ABSTRACT
Background
Secukinumab (SEC) is effective for ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in randomized trials, but real-life data are lacking.
Research design and methods
Real-life, prospective observational study on 169 consecutive outpatients at baseline (T0) and at 6 (T6) and 12 months (T12) after starting SEC (39 AS, 23%; 130 PsA, 77%).
Results
Significant improvement was seen at T6 and T12 for all clinical variables, including TJC, SJC, ESR, CRP, DAPSA, ASDAS-CRP, and BASDAI, as well as in patient-reported outcomes like VAS-pain. By multivariable regression analysis, in AS patients high BASDAI at T0 correlated with diagnostic delay (R2 = 0.4; p = 0.009) and peripheral joint involvement (R2 = 0.4; p = 0.04). During follow-up, reduction of BASDAI positively correlated with high ESR (R2 = 0.65; p = 0.04). ASDAS-CRP at T0 positively correlated with high ESR (R2 = 0.34; p = 0.004). Reduction of ASDAS-CRP from T0 to T6 correlated with current smoking status (R2 = 0.42; p = 0.003). In PsA patients, reduction of DAPSA score from T0 to T12 is negatively correlated with the presence of metabolic syndrome (R2 = 0.41; p = 0.0025). SEC was well tolerated; 10 patients discontinued treatment for non-severe adverse events.
Conclusions
Secukinumab is effective and safe in patients with AS and PsA in a real-life setting.
1. Introduction
Spondyloarthritis (SpA) is a group of disorders with manifestations of both inflammation and structural damage and which share a common genetic background, similar pathogenetic mechanisms, and clinical picture [Citation1]. Among these pathologies, ankylosing spondylitis (AS) is a chronic immune-mediated rheumatic disease characterized by inflammation and formation of new bone, predominantly in the axial skeleton [Citation2], while psoriatic arthritis (PsA) is defined as a chronic inflammatory arthropathy that is typically associated with psoriasis (PsO) [Citation3]. In both former conditions, joint manifestations include oligo- or polyarthritis, dactylitis, enthesitis, and axial involvement [Citation4].
Although the introduction of TNF inhibitors (TNF-i) around two decades ago has dramatically changed the management of AS and PsA, several unmet needs still remain. Some patients do not respond to these agents or may experience a secondary loss of response following an initial benefit. Moreover, patients may be affected by extraarticular involvement or comorbidities that may recommend against the use of TNF-i [Citation5]. Switching to another TNF-i is often considered as a valid option, even if side effects may lead to treatment discontinuation [Citation6]. The development of drugs targeting the interleukin (IL)-23/IL-17 axis, which is involved in the pathogenesis of SpA, has provided an additional therapeutic option [Citation7]. Briefly, IL-23 induces the differentiation of IL-17-producing T cells (Th17), which are one of the main cells responsible not only for enthesitis, synovial inflammation, and joint erosion but also for the formation of new bone [Citation8]. Proinflammatory cytokines, such as IL-17, are decisively involved in all clinical manifestations of SpA [Citation9]. Th-17 responses include dysregulation of several IL-17 cytokines, and among these, IL-17A is crucial for the regulation of both innate and adaptive immune pathways [Citation10].
Secukinumab (SEC) is the first inhibitor of IL-17 that has demonstrated good efficacy in both AS and PsA in randomized clinical trials (RCTs) [Citation11,Citation12]. Nevertheless, RCTs may suffer from selection bias as those selected to participate may present similar features or may be without relevant comorbidities and/or may have not failed multiple therapeutic strategies [Citation13]. RCTs may be potentially biased since they can enlist patients who may not be totally representative of real-life settings for patients with AS and PsA. Therefore, an objective need for real-life data on the wide spectrum of patients affected by these diseases is needed in order to evaluate the effectiveness and safety of the drug and integrate this information with all available levels of evidence.
In the present prospective observational study, the Spondyloarthritis Roman Group (STRONG) aimed to: (i) evaluate the effectiveness and the drug survival of SEC in patients affected by moderate to severe AS and PsA in a real-life clinical setting; (ii); identify predictors of clinical response and treatment discontinuation; and (iii) determine the safety of the drug in these patients.
