ABSTRACT
Objective: Our pharmacogenomic study evaluated the influence of the presence/absence of genetic variants of psoriasis-risk loci on the clinical response to secukinumab. Differences in the single-nucleotide polymorphism (SNP) pattern characterizing HLA-Cw6+ or HLA-Cw6− patient subpopulations, showing high or low responses to secukinumab, were also analyzed.
Methods: 417 SNPs were analyzed by Next-Generation Sequencing technology, in a cohort of 62 psoriatic patients and undergone secukinumab treatment.
Univariate regression analysis was employed to examine the association between SNP and clinical response to secukinumab. Multivariate analysis was also performed to assess multivariate differences in SNP pattern of HLA-Cw6+ or HLA-Cw6− patients showing high or low responses to secukinumab.
Results: Eight SNPs in HLA-C and upstream region (rs13207315, rs6900444, rs12189871, rs12191877, rs4406273, and rs10484554), including HLA-Cw6 classical allele (rs1131118), and three in MICB-DT (rs9267325), DDX58 (rs34085293) and TYK2 (rs2304255) genes, associating with excellent response to secukinumab were identified. Importantly, rs34085293 or rs2304255 SNP status defined a subgroup of super-responder patients. We also found that HLA-Cw6+ and HLA-Cw6− patients carried out specific patterns of SNPs associating with different responses to secukinumab.
Conclusion: Assessment of HLA-Cw6, together with other allelic variants of genes, could be helpful to define patients which better benefit from anti-IL-17 therapy.
Abbreviations: PASI: Psoriasis Area and Severity Index; SNP: Single-Nucleotide Polymorphism Rs: Reference SNP; PASI75: 75% reduction in Psoriasis Area and Severity Index; PASI90: 90% reduction in Psoriasis Area and Severity Index; PASI100: 100% reduction in Psoriasis Area and Severity Index; NGS: Next-Generation Sequencing; OR: Odds Ratio; CAP: Canonical Analysis of Principal coordinates; BMI: Body Mass Index; LD: Linkage Disequilibrium
1. Introduction
Psoriasis is an immune-mediated skin disorder caused by inherited susceptibility alleles [Citation1]. Most psoriasis susceptibility loci are related to inflammatory and immune genes. Current opinion on the pathogenesis of psoriasis emphasizes the role of cytokine signaling to drive an inflammatory cycle, in which infiltrating dendritic cells, by releasing IL-23, induce the expansion of autoreactive T lymphocytes, typically represented IL-17-producing T cells [Citation2]. Following their expansion, T cells, together with innate lymphoid cells, γδ-T cells, mast cells, and neutrophils release very high amounts of IL-17 [Citation3,Citation4]. IL-17, in turn, mediates most of the epidermal hyperplasia by impairing differentiation of keratinocytes, and inducing their maturation and aberrant cornification. IL-17 also promotes the release of neutrophil- and T-cell-recruiting chemokines and antimicrobial peptides, and synergizes with the pro-inflammatory cytokines IFN-γ and TNF-α[Citation5].
The pathogenic role of IL-17 in psoriasis has been definitely confirmed by the clinical efficacy of secukinumab, the first monoclonal antibody approved for the treatment of psoriasis and targeting IL-17A [Citation6]. Although secukinumab is highly effective [Citation7], variable therapeutic responses have been observed in the psoriatic population, especially in a real-life setting [Citation8].
Biological therapies for psoriasis show significant variability in efficacy in patients, and among factors determining this variability, the presence and/or absence of specific single-nucleotide polymorphisms (SNPs) in psoriasis-risk genes play an important role. To date, SNPs located in HLA-C, TNFAIP3, TNFA, IL12B, IL-23A, and IL-23 R TAP1 genes have been found to influence the response of psoriatic patients to anti-TNF-α or anti-IL-12/23 drugs [Citation9–12].
Among psoriasis-related SNPs, the MHC class I allele HLA-Cw6, the strongest genetic risk variant predisposing to psoriasis, has been reported to associate with better response to the anti-IL-12/IL-23p40 drug ustekinumab [Citation13,Citation14]. Similarly, anti-TNFs were found to be more effective in HLA-Cw6-positive patients, even though contrasting results have been reported [Citation15]. A recent comparative study showed that HLA-Cw6-negative patients are more likely to respond to adalimumab than to ustekinumab [Citation15]. Moreover, the combination of HLA-Cw6 allele with different genotypes, for instance those related to IL12B gene, determined optimal response to ustekinumab [Citation16]. An interaction between the HLA-Cw6 and TNFAIP3 genotypes on disease improvement among patients treated with anti-TNFs has also been described [Citation17]. To date, few pharmacogenetic studies on response to the anti-IL-17A drugs in the psoriatic population have been performed [Citation18,Citation19].
