ABSTRACT
Introduction: Perception of illness varies among individuals and psoriasis of the same severity can be perceived in different ways by patients, making it essential to evaluate quality of life (QoL) since it can provide information on the impact of the disease on the patient’s overall well-being. The use of patient-reported outcomes in clinical trials provides the ability to integrate objective clinical assessment with the patient’s perception of their own state of health.
Areas covered: The introduction of anti-IL17 agents in clinical practice has given patients the possibility to achieve a PASI90 response (almost clear skin) or even higher (complete clear skin) in the majority of patients. There is accumulating evidence in support of PASI90 response as the new standard goal for therapy based on its greater correlation with health-related QoL. The present review summarizes current knowledge of the effects of secukinumab on the QoL of patients with psoriasis using patient-reported outcome measures.
Expert Opinion: Secukinumab, the first approved drug of this new class, has fully reached a new therapeutic paradigm not only in terms of clinical efficacy, but also in terms of patient satisfaction and self-rated health.
1. Introduction
Psoriasis is an immune-mediated chronic inflammatory skin disease that affects about 2–3% of the world’s population [Citation1]. Psoriasis is associated with a large number of systemic comorbidities such as psoriatic arthritis, depression, and metabolic diseases [Citation2]. Chronic plaque psoriasis involves 90% of patients resulting in raised, well-demarcated erythematous plaques that can cover the majority of the body with significant stress and social stigma [Citation2]. Psoriasis can affect any area of the skin, but involvement of some localizations as scalp, nail, palms, soles, and joints is associated with significant impact on the quality of life (QoL).
The etiology of psoriasis is multifactorial, comprising both genetic and environmental factors. The prevailing hypothesis is that keratinocyte stress, dendritic cell activation, and autoreactive skin T cells cause of normal skin barrier immunity mechanisms that produce a pro-inflammatory state [Citation3]. Activation of keratinocytes and dendritic cells leads to upregulation of inflammatory molecules such as TNF-α, IFN-α, and IFN-γ with consequent expression of IL-12 and IL-23. Acting on IL-23 receptors, this cytokine stimulates Th17 to release IL-17 [Citation4–7]. IL-17 is produced by Th17, but also by neutrophils and mast cells, and binds to IL-17 receptors on various cells, including keratinocytes to stimulate their activation. The effect of these molecules is to induce keratinocyte proliferation, inflammatory cytokine production, innate immune system activation, and recruitment of immune cells [Citation4,Citation8–10].
IL-17A, whose key target is keratinocytes, is the main effector cytokine of the IL-17 family [Citation11]. Its upregulation in patients with psoriasis is implicated in plaque histopathology [Citation12–14]. IL-17A and IL-17 F, in the form of homodimers or heterodimers, bind to the IL-17 R receptor complex, and once activated the receptors send a signal to stimulate transcription of adaptor protein ACT1, nuclear factor-κB (NF-κB), and TNF receptor-associated factor 6 (TRAF6). This process guides the release of IL-6 and IL-8 [Citation7]. In summary, the IL-23/Th17 axis and its changes play a central role in the etiopathogenesis of psoriasis. IL-23 and IL-17 are the key cytokines in the development of chronic inflammation underlying plaque psoriasis. These relations are depicted in .
Figure 1. Psoriasis inflammatory cascade. Abbreviations: TNF, tumor necrosis factor; IL, interleukin; Th, T helper; IFN-γ, interferon gamma; γδ T-cells, gamma delta T cells. Elaborated from [Citation14]
![Figure 1. Psoriasis inflammatory cascade. Abbreviations: TNF, tumor necrosis factor; IL, interleukin; Th, T helper; IFN-γ, interferon gamma; γδ T-cells, gamma delta T cells. Elaborated from [Citation14]](/cms/asset/bfca9dee-5128-4829-8348-d44430bbb7ce/iebt_a_1849131_f0001_oc.jpg)
2. Secukinumab
Secukinumab is approved for the treatment of psoriasis, psoriatic arthritis (PsA), and ankylosing spondylitis (AS), and is thus widely used for treatment of both dermatological and rheumatological conditions. Secukinumab, due to its specific inhibition of IL-17A, improves the complete spectrum of psoriatic manifestations, and demonstrates a rapid onset of action and sustained responses with a favorable safety profile [Citation6].
Secukinumab was the first biological therapy to establish PASI90 (an improvement of at least 90% of the baseline PASI – Psoriasis Area Severity Index) as a primary endpoint in clinical trials. Before its entry in the market, anti-TNF-α molecules and ustekinumab had always used PASI50 and PASI75 as endpoints to monitor clinical response. Since psoriasis is a disease with major impact on patient QoL, the interest was largely focused on variations in the Dermatology Life Quality Index (DLQI). DLQI is a tool, consisting of 10 simple questions, which measures the impact of skin disease on normal daily activities, including social, emotional, and work-related aspects. A DLQI score of ‘0’ or ‘1’ (DLQI 0/1) corresponds to no effect on a patient’s life [Citation15]. PASI90 is considered by the European Medicines Agency as the therapeutic goal since this directly correlates with a DLQI 0/1, which indicates no impact of psoriasis on the patient’s QoL [Citation16].