2. Patients and methods
2.1. Study design
A prospective observational study was carried out by the STRONG on consecutive outpatients diagnosed with AS and PsA attending the Rheumatology Units of seven tertiary referral centers in the Lazio region of Italy. The local Ethics Committee of the Policlinico Tor Vergata, University of Rome Tor Vergata approved the study and the committee’s reference number is 186/16. Patients were enrolled between September 2018 and May 2019 when starting treatment with SEC. Patients received SEC according to the European League Against Rheumatism (EULAR) and/or Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) guidelines [Citation14]. Inclusion criteria were: age ≥18 years, diagnosis of AS or PsA for at least 6 months in accordance with modified New York criteria [Citation15] and Classification for Psoriatic Arthritis (CASPAR) criteria, respectively [Citation16], and an indication to initiate treatment with SEC. Patients with PsA were classified as affected by axial involvement if they presented clinical symptoms of inflammatory back pain supported by imaging findings for axial spondyloarthritis [Citation17]. Furthermore, AS patients were considered as affected by peripheral disease if they had current peripheral arthritis [Citation18]. The use of concomitant conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) was allowed if the dosage was stable over the previous 3-month period. Although discontinuation or reduction in the csDMARD dosage was consented during the study period based on the patient’s clinical conditions, an increase in the dose was not allowed. Non-steroidal anti-inflammatory drugs (NSAIDs) were permitted for a maximum of 3 days a week. Low-dose glucocorticoids (daily dose ≤10 mg of prednisone or equivalent) were permitted. Intra-articular glucocorticoid injections were not allowed. We excluded patients with the following: history of malignancy (in the last 5 years), systemic infections, infectious disorders (including active or latent tuberculosis), active uveitis, and active inflammatory bowel disease (IBD).
The study was approved by the local ethics committees of the institutions involved. Informed consent was obtained from all patients before inclusion in the study, which was conducted in accordance with the ethical principles of the Declaration of Helsinki and consistent with guidelines for good clinical practice.
2.2. Screening and evaluation
Patients underwent screening tests before enrollment including chest radiography, laboratory tests (including screening for HIV and hepatitis B and C), Quantiferon TB Gold test, and pregnancy test for women of childbearing age. Patients were evaluated at baseline (T0) and after 6 (T6) and 12 (T12) months of SEC treatment. Relevant anamnestic, clinical, and biochemical data were collected, including biologic treatment line (SEC as a first biologic and as second or later line biologic).
Patient comorbidities were evaluated in accordance with the classification of diseases outlined in the Charlson Comorbidity Index [Citation19]. The presence of comorbidities and concomitant therapies was investigated (yes/no) and collected in a dedicated database. Concomitant cardiovascular disease, metabolic syndrome (MetS), and psychiatric and neurologic conditions were evaluated. MetS was investigated in accordance with internationally recognized standards [Citation20]. Clinical variables such as PsO and smoking status were considered (yes/no) if present at patient enrollment. Information on previous biologic therapies with TNF-i, concomitant csDMARDs (methotrexate, leflunomide, sulfasalazine, and cyclosporine A) and glucocorticoids were recorded at baseline and throughout the study.
2.3. Outcome measures
Clinical indexes and patient-reported outcomes (PROs) such as visual analogue scale of pain (VASp) and global health (VASgh) were assessed. Clinical evaluation included tender joint count (TJC) (68 joints) and swollen joint count (SJC) (66 joints), dactylitis (yes/no), and enthesitis (yes/no). Enthesitis and dactylitis were assessed by physical examination for the presence (positive confirmation) or absence of pain (negative confirmation) for enthesitis and pain and swelling for dactylitis according to expanded Leeds index. The following entheses were bilaterally evaluated: lateral epicondyle, medial femoral condyle, Achilles tendon insertion, insertion of quadriceps tendon on the superior pole of the patella, proximal insertion of the patellar tendon on the inferior pole of the patella, and the insertion of the plantar fascia on the calcaneus, as previously described [Citation21]. Leeds Enthesitis Index (LEI) was also evaluated [Citation22]. Joint involvement, dactylitis, and enthesitis were also confirmed by ultrasound. As composite clinical indexes, AS disease activity score with C-reactive protein (ASDAS-CRP) [Citation23], and Bath AS Disease Activity Index (BASDAI) [Citation24] were calculated for AS patients, while the Disease Activity in Psoriatic Arthritis (DAPSA) [Citation25] score was calculated for PsA patients. Inflammatory markers such as erythrocyte sedimentation rate (ESR; normal range 0–28 mm/h) and CRP (normal range 0–3 mg/L) were evaluated. Height, weight, and body mass index (BMI) were also collected.
2.4. Administration of SEC
All AS patients were treated with SEC administered subcutaneously by self-injection at a dose of 150 mg; the same dose was used for PsA patients naïve for biologic therapies. PsA patients with previous failure of a TNF-i were treated with SEC administered subcutaneously at a dose of 300 mg with initial administrations at weeks 0, 1, 2, 3, and 4, followed by monthly maintenance dosing. Each 300 mg dose was administered as two subcutaneous injections of 150 mg in accordance with the Summary of Product Characteristics [Citation26]. Safety was evaluated by assessing adverse events through standard clinical evaluation and laboratory testing.