Here, we report a pharmacogenomic study aimed at evaluating the simultaneous presence of SNPs in psoriasis-risk loci, associating with clinical response to secukinumab, in a cohort of 62 patients affected by moderate-to-severe plaque psoriasis. SNPs potentially predicting the response to secukinumab were identified.
2. Materials & methods
2.1. Patients and ethics statement
Our study included 62 patients recruited between September 2015 and June 2018, at the Dermatology Unit, University of Rome ‘Tor Vergata’. All patients were Caucasians, aged > 18 years with moderate-to-severe plaque-type psoriasis defined at enrollment by: Psoriasis Area Severity Index (PASI) score > 10, Body Surface Area (BSA) > 10%, Dermatology Life Quality Index (DLQI) > 10. Patients with a baseline PASI < 10, who presented involvement of sensitive areas were also included. The enrolled patients required biologic treatment with secukinumab, as failed to respond, had contraindications for, or did not tolerate at least one conventional treatment.
Secukinumab was administered following AIFA (Agenzia Italiana del Farmaco) criteria in a standard dosing regimen (300 mg subcutaneous, five times within 4 weeks followed by once monthly injections), used in monotherapy and not combined with conventional systemics or topical therapies.
For each patient, personal data, as well as anthropometric and clinical data were collected and were annotated in an electronic database specifically programmed and created ad hoc for the study. The severity of psoriasis and response to treatment were evaluated using the PASI score at baseline and, then, at follow-up visits on weeks 8, 16, 24, 40, 56, 64, 72, 88, and 100. Clinical efficacy was assessed in terms of the 75%, 90%, and 100% improvement of PASI score compared to baseline (PASI75, PASI90, and PASI100). 2-ml blood samples were collected from each psoriatic patient to isolate DNA.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Tor Vergata University Ethics Committee (approval no. 20,745, v. 25 March 2005). Thus, clinical data, as well as blood were collected from patients after written informed consent.
2.2. SNP analysis
DNA was extracted from blood by QIAcube system with QIAmp DNA kit (Qiagen, Hilden, Germany), and 10 ng were used for sequencing by NGS technology. The customized designed SNP panel was composed of n = 122 SNPs (Supplementary Table 2), present in n = 89 amplicons (size range 125–375 bp), potentially implicated in immune responses (antigen presentation, T-cell signaling, innate immunity), as well as inflammatory pathways (cytokine-dependent signaling) and skin barrier function. Sequencing of amplicons permitted the identification of additional 295 SNPs located nearby the primary SNPs. The SNP array analysis thus identified 417 genetic variants in total. The analyzed SNPs were selected based on an extensive review of articles on the association between psoriasis and SNPs or response to biologics [Citation9,Citation15,Citation20–27].
NGS was performed using the Ion GeneStudio™ S5 Plus platform (Thermo Fisher Scientific, Massachusetts, USA). Libraries were amplified by the Ion AmpliSeq™ Library kit Plus (Thermo Fisher) and quantified using the Qubit 4 Fluorometer and 2100 Bioanalyzer with dsDNA HS assay and High Sensitivity DNA kit (Thermo Fisher), respectively. Sequencing data were processed with Ion Torrent Suite software v.5.10.
Positive calls were selected with a read depth > 30X and allelic frequency of 0.3. Reads were aligned to human genome sequence (build GRCh37/hg19) and analyzed using Variant Caller plugin. Variants’ annotations were verified with ANNOVAR from the UCSC Genome Browser on hg19 assembly.
2.3. Statistical analysis
Drug response data were analyzed by an intent-to-treat last observation carried forward method. SNPs that showed an identical pattern in patients have been merged to reduce the number of genetic variables that needed to be managed.
Differences between groups (allele-positive or -negative patients) based on the clinical response to secukinumab obtained at the different time-points of treatment were evaluated by χ2 test. Univariate and multivariate logistic regression analyses were performed to combine genetic data or demographic variables, clinical factors, and responses to secukinumab, expressed as PASI75, PASI90, or PASI100. The association between drug response and all the variables collected was estimated by calculating the odds ratio (OR), its standard error, and 95% confidence interval (CI), using the STATA 14.2 software (StataCorp, College Station, TX, USA). Deviation from null hypothesis was considered significant at p-value < 0.05.
