bladder cancer: the pathologist’s point of view. Eur Urol Suppl. 2017;16:210–222.
- Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22(Suppl 2):S96–S118.
- Lopez-Beltran A, Henriques V, Montironi R, et al. Variants and new entities of bladder cancer. Histopathology. 2019;74:77–96.
- Udager AM, McDaniel AS, Hovelson DH, et al. Frequent PD-L1 protein expression and molecular correlates in urinary bladder squamous cell carcinoma. Eur Urol. 2018;74(4):529–531.
- Reis H, Serrette R, Posada J, et al. PD-L1 expression in urothelial carcinoma with predominant or pure variant histology: concordance among 3 commonly used and commercially available antibodies. Am J Surg Pathol. 2019;43(7):920–927.
- Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol. 2018;36(34):3353–3360.
- Necchi A, Raggi D, Gallina A, et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol. 2019. pii: S0302-2838(19)30825-5.
- Li H, Zhang Q, Shuman L, et al. Evaluation of PD-L1 and other immune markers in bladder urothelial carcinoma stratified by histologic variants and molecular subtypes. Sci Rep. 2020;10(1439). DOI:10.1038/s41598-020-58351-6
- Joseph RW, Millis SZ, Carballido EM, et al. PD-1 and PD-L1 expression in renal cell carcinoma with sarcomatoid differentiation. Cancer Immunol Res. 2015;3(12):1303–1307.
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol. 2013;8(6):803–805.
- Necchi A, Madison R, Raggi D, et al. Comprehensive assessment of immuno-oncology biomarkers in adenocarcinoma, urothelial carcinoma, and squamous-cell carcinoma of the bladder. Eur Urol. 2020. pii: S0302-2838(20)30003-8.
- Sjödahl G, Jackson CL, Bartlett JM, et al. Molecular profiling in muscle-invasive bladder cancer: more than the sum of its parts. J Pathol. 2019;247(5):563–573.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322.
- Dadhania V, Zhang M, Zhang L, et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine. 2016;12:105–117.
- Breyer J, Wirtz RM, Otto W, et al. In stage pT1 nonmuscle- invasive bladder cancer (NMIBC), high KRT20 and low KRT5 mRNA expression identify the luminal subtype and predict recurrence and survival. Virchows Arch. 2017;470:267–274.
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76.
- Hodgson A, Liu SK, Vesprini D, et al. Basal-subtype bladder tumours show a ‘hot’ immunophenotype. Histopathology. 2018;73(5):748–757.
- Pfannstiel C, Strissel PL, Chiappinelli KB, et al. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol Res. 2019;7(6):923–938.
- Tan TZ, Rouanne M, Tan KT, et al. Molecular subtypes of urothelial bladder cancer: results from a meta-cohort analysis of 2411 tumors. Eur Urol. 2019;75(3):423–432.
- Mariathasan S, Turley SJ, Nickles D, et al. TGF-β attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548.
- Miyamoto DT, Gibb E, Mouw KW, et al. Genomic profiling of muscle invasive bladder cancer to predict response to bladder-sparing trimodality therapy. Genitourinary Cancers Symposium. San Francisco: ASCO; 2018. DOI:10.1200/JCO.2018.36.6_suppl.513
- Sweis RF, Spranger S, Bao R, et al. Molecular drivers of the non-T-cell-inflamed tumor microenvironment in Urothelial bladder cancer. Cancer Immunol Res. 2016;4:563–568.
- Gajewski TF. The next hurdle in cancer immunotherapy: overcoming the non-T-cell-inflamed tumor microenvironment. Semin Oncol. 2015;42:663–671.
- Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940.
- Diaz LA, Marabelle A, Delord J-P, et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. J Clin Oncol. 2017;35:3071.
- Shah MA, Bennouna J, Shen L, et al. Pembrolizumab for previously treated metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: phase 2 KEYNOTE-180 study. J Clin Oncol. 2016;34:TPS4139–TPS.
- Doi T, Bennouna J, Shen L, et al. KEYNOTE-181: phase 3, open-label study of second-line pembrolizumab vs single-agent chemotherapy in patients with advanced/metastatic esophageal adenocarcinoma. J Clin Oncol. 2016;34:TPS4140–TPS.
- Schalper KA, Kaftan E, Herbst RS. Predictive biomarkers for PD-1 axis therapies: the hidden treasure or a call for research. Clin Cancer Res. 2016;22:2102–2104.
- Lovitch SB, Rodig SJ. The role of surgical pathology in guiding cancer immunotherapy. Annu Rev Pathol. 2016;11:313–341.