2.5. Statistical analysis
The differences between various parameters at T0, T6, and T12 were evaluated using the non-parametric Wilcoxon test for paired numerical variables. Parameters recorded were correlated with disease activity at baseline and with the difference between disease activity at T0 and T6 using a multivariable regression model. Backward selection was utilized to determine which variables were fit into the multivariable model. Statistical analyses were performed using GraphPad Prism (ver. 6) and R Studio (R Foundation for Statistical Computing). Two-tailed p values <0.05 were considered statistically significant. Median survival time and survival curves were obtained using Kaplan–Meier curve, along with a log-rank test between the curves. The groups were further subdivided by gender, BMI (overweight vs normal weight), smoking (yes vs no), presence of comorbidities (yes vs no) and lines of bDMARDs treatment (first-line vs second or more lines), diagnostic delay (≤12 months vs >12 months), CRP (positive vs negative), and log-rank tests performed between each pair of drug survival curves.
3. Results
3.1. Patient characteristics
Of the 175 consecutive patients who started treatment with SEC in the enrollment period, 6 were excluded for missing data. Therefore, 169 outpatients fulfilling inclusion criteria were enrolled. These included 39 patients (23%) with AS, and 130 patients (77%) with PsA. Baseline clinical and laboratory characteristics are summarized in . Peripheral arthritis was present in 138 (81.7%) cases [AS 35.9% (n = 14); PsA 95.4% (n = 124)], axial disease in 101 cases (59.7%) [AS 100% (n = 39); PsA 47.7% (n = 62)], enthesitis as a prominent manifestation in 57 (33.7%) patients [AS 28.2% (n = 11); PsA 35.4% (n = 46)] and dactylitis in 63 (37.3%) patients [AS 23% (n = 9); PsA 41.5% (n = 54)]. Erosive disease was present in 26 (15.4%) [AS 7.7% (n = 3); PsA 17.7% (n = 23)] of patients at baseline, and no cases of arthritis mutilans or prominent distal interphalangeal joint (DIP) involvement were registered in the PsA group. The most frequent comorbidities were MetS described in 33 (19.5%) patients [AS 23% (n = 9); PsA 18.5% (n = 24)] and cardiovascular disease described in 26 (15.4%) patients [AS 20.5% (n = 8); PsA 13.8% (n = 18)].
Table 1. Baseline clinical and serological characteristics of patients.
At baseline, 59 patients (34.9%) were undergoing concomitant csDMARD therapy [AS 20.5% (n = 8); PsA 39.2% (n = 51)] at a stable dosage for at least 3 months, 41 (24.3%) were taking glucocorticoids [AS 15.4% (n = 6); PsA 26.9% (n = 35)] and 59 (34.9%) were taking NSAIDs [AS 38.5% (n = 15); PsA 33.8% (n = 44)]. Thirty-five (20.7%) patients were biologic-naïve [AS 20.6% (n = 8); PsA 20.8% (n = 27)]; the remainder had previously failed up to 4 TNF-i: SEC was biologic second-line treatment for 46 (27.2%) patients [AS 33.3% (n = 13); PsA 25.4% (n = 33)], third line for 52 (30.8%) [AS 28.2% (n = 11); PsA 31.5% (n = 41)], and fourth line or greater for 36 (21.3%) [AS 17.9% (n = 7); PsA 22.3% (n = 29)].
3.2. Therapeutic effectiveness
Improvement was seen at T6 and T12 for all the variables evaluated, including TJC, SJC, ESR, CRP, DAPSA, ASDAS-CRP, and BASDAI, as well as in PROs such as VASp throughout the study (–). A difference was observed in joint count: TJC was 6.7 ± 5.7 at baseline, 4.2 ± 5.8 at T6, and 2.4 ± 2.5 at T12 (p < 0.0001 and p = 0.002 vs. T0, respectively); SJC was 2.2 ± 3.5 at baseline, 1.1 ± 2.2 at T6, and 0.36 ± 0.86 at T12 (p < 0.0001 and p = 0.0004 vs T0, respectively). CRP improved: mean CRP was 2.1 ± 6.7 at baseline, 1.6 ± 4.7 at T6 and 1.0 ± 2.0 at T12 (p = 0.04 and p = 0.038 vs T0, respectively). No significant differences were observed in ESR values from baseline to T6 or T12.
Figure 1. Evaluation of joint count and inflammatory markers during follow-up in patients with PsA and AS pooled together. T0: 169 patients, T6: 145 patients, and T12: 129 patients. The Wilcoxon test was used to compare matched values (T0, T6, and T12).
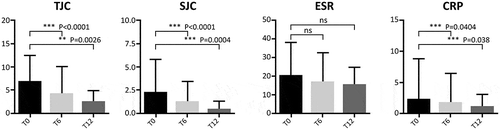
Figure 2. Evaluation of DAPSA during follow-up in patients with PsA. T0: 130 patients, T6: 109 patients, and T12: 99 patients. The Wilcoxon test was used to compare paired disease activity scores at different time points (T0, T6, and T12).