CAP, based on the Bray-Curtis similarity matrix [Citation28], was performed in order to assess multivariate differences in the genetic pattern of psoriatic population among the HLA-Cw6+ or HLA-Cw6− patients showing high or low clinical responses to secukinumab. The canonical correlations were tested using 4999 random permutations of the genetic data, expressed as presence or absence of the SNPs. The analyzed data matrix included 94 SNPs, since SNPs showing identical patterns in psoriatic patients were deleted. Moreover, distinctness of the four patient groups (HLA-Cw6+ high-responders, HLA-Cw6+ low-responders, HLA-Cw6− high-responders, HLA-Cw6− low-responders) was assessed using leave-one-out allocation success [Citation29]. The product-moment correlations of the selected 94 SNPs with the two canonical discriminant axes (ρ1 and ρ2) were calculated, and only the most relevant correlation (i.e. √ρ12+ ρ22 > 0.35) was considered as valuable and included in the plot. The multivariate analysis was carried out using PRIMER 6© v.6.1.5 and PERMANOVA +© v.1.0.1 software (PRIMER-E, Plymouth, UK).
3. Results
3.1. SNPs in HLA-C and upstream region associate with clinical response to secukinumab
Participants’ demographic and disease characteristics are summarized in Supplementary . At baseline, mean PASI was 18.9 ± 12.2, disease duration was 22.4 ± 14.9 years, and age at disease onset was 22.7 ± 12.2 years. Mean weight and body mass index (BMI) were 79.9 ± 17.5 and 26.8 ± 6.3, respectively. Most of the patients (74.5%) were naïve to biological therapy. Hypertension was the most frequent co-morbidity, together with obesity and type-2 diabetes. PASI follow-up scores were available for sixty (96.8%) patients at the central observational point (56 weeks) and for thirty-one (50%) at the last follow-up visit (100 weeks). The lack of efficacy (secondary lack of efficacy, defined as loss of PASI75 starting from week 16) was the main reason for discontinuation of the drug (7 out of 62 patients, 11.3%).
Table 1. Multivariate logistic regression analysis (step-wise analysis) of predictors of PASI response after treatment with secukinumab
In the primary analysis, we tested the influence of genetic variants predisposing to psoriasis on the response to secukinumab. Univariate logistic regression analysis between single independent variables (SNP status) and dependent variables, such as PASI75, PASI90, and PASI100 responses at weeks 8–100, identified eight SNPs in HLA-C and upstream region associating with an optimal response to secukinumab . Psoriatic patients carrying rs13191343 or rs13207315 SNPs in HLA-C promoter region , more successfully achieved PASI75 and PASI90 end-points at weeks 16, 24, 40, and at week 56, as well as PASI100 at weeks 16 and 24 (statistical values in Supplementary Figure 1). For both SNPs, we observed the same pattern of presence in the psoriatic population and, thus, identical regression curves . A third SNP in the HLA-C promoter region, the rs6900444, also influenced the response to secukinumab, as positive subjects reached faster PASI90 at weeks 8, 16, and week 24, when compared to negative patients (Supplementary Figure 2).
Figure 1. Association analysis between rs13191343, rs13207315, rs1131118, rs12189871, rs4406273, rs12191877, and rs10484554 SNPs and clinical responses to secukinumab treatment. Univariate logistic regression analysis was performed to evaluate the association between (A) rs13191343 (HLA-C_promoter1) or rs13207315 (HLA-C_promoter2), (B) rs1131118 (HLA-Cw6), rs12189871 (HLA-Cw6_LD1) or rs4406273 (HLA-Cw6_LD3), and (C) rs12191877 (HLA-Cw6_LD2) or rs10484554 (HLA-Cw6_LD5) with the clinical response to secukinumab after 8, 16, 24, 40, 56, 64, 72, 88 and 100 weeks of treatment, in a cohort of patients (n = 62) affected by moderate-to-severe psoriasis. Graphs show the percentage and the number of patients (n Pz) carrying (SNP-POS, dark gray line) or not (SNP-NEG, gray line) the SNPs and achieving 75% reduction of PASI score (PASI75), 90% reduction of PASI (PASI90) and 100% reduction (PASI100). rs13191343 and rs13207315, rs1131118, rs12189871 and rs4406273, as well as rs12191877 and rs10484554 showed the same pattern of presence/absence in the psoriatic population and, thus, identical logistic regression curves. *p value < 0.05 were considered significant
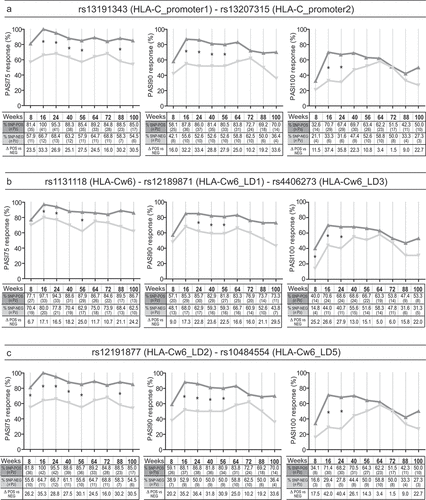
Of note, we observed a significant association between the HLA-Cw6 psoriasis classical allele (rs1131118), and response to secukinumab . In fact, allele-positive patients better achieved PASI75 at weeks 16, 24, and 56, PASI 90 at weeks 24, 40, and 56, and PASI100 at weeks 8, 16, and 24 (statistical values in Supplementary Figure 3a). Other two SNPs co-segregating with HLA-Cw6, namely rs12189871 and rs4406273 showed the same pattern of presence in patients and, thus, identical regression curves .