The efficacy of secukinumab in both the short and long term has been demonstrated in several clinical trials [Citation17–19]. In phase III clinical trials at 12 weeks (ERASURE, FIXTURE and CLARITY), PASI75 response was achieved in 76–88% of patients, while PASI90 response was achieved by 54–67%, and PASI100 in 24–38%. In phase III clinical trials at week 52 (CLEAR and SCULPTURE), PASI75 response was achieved in 75–93% of patients, PASI90 in 60–76%, and PASI100 in 36–46%. The results after 5 years of follow-up in SCULPTURE demonstrated a high level of clearance even in the long term, with PASI75 achieved in 80%, PASI90 in 59%, and PASI100 achieved in 36% of patients. Thus, secukinumab has a rapid onset of action, within 12 weeks in a large number of patients, with further improvement up to week 52 and the ability to maintain good efficacy in the long term.
SCALP, GESTURE, and TRANSFIGURE examined efficacy on ‘difficult-to-treat’ lesions of the scalp, palmoplantar regions, and nails, respectively. In SCALP, with a mean Psoriasis Scalp Severity Index (PSSI) at baseline of 33.2, secukinumab 300 mg led to rapid improvement with 53% of the patients achieving a PSSI90 response at week 12 vs. 2% of patients with placebo, and 59% of patients achieved a PSSI90 response at week 24 [Citation20]. In TRANSFIGURE, improvements in nail psoriasis severity index (NAPSI) were scored as – 45%, – 38%, and – 11%, for secukinumab 300 mg, 150 mg, and placebo, respectively, at 16 weeks [Citation21]. Further reductions in NAPSI were noted at 32 weeks: – 63.2% and – 52.6% for secukinumab 300 mg and 150 mg, respectively. GESTURE investigated 205 patients with palmoplantar psoriasis treated with secukinumab using the Palmoplantar Investigator’s Global Assessment (ppIGA) score; 59% of patients demonstrated ppIGA 0/1 improvements after 2.5 years of treatment [Citation22]. The reduction from baseline was sustained up to 2.5 years with a mean NAPSI improvement of −73.3% and −63.6% (as observed) and −70.5% and −52.9% (last observation carried forward) with secukinumab 300 and 150 mg groups, respectively [Citation23]. Lastly, it should be noted that a proportion of patients experience loss of efficacy after around 6 months despite continuous treatment with secukinumab [Citation24]. Drug survival is also lower in patients who had previously been treated with two or more biologics [Citation25].
The safety profile for secukinumab is also favorable in patients with moderate to severe psoriasis throughout 5 years of treatment, showing no increase in yearly rates of adverse events [Citation17]. Pooled safety data of 96,054 patient-years including studies on the three indications of secukinumab (psoriasis, PsA, and AS) validated its safety during long-term use in these chronic conditions [Citation26]. As shown for other monoclonal antibodies used for psoriasis, the most frequent adverse event was upper respiratory tract infection. In psoriasis, PsA, and AS, a low incidence was seen for serious infections (1.4, 1.9, and 1.2, respectively), Candida infections (2.2, 1.5, and 0.7, respectively), inflammatory bowel disease (0.01, 0.05, and 0.1, respectively), and major adverse cardiac events (0.3, 0.4, and 0.6, respectively). No cases of reactivation of tuberculosis were reported. However, it should be mentioned that according to the Summary of Product Characteristics, secukinumab is not recommended in patients with inflammatory bowel disease, and cases of new or exacerbations of inflammatory bowel disease have been reported with secukinumab [Citation27,Citation28].
3. Utility of patient-reported outcomes in psoriasis
Patient-reported outcome measures (PROMs) are used to evaluate the health and quality of life of patients. In 2009, the FDA defined patient-reported outcome as ‘any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else’ [Citation29]. The first PROMs date to the 1970s and were developed to assess pain experienced by patients in the context of clinical trials [Citation30]. The use of PROMs in clinical trials has become much more common in recent years [Citation31]. PROMs, indeed, give clinicians the ability to integrate objective clinical outcomes with the patient’s perception of their own state of health. Accordingly, PROMs make it possible to understand if a treatment or medical intervention ‘make the difference’ for a patient, in terms of specific or general health conditions and quality of life [Citation31]. PROMs are typically measured using standardized, self-administered questionnaires, and usually investigate domains such as QoL, disease symptoms, mental health/mental distress, work productivity, and the ability to perform activities of daily living [Citation32]. Finally, PROMs must be monitored to evaluate treatment outcomes and determine the most cost-effective options to assist policymakers in allocating resources [Citation33]. Likewise, aggregated PROM data can play a role in health-care evaluation where they are increasingly utilized in routine clinical practice to examine the impact, appropriateness, quality, and performance of health-care services, also allowing benchmarking between service providers [Citation34].