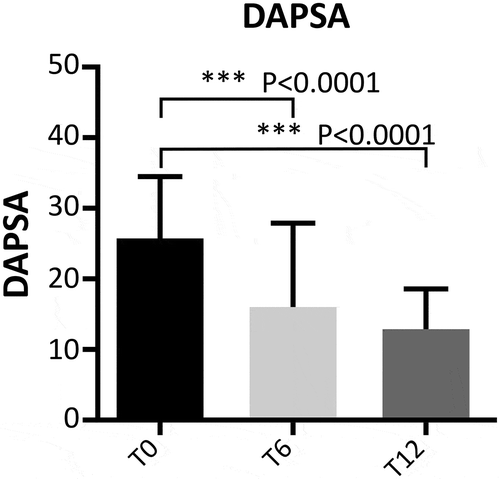
Figure 3. Evaluation of BASDAI (Panel A) and ASDAS-CRP (Panel B) during follow-up. BASDAI Bath Ankylosing Spondylitis Disease Activity Index, ASDAS-CRP Ankylosing Spondylitis Disease Activity Score with C-reactive protein. T0: 169 patients, T6: 145 patients, and T12: 129 patients. The paired scores at T0, T6, and T12 were compared using the Wilcoxon test in patient with PsA and AS, pooled together.
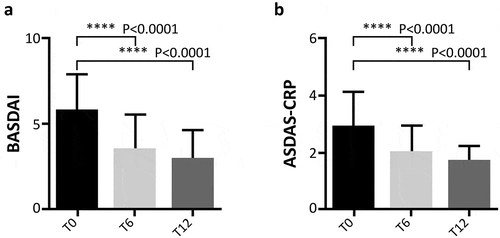
Concerning clinical values of patients affected by PsA, DAPSA was calculated at baseline and during follow-up. Differences emerged from baseline (25.5 ± 9.3) to T6 (15.2 ± 10.5) and T12 (12.7 ± 5.8) for DAPSA scores (p < 0.0001 for both comparisons) (). At T12, DAPSA low-disease activity was reached in 52 (52.5%) patients and DAPSA remission was achieved in 19 (19.2%) patients. In patients affected by AS, BASDAI and ASDAS-CRP decreased during the follow-up period: from 5.9 ± 2.0 at baseline to 3.5 ± 2 at T6 and 3 ± 1.7 at T12 (p < 0.0001 for both comparisons) for BASDAI ()) and from 2.9 ± 1.2 at baseline to 2.0 ± 0.9 at T6 and 1.7 ± 0.5 at T12 for ASDAS-CRP (p < 0.0001 for both comparisons) ()).
The number of patients on csDMARDs was higher at T0 [34.9% (n = 59); AS 20.5% (n = 8) and PsA 39.2% (n = 51)] than at T6 [29% (n = 42); AS 13.9% (n = 5) and PsA 33.9% (n = 37)] and T12 [22.2% (n = 29); AS 0% (n = 0) and PsA 29.3% (n = 29)], as was the number of patients treated with glucocorticoids, which was 41 (24.3%) at T0 [AS 15.4% (n = 6); PsA 26.9% (n = 35)], 19.6% (n = 28) at T6 [AS 13.9% (n = 5) and PsA 21.1% (n = 23)], and 15.4% (n = 20) at T12 [AS 0% (n = 0) and PsA 20.2% (n = 20)]. No patient required an increase in concomitant treatments (e.g. csDMARDs, NSAIDs, or topical or oral glucocorticoids).
Multivariable regression analysis with stepwise backward selection was used to determine which variables fit into the multivariable model. In a multivariable regression analysis, disease activity indexes and their improvement over 6 and 12 months were correlated with the following parameters: sex, age, BMI, smoking, diagnosis, SEC dose, axial and peripheral disease, cardiovascular comorbidities, MetS, biologic line of treatment, CRP, ESR, disease duration, and diagnostic delay. High DAPSA score at T0 was positively correlated with female sex and high levels of ESR at T0 (R2 0.26; p = 0.002 and p = 0.075, respectively). DAPSA score reduction from T0 to T6 was positively correlated with high basal ESR and negatively correlated with MetS (R2 0.41; p = 0.019 and p = 0.002, respectively). Logistic regression was performed for DAPSA low-disease activity and for DAPSA remission, but did not show any statistically significant predictors.
For BASDAI, high level at T0 positively correlated with diagnostic delay and the presence of joint peripheral involvement (R2 = 0.4; p = 0.009 and p = 0.04, respectively), and was negatively correlated with male sex (R2 = 0.4; p = 0.002). During follow-up, reduction in BASDAI from T0 to T6 positively correlated with diagnostic delay and ESR (R2 = 0.65; p = 0.016 and p = 0.0483, respectively). ASDAS-CRP at T0 was positively correlated with ESR level and female sex (R2 = 0.34; p = 0.004 and p = 0.06, respectively). The reduction of ASDAS-CRP score from T0 to T6 was negatively correlated with smoking habit (R2 = 0.42; p = 0.003).