Rs12191877 or rs10484554 in HLA-C upstream region associated with a better response to secukinunab, as positive patients better reached PASI75 than allele-negative patients at weeks 8, 16, 24, 40, 56, and 88, PASI90 at weeks 16, 24, 40 and week 56, and PASI100 at weeks 16 and 24 (, statistical values in Supplementary Figure 3b).
Association data and regression curves relative to rs12191877 and rs10484554 variants were identical, due to their identical status in patients. They were not detected in all the HLA-Cw6+ subjects, even though they were previously described in linkage disequilibrium (LD) with HLA-Cw6 [Citation30,Citation31].
3.2. Optimal response to secukinumab associates with allelic variant status of MICB-DT, DDX58 and TYK2
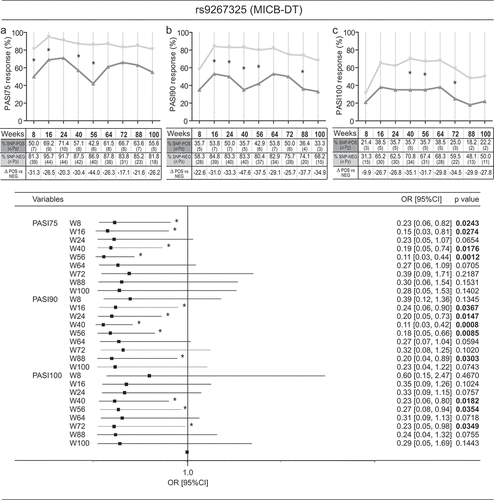
Significant results of association between SNP status and response to secukinumab were observed for three SNPs in MICB-DT, DDX58, and TYK2 genes. A strong association was found for rs9267325 in MICB-DT, whose absence in psoriatic patients determined a better response to secukinumab when compared to positive patients. Allele-negative patients faster achieved PASI75 at weeks 8, 16, 40, and 56, PASI90 at weeks 16, 24, 40, 56, and 88, and PASI100 achievement at weeks 40, 56, and 72 . In addition, rs9267325-negative patients achieved better PASI75, PASI90, and PASI100 if they did not previously receive other biological therapies. In fact, multivariate analysis revealed an association between rs9267325 and absence of previous treatments with response to secukinumab, in terms of PASI75 and PASI90 achievement . The age is also associated with a better response to secukinumab in absence of rs9267325 SNP .
Figure 2. Association analysis between rs9267325 SNP and clinical response to secukinumab. Rs9267325 (MICB-DT_v2)-negative (SNP-NEG, gray line) patients reached a significant better response to secukinumab than positive patients (SNP-POS, dark gray line), in terms of achievement of PASI75 (A), PASI90 (B) and PASI100 (C), as evaluated by logistic regression analysis. The univariate logistic regression analysis is also summarized in the forest plot in the lower panel. The condition of good response to secukinumab is more likely to occur in the group of patients not carrying the allele, as indicated by the odds ratio (OR) < 1. Squares on the x-axis shows odd ratio (OR), squares indicate OR estimates for each observation point and the error bars represent 95% confidence interval (CI). *p value < 0.05 were considered significant and indicated in font bold
None of the analyzed SNP associated with response to secukinumab in those patients showing particular sites of psoriasis manifestation (i. e. scalp, genital, palmo-plantar areas) (not shown).
We next observed that rs34085293 in DDX58 or rs2304255 in TYK2 were carried out by a subgroup of patients which highly responded to secukinumab, as they efficiently reached PASI100 and maintained high the response up to week 100 . Concerning rs34085293, PASI100 end-point was significantly reached by most of the allele-positive patients at weeks 24, 40, 56, 64 up to and 88 . Similarly, the presence of rs2304255 SNP in TYK2 gene determined PASI100 achievement at weeks 64, 72, 88, and 100 .