PROMs are particularly valuable in psoriasis. Chronic itchy and painful symptoms (mainly if arthritis is associated) can lead to stress, interference with activities of daily living including dressing, bathing, and sleeping, depression, and suicidal ideation. Furthermore, patients can experience feelings of shame, embarrassment, stigmatization, and poor self-esteem, particularly when the face, neck, and other visible portions of the body are involved. All these elements make psoriasis a disabling condition for many patients [Citation35–37]. While in the scientific literature there are several reviews on the efficacy and safety of secukinumab in the treatment of plaque psoriasis, there are few publications that have collected data on patient-reported outcomes. Following the previous overview of secukinumab and the importance of patient-related outcomes, the available data on PROMs with secukinumab, which often represent secondary endpoints in clinical trials, will be summarized. Toward this end, PubMed was searched for articles on secukinumab and PROMs while ClinicalTrials.gov was searched for trials. Additional publications were collected from references identified in articles and related citations in PubMed. New data presented at the 2019 American Academy of Dermatology (AAD) and the European Academy of Dermatology and Venereology (EADV) meetings was also included. This literature search identified seven clinical trials and a real-world evidence registry that investigated the use of secukinumab on plaque-type psoriasis and reported PROMs.
4. Studies on secukinumab and PROMs
4.1. SCULPTURE
The core trial of SCULPTURE was a 52-week randomized, double-blind, study that compared a retreatment-as-needed versus a fixed-interval regimen of secukinumab in 966 subjects with plaque psoriasis [Citation17,Citation38]. Subsequently, 168 patients entered the extension phase and continued treatment with the same dose (300 mg every 4 weeks) as the core study for up to 5 years. From the fourth year, the treatment became open-label and patients self-injected secukinumab at home. Changes in DLQI score and DLQI 0/1 response from baseline to week 12 (core study) and to week 52 (extension study) were the PROMs reported as secondary endpoints. Significantly higher improvement of health-related quality of life (HR-QoL) in the fixed-interval groups versus the retreatment-as-needed group. DLQI scores within the latter group increased at the beginning of relapse and improved when PASI75 was achieved again [Citation38]. At week 52, more than two-thirds of patients (72.7% n = 165) achieved DLQI 0/1 and 65.5% (n = 119) maintained the response for 5 years [Citation17].
4.2. ERASURE
ERASURE was a multicenter, randomized, double-blind, phase 3 trial that compared the efficacy of secukinumab at doses of 300 mg and 150 mg versus placebo. DLQI questionnaires were administered to assess DLQI score and DLQI 0/1 response. Self-assessment of symptoms was carried out using the validated Psoriasis Symptom Diary (PSD) [Citation19,Citation39]. The PSD was designed to evaluate daily signs and symptoms of psoriasis reported by the patient including itching, pain, and scaling, and is based on 16 items. Each symptom was assessed separately, using a numerical score scale of 0 to 10, with a 24-h reporting period [Citation40,Citation41]. At week 12, subjects treated with secukinumab experienced greater improvement of patient-reported itching, pain, and scaling on the PSD (P < 0.001 for all comparisons) than placebo () [Citation19]. After 12 weeks of treatment, a DLQI score of 0/1 was achieved in significantly more patients in both secukinumab-dose groups compared with placebo (P < 0.001 for all comparisons: ). The mean DLQI score decreased in the 300 mg and 150 mg secukinumab groups and in the placebo group from 13.9, 13.4, and 12.0 at baseline to 2.5, 3.3, and 10.9 at week 12, respectively [Citation19].