3.3. Drug survival
Crude drug survival was estimated by K–M curves and the 1-year drug survival ()), was 76.4% with 23.7% (n = 40) of patients having discontinued therapy before the end of the first year ()). Most patients (65%, n = 26) discontinued the drug for reasons of lack of efficacy; the remaining patients (35%, n = 14) discontinued treatment for different reasons: 10 patients (25%) for non-serious adverse events, 1 (2.5%) patient for pregnancy, 1 patient for occurrence of IBD (2.5%), and 2 patients for dental treatments (5%).
Figure 4. Twelve months retention rate of secukinumab in AS and PsA patients according to gender. Panel A. Global retention rate (T0: 169 patients, T6: 145 patients, and T12: 129 patients); Panel B. Global retention rate according to gender (T0: 169 patients, T6: 145 patients, and T12: 129 patients); Panel C. Retention Rate according to gender in AS patients T0: 39 patients, T6: 36 patients, and T12: 30 patients.; Panel D. Retention Rate according to gender in PsA patients (T0: 130 patients, T6: 109 patients, and T12: 99 patients).
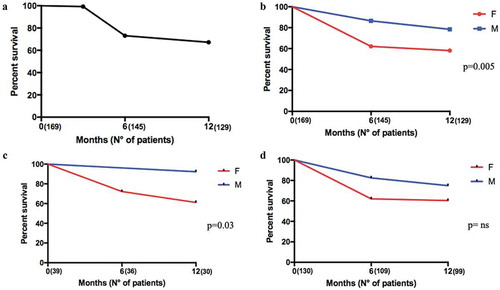
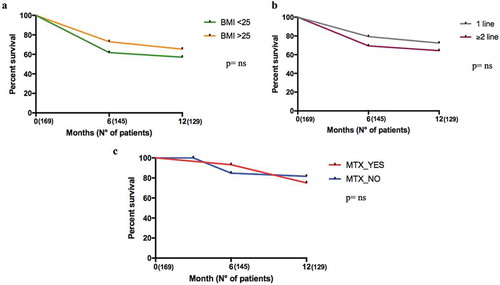
Male patients had a higher persistence rate than female (); p = 0.005). In particular, this was relevant in the AS population: males had a higher persistence rate than females; this was not demonstrated in PsA patients (); p = 0.03 and )). There were no differences in drug survival between the groups when divided according to BMI (overweight vs normal weight, lines of treatment (,)), smoking, presence of comorbidities, diagnostic delay, and CRP positivity (data not shown).
Figure 5. Twelve months retention rate of secukinumab in AS and PsA patients according to BMI and to line of biologics. Panel A. Retention Rate according to BMI (T0: 169 patients, T6: 145 patients, and T12: 129 patients); Panel B. Retention Rate according to lines of biologics (T0: 169 patients, T6: 145 patients, and T12: 129 patients); Panel C. Retention Rate according to the presence or absence of Methotrexate (T0: 169 patients, T6: 145 patients, and T12: 129 patients).
3.4. Safety
SEC was well tolerated. One PsA patient discontinued treatment for the occurrence of new-onset IBD. Only 10 patients (5.9%), all diagnosed with PsA, discontinued treatment for non-serious adverse events: 5 (2.9%) for allergy/intolerance; 1 (0.6%) for pulmonary nodule evaluation; 3 (1.8%) for infectious diseases (infections of the upper respiratory tract; none were candidiasis); 1 (0.6%) for increase in transaminases. There were no reports of severe adverse events during the treatment period. Only 6 patients (3.6%) had a previous diagnosis of cancer. Two of the four patients with primary failure also had a previous history of cancer. Causes of discontinuation and adverse events are summarized in .
Table 2. Causes of treatment discontinuation in the study population.
4. Discussion
In this prospective, observational, multicenter study in patients with moderate to severe AS or PsA, SEC was effective for both articular and axial symptoms. At T6 and T12, SEC reduced all clinical parameters analyzed, in addition to PROs such as pain-VAS and inflammatory indexes. In our population-based study, 34% of patients showed entheseal involvement. IL-17 is a key player in the pathogenesis of enthesitis, as confirmed in animal models, and IL-17 may participate in the tissue repair response to microtrauma in healthy conditions [Citation27]. SEC was effective in reducing the extent and frequency of enthesitis [Citation12,Citation28,Citation29] and could be adopted in patients with AS and PsA with prevalent entheseal involvement. The reduction of inflammatory markers, such as CRP, considered as a biomarker of radiographic progression in SpA, may improve long-term outcomes in these diseases [Citation30]. Based on DAPSA, BASDAI, and ASDAS scores, low-disease activity was mostly reached within the first 6 months of treatment and sustained during the 12-month follow-up period.