Figure 3. Rs34085293 and rs2304255 SNPs associate with an optimal clinical response to secukinumab treatment. The presence of rs34085293 (DDX58_v1) and rs2304255 (TYK2_v3) variants determined an optimal response to secukinumab treatment at several time-points of observation. The upper and lower graphs show respectively the percentage and the number (n Pz) of DDX58_v1-positive (SNP-POS, dark gray line) and -negative (SNP-POS, gray line) subjects (A) or TYK2_v3-positive (SNP-POS, dark gray line) and -negative (SNP-POS, gray line) (B) patients achieving 100% reduction in the PASI score (PASI100). The relative univariate logistic regression analyses are summarized in forest plots in the right panels, where the x-axis shows odd ratio (OR), squares indicate OR estimates for each observation point and the error bars represent 95% confidence interval (CI). *p value < 0.05 were considered significant and indicated in font bold
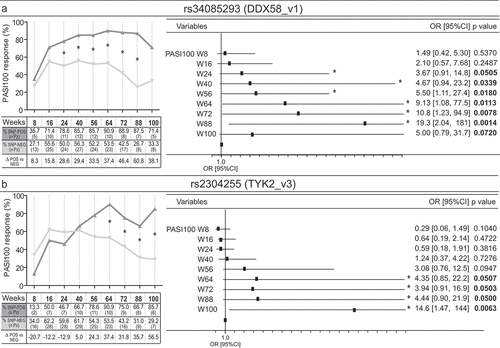
Of note, both rs34085293 or rs2304255 polymorphisms were preferentially found in patients having the following demographic and clinical profile: males (10 out of 14 for rs34085293 and 10 out of 15 for rs2304255), early age of disease onset (13 out of 14 or 15 for rs34085293 or rs2304255, respectively), and naïve to previous biological treatment (12 of 14 for rs34085293 and 11 out of 15 for rs2304255). The other demographic and disease characteristics (age, BMI, disease duration, co-morbidities) seemed to be not associated with rs34085293 or rs2304255 SNP status.
Finally, the absence of two SNPs in LTA gene, namely rs1800683 and rs909253, determined a better response to secukinumab, in terms of PASI75 achievements at weeks 16, 24, and 40 (Supplementary Figure 4).
3.3. HLA-Cw6 status identifies specific SNP patterns in psoriatic patients associating with optimal response to secukinumab
In order to understand whether specific SNP patterns characterized patients depending on HLA-Cw6 allele status and/or high or low clinical responses to secukinumab, we performed canonical analysis of principal coordinates (CAP). The analysis showed a significant clustering of patients belonging to HLA-Cw6+ and HLA-Cw6− groups, and, specifically, in four established subgroups: HLA-Cw6+ high-responders, HLA-Cw6+ low-responders, HLA-Cw6− high-responders, and HLA-Cw6− low-responders . HLA-Cw6+ high-responders mainly distributed in the lower left quadrant of plot area (91.3%). The HLA-Cw6+ low-responder cluster was mostly found in the upper left quadrant (83.3%). On the other hand, low-responders (54.5%) and high-responders (58.3%) HLA-Cw6− patients clustered in the lower and upper right quadrants, respectively, even though their distribution patterns partially overlapped . Among HLA-Cw6− patients, 5 out of 11 low-responders and 5 out of 13 high-responders did not segregate in their respective clusters, likely due to their similar genetic SNP pattern. It is noteworthy that the five HLA-Cw6− low-responders clustering with HLA-Cw6− high-responders showed severe obesity and discontinued treatment for loss of efficacy within the 2 years of observation.