Figure 2. QoL improvement at week 12 in FIXTURE, ERASURE, and CLEAR. Data assessed with non-responder imputation
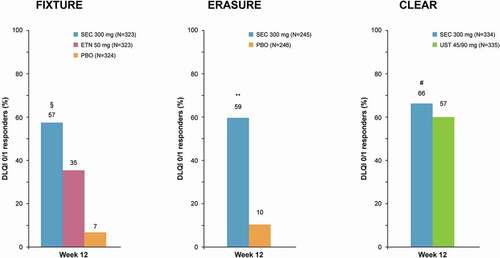
Table 1. ERASURE: Results from psoriasis symptoms diary at week 12
4.3. FIXTURE
In this phase 3, randomized, double-blind, placebo-controlled study, adult patients with plaque psoriasis were randomly assigned (1:1:1:1) to secukinumab 300 mg, secukinumab 150 mg, etanercept (active reference group), or placebo [Citation19]. Subjects completed the PSD and DLQI questionnaire [Citation19,Citation39] Significant improvement in itching, pain, and scaling was seen in patients treated with secukinumab at both doses compared to etanercept and placebo (P < 0.001; ). Those treated with secukinumab also showed a higher proportion with a DLQI score of 0/1 at week 12 than the etanercept or placebo groups (P < 0.001 for all comparisons; ). Mean DLQI score decreased in the 300 mg and 150 mg secukinumab, etanercept, and placebo groups from 13.3, 13.4, 13.4, and 13.4 at baseline to 2.9, 3.7, 5.5, and 11.5 at week 12, respectively. Lastly, Strober et al. reported the DLQI 0/1 response rate at week 52 in patients from the ERASURE study added to those of FIXTURE. Overall, 79.9% in the secukinumab 300 mg group and 70.8% in the secukinumab 150 mg group achieved DLQI 0/1 vs. 59.4% in the etanercept group (p < 0.0001 and p = 0.0036, respectively) [Citation39,Citation42].
Table 2. FIXTURE: Results from psoriasis symptoms diary at week 12
4.4. CLEAR
The CLEAR study was a 52 week, phase III, randomized, double-blind, clinical trial that aimed to demonstrate the superiority of secukinumab over ustekinumab in patients with plaque-type psoriasis [Citation17,Citation43]. After 52 weeks, participants treated with secukinumab continued in the open-label extension phase and received secukinumab 300 mg at week 52, followed by 300 mg every 4 weeks to week 100 [Citation43]. In this trial, the effectiveness of secukinumab on PROMs as secondary endpoints was investigated. Psoriasis-related symptoms of pain, itching, and scaling were collected in the PSD. Generic HR-QoL was assessed by the European Quality of Life 5 Dimension 3 Level (EQ-5D-3 L) Health tool. The EQ-5D is a preference-based instrument for measurement of the HR-QoL that provides simple measures for clinical and economic assessment. The EQ-5D consists of two distinct sections: the first provides a subjective evaluation relative to five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and each item provides answers graded from 1 (no problems) to 3 (extreme limitation). The second section is a Visual Analog Scale (VAS) investigating perceived health status with a range from 0 (worst possible) to 100 (best possible) [Citation44,Citation45]. At all timepoints, QoL measured by EQ-5D-3 L in the secukinumab groups showed that, in all EQ-5D-3 L domains, significant improvements were greater relative to those in the ustekinumab group (mean absolute change from baseline at week 52 was 13.8 versus 10.6 [P = 0.0301]) [Citation18]. Greater improvements for secukinumab compared to ustekinumab were reported at the 52-month follow-up visit for all EQ-5D domains: mobility (mean difference −14% vs −11%), self-care (mean difference −8% vs −4%), usual activities (mean difference −30% vs −20%), pain/discomfort (mean difference −45% vs −38%), and anxiety/depression (mean difference −34% vs −30%; ) [Citation19].
Figure 3. QoL improvement at 1-year follow-up with EQ-5D-3 L in CLEAR. Data assessed with non-responder imputation. Ustekinumab dose: 45 mg for patients weighing ≤100 kg at baseline or 90 mg for patient weighing >100 kg. QoL, quality of life; SEC, secukinumab; UST, ustekinumab
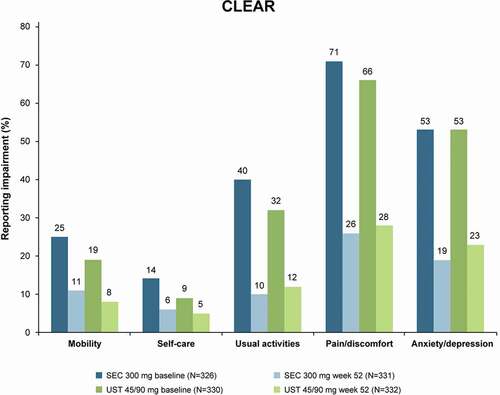
Skin-specific quality of life was assessed by the DLQI, and work productivity was measured by the Work Productivity and Activity Impairment Questionnaire-Specific Health Problem (WPAI-SHP) [Citation44,Citation45]. The WPAI questionnaire is a self-administered instrument used to assess the impact of disease on productivity. The WPAI is based on six questions and measures work productivity loss in terms of time and quality due to general health issues [Citation46,Citation47]. Starting from week 16, higher response rates were obtained in the secukinumab vs. ustekinumab groups on self-assessments of psoriasis-related pain, itching, and scaling. Secukinumab was associated with a greater mean percentage improvement of patient-reported itch (77.6% vs. 68.3%), pain (80.1% vs. 58.8%), and scaling (82.6% vs. 71.8%) from baseline to week 52 [Citation18]. Low mean scores of psoriasis-related pain, itching, and scaling severity were seen for up to 2 years of secukinumab treatment [Citation18]. Similarly, at all time points, patient treated with secukinumab had greater mean percentage improvements in DLQI scores and all subscale scores, which were sustained for up to 1 year [Citation18]. Furthermore, patients treated with secukinumab experienced an overall increase in work productivity and improvement in their abilities to perform daily activities as assessed by the WPAI-PSO [Citation18]. At 12 weeks, the percentage of patients achieving a DLQI 0/1 response in the FIXTURE, ERASURE, CLEAR, and SCULPTURE studies ranged between 57%-66%; at week 52, 66%-72% of patients achieved a DLQI 0/1 response, and 66% at 5 years.