Most patients (79.3%) evaluated in our study had been previously treated with biologic agents (TNF-i) and only 20.7% of patients were bio-naïve. In this context, the efficacy of SEC was independent of the line of treatment used and previous use of bDMARDs and can therefore be considered effective as first-line therapy and in patients with failure of one or more previous bDMARDs. The clinical efficacy of SEC has been previously demonstrated in RCTs, but only partial data in a real-life setting are present [Citation11,Citation12]. Data from national registries, such as the ATTRA registry, have compared the characteristics of patients starting treatment with SEC or TNFi [Citation31]. The indications for use of TNF-i and SEC in clinical practice have been assessed, and abstracts have been presented on the efficacy and safety of SEC in PsA and axial SpA [Citation32].
The use of combination therapy with corticosteroids, MTX, or other csDMARDs and bDMARDs is still controversial in the management of SpA. Its rational use is based on indirect evidence or expert opinion [Citation33]. However, these drugs may be warranted when peripheral joint manifestations are not well controlled, even in the absence of effective or other treatment options, or as an adjunct to bDMARDs [Citation34]. We observed a progressive decrease in concomitant medications during follow-up. The low number of patients treated with csDMARDs seen herein and the tapering of low dose of glucocorticoids after 6 months of SEC treatment are of clinical relevance. A reduction in the concomitant use of csDMARDs and concomitant glucocorticoids was observed during follow-up, and a high proportion of patients were free of concomitant therapy, thus demonstrating the effectiveness of SEC as monotherapy. Furthermore, no difference in survival rates was observed in patients on SEC with or without MTX and no patient required an increase in concomitant csDMARDs, NSAIDs, or glucocorticoid therapy. This observation can allow clinicians to consider SEC even in patients who are unsuitable for csDMARDs or glucocorticoids, as monotherapy.
Response to SEC treatment was significantly lower in PsA patients with MetS as a comorbidity and in AS patients who were smokers. MetS is a cluster of classic cardiovascular (CV) risk factors identified as a strong predictor of CV disease, stroke, and type 2 diabetes. MetS was strongly associated with a lower probability of achieving MDA in PsA patients in therapy with TNFi, and different studies have reported a higher prevalence of MetS in PsA patients compared to other rheumatic diseases [Citation35]. Our cohort of patients, in line with previous findings, suggests that MetS may be a negative predictor factor for achieving MDA not only in patients undergoing TNFi therapy, but also for SEC. In randomized clinical trials, no evidence for the role of SEC in altering the metabolic profile in PsA patients has emerged and further study is needed to confirm our results [Citation36].
Smoking is associated with higher BASDAI scores in AS patients. Smoking has an adverse effect on functional ability in AS patients: longer smoking duration is associated with a higher risk of greater disease activity [Citation37]. In our patients, high disease activity in AS patients at baseline was positively correlated with diagnostic delay and peripheral arthritis, and negatively correlated with male sex. These outcomes suggest the efficacy of the drug even in long-standing disease and may help to guide the optimal use of SEC in AS patients.
The gender influence in bDMARDs clinical response still remains a challenge. In patients with PsA, female gender has been associated with a prevalent polyarticular phenotype, higher SJC, and peripheral erosive joint involvement compared to males, but with lower rates of extraarticular manifestations and axial involvement [Citation38,Citation39]. These findings are confirmed in our study, as women had higher DAPSA scores and ESR levels at T0. Furthermore, regarding treatment, female gender generally associates with poor rates of response to TNF-i and a lower probability of achieving remission compared to men [Citation40], especially when comorbidities, such as MetS, are present. In our cohort, we did not estimate gender differences in the efficacy of SEC, since sex was not a relevant factor in patient outcomes.
In contrast, high levels of ESR at baseline were associated with a substantial reduction of clinical disease activity. This suggests that patients with higher inflammatory markers at baseline may have a better response to SEC compared to those with low inflammatory indexes. Cigarette smoking has an unequivocal and strong association with onset, severity, duration, and treatment failure in several inflammatory conditions, including PsA and AS. Additionally, smoking habit has been demonstrated to correlate with disease activity in SpA [Citation41] and has also been associated with radiographic progression in AS [Citation41]. High BMI is strongly associated with a lower rate of achievement and maintenance of MDA. While MetS is a risk factor for non-response, BMI itself did not affect treatment response in our patients, and thus SEC may be also considered in overweight patients, although further studies are needed to confirm this observation.
A higher retention rate was observed in AS male patients than in AS women, but this did not emerge in PsA patients. Male sex has been identified as a predictor of higher treatment retention in both AS and PsA patients treated with TNF-i [Citation42]. Gender had no influence in PsA patients, sustaining the hypothesis that, in female patients, bDMARDs other than TNF-i may be suggested and could be more effective with a higher retention rate than TNF-i.