Figure 4. HLA-Cw6 status identifies specific SNP patterns in psoriatic patients correlating with clinical response to secukinumab. Clustering of patients based on presence of SNPs and response to secukinumab was performed by CAP on a cohort of psoriatic patients (n = 53), classified as high responders to secukinumab (PASI90 or PASI100 achievement at week 56) or low responders (unsatisfactory responses or achievement of PASI75 or less at week 56). (A) CAP ordination plot shows a significant clustering of psoriatic patients belonging to HLA-Cw6+ and HLA-Cw6− groups along x-axis, and, in particular, to four established subgroups: HLA-Cw6+ high-responders (downward triangles), HLA-Cw6+ low-responders (triangles), HLA-Cw6− high-responders (diamonds), and HLA-Cw6− low-responders (squares) to secukinumab. The percentage of patients carrying similar SNP profiles, based on the response to secukinumab and HLA-Cw6 status, and relative to leave-one-out allocation of observations to groups, are shown in panel A. The length of each vector line corresponds to the strength of the correlation and direction for each SNP (squared canonical correlations δ12 = 0.89 and δ22 = 0.25; p = 0.0002; 69.8% of sampling units correctly classified). Univariate logistic regression analysis was employed to evaluate the association between rs1004819 (IL23R_v4) (B), rs2201841 (IL23R_v5) (C), rs71562288 (TRAF3IP2_v1) (D) or rs2304255 (TYK2_v3) (E) and PASI90 response at 8, 16, 24, 40, 56, 64, 72, 88 and 100 weeks of treatment, in HLA-Cw6+ (left column) and HLA-Cw6− (right column) patient groups. *p values < 0.05 were considered significant
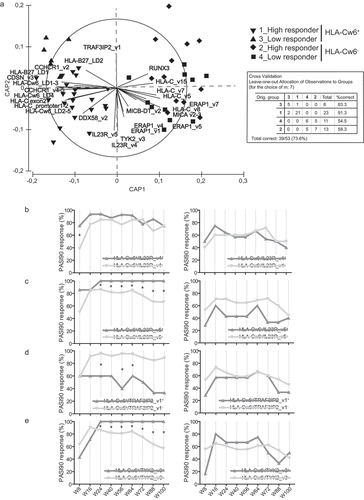
As shown in , HLA-Cw6+ and HLA-Cw6− groups totally segregated along the x-axis and showed a distinct pattern of SNP presence. In particular, the DDX58_v2, HLA-Cw6_LD2-LD5, HLA-C_promoter1-promoter2, HLA-C exon2, HLA-Cw6_LD4, CCHCR1_v4-v5, HLA-Cw6_LD1-LD3, CDSN_v3, HLA-B27_LD1, CCHCR1_v2, and HLA-B-27_LD2 SNPs were mostly carried out by HLA-Cw6+ patients (see Supplementary Table 2 for corresponding identification number). On the other hand, MICB-DT_v2, ERAP1_v1, ERAP1_v4, MICA_v2-v3, HLA-C_v8, ERAP1_v5, and ERAP1_v7, HLA-C_v5 and HLA-C_v7, HLA-C_v15, and RUNX3 variants characterized HLA-Cw6− patients. Importantly, most of the SNPs associating with HLA-Cw6 were predominantly found in the high-responders, with the exception of HLA-B27_LD1, CCHCR1_v2, and HLA-B27_LD2, which were also present in low-responder group. Although HLA-Cw6_LD4, HLA-C exon2, CCHCR1_v4–5, and DDX58_v2 variants characterized HLA-Cw6+patients of the high-responder group , univariate regression analysis demonstrated significant association of these SNPs with optimal response to secukinumab only at discontinuous time-points of observation (data not shown). While MICB-DT_v2, ERAP1_v1, ERAP1_v4, MICA_v2-v3 variants were present in HLA-Cw6− patients showing moderate response to drug, HLA-C_v5, HLA-C_v7, HLA-C_v8, and HLA-C_v15, ERAP1_v5, and ERAP1_v7, as well as RUNX3 SNPs characterized HLA-Cw6− patients belonging to both low-responder and high-responder groups. .
Of note, the IL23R_v4 and IL23R_v5 genetic variants were mutually exclusive with TRAF3IP2_v1 allele in patients. TRAF3IP2_v1 and IL23R_v4 or IL23R_v5 equally distributed in both HLA-Cw6+ and HLA-Cw6− groups, with their representative arrows pointing to the area plot in the upper and lower quadrants, respectively, between the HLA-Cw6+ and HLA-Cw6− groups . Also, TYK2_v3 was found in patients of both HLA-Cw6+ and HLA-Cw6− clusters, and it was mostly present in patients not showing TRAF3IP2_v1 allele.
In order to understand whether the IL23R_v4, IL23R_v5, TRAF3IP2_v1 and TYK2_v3 allele influenced the response to secukinumab of HLA-Cw6+ and HLA-Cw6− patients, we performed univariate regression analysis of these SNPs and response to the drug, in terms of PASI90 achievement, using separate HLA-Cw6− or HLA-Cw6+ patient databases. We found that IL-23 R_v5, and not IL-23 R_v4 allele presence significantly associated with response to secukinumab in HLA-Cw6+ population ). PASI90 achievement in HLA-Cw6+ population also significantly depended on TRAF3IP2_v1 allele absence and TYK2_v3 presence ). TRAF3IP2_v1 or TYK2_v3 alleles also significantly influenced PASI100 achievement by HLA-Cw6+ patients at weeks 56, 64, 72, and 100 (data not shown). IL23R_v4, IL23R_v5, TRAF3IP2_v1, and TYK2_v3 allele status did not influence response of HLA-Cw6− population to secukinumab ).
The list of the SNPs significantly associating with a better secukinumab response and the number of psoriatic patients carrying these genetic variants is summarized in Supplementary Table 3.