Regarding a DLQI of 0/1, indicating no impact of skin problems on QoL, at all timepoints up to week 52, there were more responders in the secukinumab group. At week 16, 71.9% of patients treated with secukinumab vs. 57.4% with ustekinumab achieved a DLQI 0/1 response (p <.0001); at week 52, 71.6% of those on secukinumab achieved the same goal vs. 59.2% on ustekinumab [Citation18]. Analogous outcomes were observed for up to 2 years (65.9% of patients) [Citation43].
As reported by Puig et al., cumulative clinical benefit was assessed by evaluating the area-under-the-curve of the percentage of PASI75, PASI90, and PASI100 responders, as well as complete relief of symptoms and DLQI 0/1 responders over 52 weeks [Citation48]. The cumulative clinical benefit was greater with secukinumab than with ustekinumab across all PASI levels, relief of symptoms, and DLQI 0/1. Consequently, the clinical benefit ratios were all higher than 1.0 for secukinumab compared to ustekinumab.
4.5. CLARITY
Another head-to-head clinical trial that investigated efficacy and safety of secukinumab versus ustekinumab is CLARITY, a multicenter, randomized, double-blinded, phase 3b study, with a larger sample size than CLEAR (n = 1102 in CLARITY, n = 676 in CLEAR) [Citation49]. DLQI 0/1 response at weeks 4, 12, and 16 was assessed as a secondary endpoint [Citation49]. A higher proportion of patients with a DLQI score of 0/1 was seen in the secukinumab group compared with the ustekinumab group at week 4 (33.9% vs.18.0%; p = 0.0001), week 12 (64.0% vs. 51.7%; p = 0.0001), and week 16 (68.4% vs. 55.9%; p = 0.0001) [Citation49].
4.6. FEATURE
FEATURE was a randomized, double-blind, placebo-controlled study. It enrolled 117 patients with plaque psoriasis and investigated secukinumab administration via ‘self-injection with a pre-filled syringe’ (PFS) with the Self-Injection Assessment Questionnaire (SIAQ) [Citation50]. This tool consists of two parts: a module self-completed before the first self-injection and module self-completed following the two self-injections at baseline and weeks 1, 4, 8, and 12. The tool queries self-confidence, satisfaction with self-injection, pain, and reaction during or after the injection, feelings about injections, self-image, and ease of use [Citation51]. The mean score (0–10) on self-assessment of feelings about self-injection was 7.87 for the baseline module and 8.70 for the module at week 12; for self-confidence, the respective scores were 7.09 and 8.23, while they were 6.83 and 8.35, respectively, for satisfaction with self-injection [Citation56]. Positive results were also obtained for the other domains. Overall, the patients’ responses suggested good acceptability of self-injections at baseline and through week 12.
4.7. PROSE
The prospective, non-randomized, patient-centric, multicenter PROSE study (NCT02752776) examined the impact of treatment with 300 mg secukinumab on patient-reported outcomes in patients with moderate to severe psoriasis who were stratified by treatment history: naive to systemic therapy (N = 663), prior systemic (N = 673), and previously exposed to biologics (N = 324) [Citation52]. QoL patient-reported outcomes, along with efficacy and safety, were evaluated over 52 weeks. Altogether, 70.8% of patients achieved a DLQI 0/1 response at Week 16, with effects that were sustained until study end (). The mean Family DLQI score showed a decrease from 11.5 to 2.5 after 16 weeks with a further decrease at study end (). Substantial improvements were also seen in all PROMs at week 16, which included the EQ-5D, Numeric Rating Scale for pain, itching and scaling, HAQ-Disability Index, Treatment Satisfaction Questionnaire for Medication, and Patient Benefit Index. Importantly, all improvements in QoL and efficacy were sustained throughout the 52-week study. Thus, in the PROSE study, treatment with secukinumab was associated with normalization of QoL in a substantial proportion of patients with psoriasis, as well as their families. Moreover, the benefits were not dependent on history of prior treatment.