On the contrary, no other factors have been identified having an impact in the survival rates. Drug survival was similar considering the different lines of treatment with SEC in both PsA and AS, as was drug survival based on BMI. High BMI is associated with reduced response to TNF-i. The lack of impact of BMI on response to IL-17 inhibition may be a factor favoring the use of IL-17 blockade over TNF inhibition in overweight patients with PsA or AS [Citation43,Citation44].
A very low rate of primary failure was observed. Moreover, SEC had a good safety profile with no serious adverse events reported. The discontinuations seen in patients with PsA, and not observed in AS patients, might be related to the relatively higher number of patients with PsA enrolled compared to AS and a higher rate of comorbidities in the former group. Therefore, any correlation between the diagnosis of PsA or AS and discontinuation rates warrants further investigation with higher numbers of patients treated in real-life settings to better understand this observation.
The limitations of this study include: (1) the relatively low number of patients with AS enrolled (in particular concerning the multivariate analysis); (2) the limited follow-up period of 12 months; (3) and the absence of imaging follow-up (radiographic evaluation of efficacy), even if ultrasound, MRI and/or X-ray were performed in all patients to confirm the diagnosis at baseline.
5. Conclusions
Our findings nonetheless confirm the effectiveness of the SEC in the treatment of PsA and AS in a real-life multicenter setting. SEC was effective in reducing the severity and frequency of enthesitis, and a reduction in the concomitant use of csDMARDs and glucocorticoids was seen during follow-up. Thus, clinicians might consider the use of SEC in patients who are unsuitable for csDMARDs or glucocorticoids, at least in monotherapy. Response to SEC was lower in PsA patients with MetS and in AS patients who were smokers. The risk of IBD with IL-17 blockade is a factor that may favor TNF inhibition over IL-17 blockade.
Author contributions
Conceptualization: M.S.C., G.L.F, F.S., and R.S.; Methodology: L.N., P.T., and P.C.; Writing – Original Draft Preparation: M.S.C., P.C., and P.T.; Formal Analysis and investigation: G.P., P.S., R.C., and A.P.D.; Resources and Data Curation: E.D.M., S.S., D.R., and A.A.; Review & Editing: M.P., V.B., B.L., and E.G.; Supervision: G.V., A.A., and R.P. All authors made substantial contributions to concept and design of the study, revised the manuscript, and gave their approval to the final version of the manuscript.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
One of the reviewers on this manuscript received research, speaking and/or consulting support from a variety of companies including Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. They also consult for others through Guidepoint Global, Gerson Lehrman, and other consulting organizations, and are the founder and majority owner of www.DrScore.com. They are a founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. Two additional peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
Editorial assistance for the manuscript was provided by Dr. Patrick Moore, on behalf of Health Publishing & Services srl, and supported by an unrestricted grant from Novartis Farma Italy SpA.
Additional information
Funding
References
- Baeten D, Breban M, Lories R, et al. Are spondylarthritides related but distinct conditions or a single disease with a heterogeneous phenotype? Arthritis Rheum. 2013;65(1):12–20.
- Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379–1390.
- Chimenti MS, Ballanti E, Perricone C, et al. Immunomodulation in psoriatic arthritis: focus on cellular and molecular pathways. Autoimmun Rev. 2013;12(5):599–606.
- Feld J, Chandran V, Haroon N, et al. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol. 2018;14(6):363–371.
- Ward MM, Deodhar A, Gensler LS, et al. Update of the American college of rheumatology/spondylitis association of America/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2019;71(10):1599–1613.
- Conti F, Ceccarelli F, Marocchi E, et al. Switching tumour necrosis factor alpha antagonists in patients with ankylosing spondylitis and psoriatic arthritis: an observational study over a 5-year period. Ann Rheum Dis. 2007;66(10):1393–1397.
- Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18(7):1069–1076.
- Abe Y, Ohtsuji M, Ohtsuji N, et al. Ankylosing enthesitis associated with up-regulated IFN-gamma and IL-17 production in (BXSB x NZB) F(1) male mice: a new mouse model. Mod Rheumatol. 2009;19(3):316–322.
- Raychaudhuri SP, Raychaudhuri SK. Mechanistic rationales for targeting interleukin-17A in spondyloarthritis. Arthritis Res Ther. 2017;19(1):51.
- Costa MB, Hungria EM, Freitas AA, et al. In situ T regulatory cells and Th17 cytokines in paired samples of leprosy type 1 and type 2 reactions. PLoS One. 2018;13(6):e0196853.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76(6):1070–1077.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146.
- Monti S, Grosso V, Todoerti M, et al. Randomized controlled trials and real-world data: differences and similarities to untangle literature data. Rheumatology (Oxford). 2018;57(57Suppl 7):vii54–vii58.