4. Discussion
The genetic basis of psoriasis has long been recognized, and, thanks to genome-wide association studies and linkage scans, more than 60 susceptibility regions predisposing to psoriasis have been identified [Citation21,Citation22,Citation24,Citation25,Citation27,Citation32,Citation33]. The major genetic determinants of psoriasis reside in psoriasis susceptibility (PSORS)1 locus, mapping on chromosome 6p21. This region spans approximately 250 kb within the MHC class I region and contains nine genes, including HLA-C, HLA-B, TNFA, LTA, and MICB, together with non-protein coding genes and pseudogenes [Citation34]. Although HLA-Cw6 is itself considered the causal PSORS1 allele, over 100 HLA-C alleles and several SNPs within HLA-C promoter region have been identified [Citation35]. Other psoriasis-risk SNPs were identified in additional PSORS loci, in genes involved in Th17/Th1 responses, innate immunity, and inflammatory pathways, as well as skin barrier and antigen presentation functions [Citation1].
In the last decade, several pharmacogenetic studies allowed the identification of polymorphisms potentially predicting the clinical response of psoriatic patients to the anti-TNF-α adalimumab, and to the anti-IL-12/IL-23 ustekinumab [Citation10,Citation11,Citation13,Citation15,Citation36]. On the contrary, pharmacogenetic studies on IL-17 blockers are scarce and contrasting. In fact, secukinumab showed good efficacy independently of HLA-C*06:02 allele in the phase-IIIb SUPREME study [Citation19,Citation37], whereas, in a real-life setting, it was shown to be more efficacious in HLA-C*06:02-positive than in negative patients [Citation38].
In the present study, we identified a set of psoriasis-risk SNPs associating with an excellent response to secukinumab . Among HLA-C SNPs, three (rs13191343, rs13207315, and rs6900444) were present at – 1268, – 1209, and at – 1196 bp from the transcription start site of HLA-C. The first two are strictly close, and positioned in HLA-C promoter region containing three putative binding sites for the Sp1 transcription factor, which could be involved in the IL-17-dependent positive regulation of HLA-C. Indeed, a previous work investigated on possible effects of other two SNPs in HLA-C promoter virtually abolishing the response to TNF-α and IFN-γ, and pointed out the significance of SNP influence on the regulation of HLA-C by cytokines in psoriasis [Citation39]. The other HLA-C variants associating with secukinumab response include the HLA-Cw6 psoriasis classical allele (rs1131118), and rs12189871 and rs4406273. While rs1131118 is present in the exon 3 of HLA-C gene, rs12189871 and rs4406273 are located 12 kb and 26 kb upstream of HLA-C . Of note, rs4406273 SNP maps within a region containing two overlapping sequences, namely LINC02571 and LOC112267902, generating two long non-coding RNA (lncRNA) with potential regulatory functions on HLA-C [Citation40]. The pattern of presence of rs1131118 in HLA-C exon 3 and rs12189871 or rs4406273 in HLA-C intergenic region was identical in all psoriatic patients, indicating that these allelic variants co-segregate, even though they are distant from each other. Rs12191877 or rs10484554, located 13 kb and 34 kb upstream of HLA-C also co-segregate, and determined a better response to secukinumab treatment.
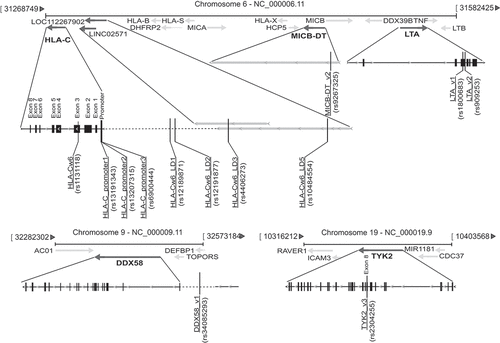
Figure 5. Scheme of SNPs associated with response to secukinumab and relative positions on chromosomes 6, 9 and 19. A schematic representation of all the identified SNPs associating with optimal response to secukinumab, and their relative positions (vertical lines) within gene and adjacent regions of chromosomes 6, 9 and 19 are shown. While HLA-C-related SNPs, together with those present in MIC-DT (rs9267325) and LTA (rs1800683 and rs909253), map within PSORS1 locus of chromosome 6, DDX58_v1 (rs34085293) and TYK2_v3 (rs2304255) SNPs localize in chromosome 9 and 19, respectively. Black boxes with continuous lines schematically represent exons and introns of genes, whereas dashed lines the intergenic regions. Gray bars indicate characterized or predicted lncRNAs, and arrows show the direction of transcription
Although no functional evidence correlating the presence of HLA-Cw6 variant and response to secukinumab exist, it is plausible that this allele allows the presentation of epitopes present in different putative autoantigens, such as the cathelicidin LL37, which is efficiently recognized by circulating CD8+ T cells with a psoriasis-like cytokine profile (IFN-γhigh and IL-17high) [Citation41]. On the other hand, SNPs present in HLA-C regulatory regions, spanning the HLA-C promoter and upstream regions, could influence the expression levels of HLA-C class I molecules on immune and resident skin cells, and, in turn, the expansion rate of CD8+ IL-17-producing T cells. Future studies exploring the functional role of specific HLA-C alleles in IL-17-dependent immune responses will be fundamental not only to unveil their pathogenic activity in psoriasis but also to understand why specific HLA-C haplotypes effectively respond to the anti-IL-17 treatments.