Figure 4. Changes in DLQI (A) and Family DLQI (B) after treatment with secukinumab in patients with psoriasis stratified by prior treatment history. Adapted from [Citation52] with permission
![Figure 4. Changes in DLQI (A) and Family DLQI (B) after treatment with secukinumab in patients with psoriasis stratified by prior treatment history. Adapted from [Citation52] with permission](/cms/asset/6d511b62-b571-4d6b-b880-d0020db563c7/iebt_a_1849131_f0004_oc.jpg)
4.8. Corrona psoriasis registry
The Corrona Psoriasis Registry was a US-driven, independent, prospective, observational cohort study that examined the real-world effectiveness of secukinumab considering clinical and patient-reported outcomes from enrollment to a 6-month follow-up visit in patients with psoriasis [Citation53]. The PROMs assessed were patient-reported itch, pain, and fatigue, which were evaluated with a VAS scale from 0 to 100; DLQI score and corresponding subscales, EQ VAS, and WPAI questionnaire with the relative percentage of impairment in each domain [Citation53]. All PROMs assessed after 6 months of treatment with secukinumab improved significantly. DLQI scores decreased from 7.7 at baseline to 2.9 after 6 months (mean difference, 4.8). After 6 months, the percentage of subjects who achieved a DLQI score of 0/1 significantly improved (55.1% vs. 12.8% at enrollment), and the percentage of patients who reported a moderate to extremely large impact of disease on QoL (DLQI 6–30) significantly decreased (18.6% vs. 57.3% at enrollment; p < 0.01) [Citation53]. Furthermore, patients experienced significant improvements in itch (mean 21.1 vs. 51.9 at enrollment), pain (mean 13.9 vs. 37.1), fatigue (mean 26.1 vs. 34.6), and EQ VAS (mean 77.1 vs. 70.2) (all p < 0.01) () [Citation53].
Table 3. CORRONA Psoriasis Registry: changes in patient-reported outcomes at 6 months
After 6 months, secukinumab showed a significant positive impact on the percentage of impairment while working (mean 5.6 vs. 14.7), overall percentage of work hours affected (mean 7.1 vs. 16), and daily activities impaired by psoriasis (mean 10.9 vs. 20.2) (all p < 0.01). Work hours missed (mean 1.7 vs. 2.6) also improved, although the difference was not statistically significant [Citation53].
5. Conclusion
The available evidence strongly suggests that treatment of psoriasis with secukinumab leads not only to resolution of clinical symptoms, but also to significant improvements in the QoL in the majority of patients. All PROMs used to evaluate QoL in clinical trials show improvement with secukinumab, indicating that the benefits extend to virtually all aspects of daily life and work, as well as social activities and family members. At the same time, improvement in the QoL is intuitive since a high proportion of patients reach PASI90 or even higher, and in the absence of plaques, it would be expected that QoL would return to ‘normal’ levels, or those in which the disease has no impact on QoL. Moreover, it seems apparent that the improvements seen in QoL are independent of prior treatment history. This is another important aspect that should not be underestimated. Thus, secukinumab has fully reached a new therapeutic paradigm not only in terms of clinical efficacy, but also in terms of patient satisfaction and self-rated health.
6. Expert opinion
QoL has been discussed in the medical literature since the 1960s [Citation54]; in 1995, Felce and Perry defined it ‘an overall general wellbeing that comprises objective descriptors and subjective evaluations of physical, material, social, and emotional wellbeing together with the extent of personal development and purposeful activity, all weighted by a personal set of values’ [Citation55]. QoL includes two components: a subjective one (psychological) and an objective one (social, economic, political, and environmental) [Citation56]. It can be grouped into five broad categories: normal life, happiness/satisfaction, achievement of personal goals, social utility, and natural capacity [Citation57]. It became a principal endpoint in health care as a consequence of the development of patients’ rights movements, and it is important for clinical, economic, and political decisions [Citation58].
QoL should be measured since, regardless of therapy, it can provide information on the impact of the disease on the patient’s well-being; furthermore, the therapeutic success can be assessed with objective clinical measures or improvement of the disease, but the most relevant benefit is the patient’s sense of well-being. Moreover, QoL helps to assess the efficacy of a therapeutic strategy in randomized-controlled trials, and is now a new endpoint to evaluate the effectiveness of treatments and can be very useful in choosing between two therapies of equal clinical efficacy. Finally, it can play a fundamental role in cost-effectiveness assessments of pharmacological intervention, providing information on health resources [Citation59].
Although psoriasis generally does not affect survival, it is associated with significant impairment on QoL [Citation60]. Psoriasis is a disease that affects people differently and the use of new treatments that last over time is essential to increase QoL [Citation59]. Physicians frequently underestimate the degree of psychological and social morbidity associated with the disease [Citation61]. On the other hand, illness perception varies among individuals and psoriasis of the same severity can be perceived in different ways by patients; it is, therefore, essential to evaluate self-rated health [Citation62].