- Coates LC, Gossec L, Ramiro S, et al. New GRAPPA and EULAR recommendations for the management of psoriatic arthritis. Rheumatology (Oxford). 2017;56(8):1251–1253.
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–368.
- Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673.
- Aydin SZ, Kucuksahin O, Kilic L, et al. Axial psoriatic arthritis: the impact of underdiagnosed disease on outcomes in real life. Clin Rheumatol. 2018;37(12):3443–3448.
- de Winter JJ, van Mens LJ, van der Heijde D, et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18:196.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887.
- Salvarani C, Girolomoni G, Di Lernia V, et al. Impact of training on concordance among rheumatologists and dermatologists in the assessment of patients with psoriasis and psoriatic arthritis. Semin Arthritis Rheum. 2016;46(3):305–311.
- Mease PJ, Van den Bosch F, Sieper J, et al. Performance of 3 enthesitis indices in patients with peripheral spondyloarthritis during treatment with adalimumab. J Rheumatol. 2017;44(5):599–608.
- van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(12):1811–1818.
- Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol. 1994;21(12):2286–2291.
- Schoels MM, Aletaha D, Alasti F, et al. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75(5):811–818.
- Secukinumab. Summary of product characteristics; cited 2019 Apr 25. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003729/human_med_001832.jsp&mid=WC0b01ac058001d124cited
- Schett G, Lories RJ, D’Agostino MA, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol. 2017;13(12):731–741.
- McInnes IB, Mease PJ, Ritchlin CT, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford). 2017;56(11):1993–2003.
- Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–1339.
- Maksymowych WP. Biomarkers for diagnosis of axial spondyloarthritis, disease activity, prognosis, and prediction of response to therapy. Front Immunol. 2019;10:305.
- Mann HF, Závada J, Šenolt L et al.; the ATTRA Registry. Real world use of secukinumab for treatment of axial spondyloarthritis and psoriatic arthritis: nationwide results from the ATTRA registry. Clin Exp Rheumatol. 2019;37(2):342–343.
- Martinis F, Caimmi C, Foti R, et al. Secukinumab for the treatment of psoriatic arthritis in real life: an italian experience. Ann Rheum Dis. 2019;78(2):A1848.
- Marchesoni A, Olivieri I, Salvarani C, et al. Recommendations for the use of biologics and other novel drugs in the treatment of psoriatic arthritis: 2017 update from the Italian society of rheumatology. Clin Exp Rheumatol. 2017;35(6):991–1010.
- Caso F, Costa L, Del Puente A, et al. Pharmacological treatment of spondyloarthritis: exploring the effectiveness of nonsteroidal anti-inflammatory drugs, traditional disease-modifying antirheumatic drugs and biological therapies. Ther Adv Chronic Dis. 2015;6(6):328–338.
- Caso F, Chimenti MS, Navarini L, et al. Metabolic Syndrome and psoriatic arthritis: considerations for the clinician. Expert Rev Clin Immunol. 2020;1:1–12.
- Nash P, McInnes IB, Mease PJ, et al. Secukinumab versus adalimumab for psoriatic arthritis: comparative effectiveness up to 48 weeks using a matching-adjusted indirect comparison. Rheumatol Ther. 2018;5(1):99–122.
- Chimenti MS, Triggianese P, Conigliaro P, et al. A 2-year observational study on treatment targets in psoriatic arthritis patients treated with TNF inhibitors. Clin Rheumatol. 2017;36(10):2253–2260.
- Lopez-Medina C, Molto A. Update on the epidemiology, risk factors, and disease outcomes of axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2018;32(2):241–253.
- Peluso R, Iervolino S, Vitiello M, et al. Extra-articular manifestations in psoriatic arthritis patients. Clin Rheumatol. 2015;34(4):745–753.
- Gremese E, Bernardi S, Bonazza S, et al. Body weight, gender and response to TNF-alpha blockers in axial spondyloarthritis. Rheumatology (Oxford). 2014;53(5):875–881.
- Zhao S, Challoner B, Khattak M, et al. Increasing smoking intensity is associated with increased disease activity in axial spondyloarthritis. Rheumatol Int. 2017;37(2):239–244.
- Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65(10):2645–2654.
- Flouri ID, Markatseli TE, Boki KA, et al. Comparative analysis and predictors of 10-year tumor necrosis factor inhibitors drug survival in patients with spondyloarthritis: first-year response predicts long-term drug persistence. J Rheumatol. 2018;45(6):785–794.
- Pantano I, Iacono D, Favalli EG, et al. Secukinumab efficacy in patients with PsA is not dependent on patients’ body mass index. Ann Rheum Dis. 2020 Mar 13. DOI:10.1136/annrheumdis-2020-217251