The introduction of anti-IL17 drugs in clinical practice bears the promise of achieving PASI90 response or better in the majority of patients, and there is accumulating evidence in support of PASI90 response becoming the new therapeutic goal standard based on its better correlation with HR-QoL and DLQI 0/1 status (absence of effect on HR-QoL) [Citation16]. Given this, evaluation of QoL thus has a primary role in evaluating the effectiveness of therapy and is also related to adherence to therapy. Lastly, it is important to highlight that by having a positive impact on the QoL of patients, new anti-interleukin agents also allow for good long-term adherence. This avoids switching therapy to another drug class that may imply the administration of additional medications such as systemic immunosuppressive agents (e.g. with use of an anti-TNF-α). Patient-reported outcomes are an important source of information for prescribers that can help them choose the most appropriate agent, among currently available biologicals, for the individual patient to treat chronic plaque psoriasis.
Article highlights
It is becoming increasingly essential to evaluate QoL in patients with psoriasis as it gives vital information about the patient’s overall well-being and efficacy of treatment.
Patient-reported outcomes can integrate objective clinical assessment with the patient’s perception of their own state of health.
The introduction of anti-IL17 agents has allowed for the majority of patients to achieve a PASI90 response or even higher, and as such PASI90 is now becoming the new goal for therapy.
Secukinumab, the first approved drug of the new class of anti-IL17 agents, has fully reached a new therapeutic paradigm in terms of clinical efficacy, patient satisfaction, and self-rated health.
This box summarizes key points contained in the article.
Declaration of interest
L Bianchi has served as a speaker and as a consultant for Abbvie, Novartis, Janssen-Cilag, Pfizer, UCB, and Leo-Pharma outside the submitted work. The other authors have no conflicts of interest to declare. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosure
A reviewer on this manuscript has disclosed receiving research, speaking, and/or consulting support from a variety of companies including Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation; They have also consulted for others through Guidepoint Global, Gerson Lehrman and other consulting organizations; They are a founder and majority owner of www.DrScore.com; They are a founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Patrick Moore, who provided editorial assistance on behalf of Health Publishing & Services Srl. This unconditional support was funded by Novartis Farma SpA.
Additional information
Funding
References
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271.
- Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5:5621.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis. Dermatol Ther (Heidelb). 2016;6:1–12.
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Krueger JG, Wharton KA Jr., Schlitt T, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144:750–763.
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776.
- Keijsers RR, Joosten I, van Erp PE, et al. Cellular sources of IL-17 in psoriasis: a paradigm shift? Exp Dermatol. 2014;23:799–803.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71(1):141–150. .
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
- Garzorz-Stark N, Psoriasis Pathogenesis: EK. Keratinocytes are back in the spotlight. J Invest Dermatol. 2019;139:995–996.
- Gaffen SL, Jain R, Garg AV, et al. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600.
- Hreggvidsdottir HS, Noordenbos T, Baeten DL. Inflammatory pathways in spondyloarthritis. Mol Immunol. 2014;57:28–37.
- Ivanov S, Linden A. Interleukin-17 as a drug target in human disease. Trends Pharmacol Sci. 2009;30:95–103.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216.
- Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29:645–648.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507–1514.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60–9 e9.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326–338.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667–674.
- Reich K, Sullivan J, Arenberger P, et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. Br J Dermatol. 2019;181:954–966.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70–80.
- Reich K, Sullivan J, Arenberger P, et al. Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study. Br J Dermatol. 2020. Accepted Author Manuscript. https://doi.org/https://doi.org/10.1111/bjd.19262
- Huang YYM, Ruth JS, Hsu S. Loss of efficacy of secukinumab for psoriasis at 24 to 32 weeks. J Am Acad Dermatol. 2016;75:e169.
- Egeberg A, Bryld LE, Skov L. Drug survival of secukinumab and ixekizumab for moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;81:173–178.
- D’Adamio S, Silvaggio D, Lombardo P, et al. The safety of anti-interleukins monoclonal antibodies for the treatment of psoriasis. Expert Opin Drug Saf. 2019;18:1031–1041.
- Fobelo Lozano MJ, Serrano Gimenez R, Castro Fernandez M. Emergence of inflammatory bowel disease during treatment with secukinumab. J Crohns Colitis. 2018;12:1131–1133.
- Secukinumab, summary of product characteristics. [cited 2020 Oct 24] Available from: https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en.pdf.
- Patient-reported outcome measures: use in medical product development to support labeling claims. U.S. food and drug administration. [published 2019 Oct 17; cited 2020 Mar 16]. Available from: http://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims.
- Atherton PJ, Sloan JA. Rising importance of patient-reported outcomes. Lancet Oncol. 2006;7:883–884.
- Snyder CF, Aaronson NK. Use of patient-reported outcomes in clinical practice. Lancet. 2009;374:369–370.
- Kingsley C, Patel S. Patient-reported outcome measures and patient-reported experience measures. BJA Educ. 2017;17:137–144.
- Franklin P, Chenok K, Lavalee D, et al. Framework to guide the collection and use of patient-reported outcome measures in the learning healthcare system. EGEMS (Wash DC). 2017;5:17.
- Field J, Holmes MM, Newell D. PROMs data: can it be used to make decisions for individual patients? A narrative review. Patient Relat Outcome Meas. 2019;10:233–241.
- Ferreira BI, Abreu JL, Reis JP, et al. Psoriasis and associated psychiatric disorders: a systematic review on etiopathogenesis and clinical correlation. J Clin Aesthet Dermatol. 2016;9:36–43.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383–392.
- Taylor WJ, Mease PJ, Adebajo A, et al. Effect of psoriatic arthritis according to the affected categories of the international classification of functioning, disability and health. J Rheumatol. 2010;37:1885–1891.
- Mrowietz U, Leonardi CL, Girolomoni G, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73:27–36 e1.
- Strober B, Sigurgeirsson B, Popp G, et al. Secukinumab improves patient-reported psoriasis symptoms of itching, pain, and scaling: results of two phase 3, randomized, placebo-controlled clinical trials. Int J Dermatol. 2016;55:401–407.
- Lebwohl M, Swensen AR, Nyirady J, et al. The psoriasis symptom diary: development and content validity of a novel patient-reported outcome instrument. Int J Dermatol. 2014;53:714–722.
- Mathias SD, Feldman SR, Crosby RD, et al. Measurement properties of a patient-reported outcome measure assessing psoriasis severity: the psoriasis symptoms and signs diary. J Dermatolog Treat. 2016;27:322–327.
- Strober B, Gottlieb AB, Sherif B, et al. Secukinumab sustains early patient-reported outcome benefits through 1 year: results from 2 phase III randomized placebo-controlled clinical trials comparing secukinumab with etanercept. J Am Acad Dermatol. 2017;76:655–661.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400–409.
- Pickard AS, Gooderham M, Hartz S, et al. EQ-5D health utilities: exploring ways to improve upon responsiveness in psoriasis. J Med Econ. 2017;20:19–27.
- Yang Y, Brazier J, Longworth L. EQ-5D in skin conditions: an assessment of validity and responsiveness. Eur J Health Econ. 2015;16:927–939.
- Reilly Associates. [cited 2020 Oct 13]. Available from: http://www.reillyassociates.net/.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365.
- Puig L, Augustin M, Blauvelt A, et al. Effect of secukinumab on quality of life and psoriasis-related symptoms: a comparative analysis versus ustekinumab from the CLEAR 52-week study. J Am Acad Dermatol. 2018;78:741–748.
- Bagel J, Nia J, Hashim PW, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week CLARITY results). Dermatol Ther (Heidelb). 2018;8:571–579.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484–493.
- Keininger D, Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the self-injection assessment questionnaire (SIAQ). Health Qual Life Outcomes. 2011;9:2.
- Augustin M, Dauden E, Mrowietz U, et al. Secukinumab treatment leads to normalization of quality of life and disease symptoms in psoriasis patients with or without prior systemic psoriasis therapy: the PROSE study results. J Eur Acad Dermatol Venereol. 2020. https://doi.org/https://doi.org/10.1111/jdv.16632.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333–341.
- Elkinton JR. Medicine and the quality of life. Ann Intern Med. 1966;64:711–714.
- Felce D, Perry J. Quality of life: its definition and measurement. Res Dev Disabil. 1995;16:51–74.
- Liu BC. Quality of life: concept, measure and results. Am J Econ Sociology. 1975;34:4–13.
- Ferrans CE. Quality of life: conceptual issues. Semin Oncol Nurs. 1990;6:248–254.
- Pais-Ribeiro JL. Quality of life is a primary end-point in clinical settings. Clin Nutr. 2004;23:121–130.
- Estoque RC, Togawa T, Ooba M, et al. A review of quality of life (QOL) assessments and indicators: towards a “QOL-Climate” assessment framework. Ambio. 2019;48:619–638.
- Krueger GG, Feldman SR, Camisa C, et al. Two considerations for patients with psoriasis and their clinicians: what defines mild, moderate, and severe psoriasis? What constitutes a clinically significant improvement when treating psoriasis? J Am Acad Dermatol. 2000;43:281–285.
- Choi J, Koo JY. Quality of life issues in psoriasis. J Am Acad Dermatol. 2003;49:S57–61.
- Larsen MH, Krogstad AL, Wahl AK. Alexithymia, illness perception and self-management competency in psoriasis. Acta Derm Venereol. 2017;97:934–940.