Significant results of association were also observed for rs9267325 in MICB-DT , whose absence in psoriatic patients determined a better response to secukinumab, with a further greater likelihood of reaching PASI100 if patients did not undergo to previous biological treatments. Interestingly, rs9267325 SNP localizes nearby MICB, within MICB-DT , from which a divergent regulatory RNA or lncRNA is generated. As consequence of absence of rs9267325, it is plausible that MICB-DT RNA cannot appropriately regulate the expression levels of MICB mRNA [Citation42]. The identification of association of rs9267325 with secukinumab response is particularly intriguing since MICB encodes an MHC class I-related protein with potential immunological functions on IL-17-producing and NKG2D-bearing NK and CD8+ T cells [Citation43].
Our pharmacogenomic analysis also revealed an association with two co-segregating SNPs of LTA (rs1800683 and rs909253) , potentially present in the 5ʹ untranslated region of LTA mRNA, and, thus, in a presumably regulatory region. Expression levels of lymphotoxin-α could influence innate immune responses in psoriatic skin, especially during the early phase of disease development [Citation44].
Importantly, we identified two SNPs in TYK2 (rs230425) and DDX58 (rs34085293) , in a subgroup of super-responder patients achieving and maintaining PASI100 up to weeks 88 and 100. Most of the rs230425-positive super-responders were also HLA-Cw6+. TYK2 and DDX58 have been initially described as mediators of anti-viral and innate immune responses. While TYK2 is a tyrosine kinase belonging to Janus kinase protein family, which associates with type I and type II cytokine receptors, including type-I IFN receptors, DDX58 is a protein involved in viral double-stranded RNA recognition and type-I IFN production. Although both molecules have antiviral activity, recent evidence shows TYK2 and DDX58 involvement in IL-23/IL-17 axis by inducing IL-23 and regulating IL-23-mediated pathways [Citation45,Citation46]. While rs34085293 maps in DDX58 intergenic region and the impact of its presence is unpredictable, rs2304255 is positioned in exon 8 of TYK2 and determines a missense substitution in the protein. Our association data of rs34085293 and rs2304255 with the clinical effect of secukinumab in the super-responder population, together with evidence on TYK2 and DDX58 involvement in IL-23/IL-17 axis, strongly suggest the importance of these pathogenic pathways in the response to anti-IL-17 drug.
A growing number of evidence showed that HLA-Cw6+ and HLA-Cw6− plaque psoriasis represents biologically distinct pathologies and endotypes [Citation47]. HLA-Cw6 is associated with phenotypic features, including early-onset psoriasis, guttate lesions, as well as arm, leg, and trunk involvement. In addition, it is now well-established that HLA-Cw6+ patients have different profiles of response to biological drugs [Citation15,Citation36].
Our study supports the notion that HLA-Cw6 status efficiently stratifies psoriatic patients, showing HLA-Cw6+ patients a specific psoriasis-risk SNP pattern, as well as optimal response to secukinumab. We found that HLA-Cw6+ and HLA-Cw6− patients differently clustered in CAP plots as consequence of their different genotypes. SNPs characterizing each group mainly localized in the HLA-C upstream region, with variants present in HLA-Cw6+ patients being mutually exclusive with those found in HLA-Cw6− patients. Nevertheless, most of the SNPs associating or not with HLA-Cw6 were predominantly located in genes of PSORS1 locus, indicating the relevance of this region in the predisposition of all patients to psoriasis.
Importantly, HLA-Cw6 status also identified specific SNP patterns associating with clinical response to secukinumab. For instance, most of the SNPs characterizing HLA-Cw6+ patients were highly represented in the high-responder subpopulation, thus confirming data obtained by univariate regression analysis. Concerning SNPs typical of HLA-Cw6− patients, MICB-DT_v2 variant (rs9267325) was present in the group showing moderate response to the drug, accordingly to the findings that its absence determined a better response to secukinumab in regression models.
Of note, despite the presence of IL23R_v5, TYK2_v3, and TRAF3IP2_v1 variants in both groups, only HLA-Cw6+ patients responded significantly better to secukinumab depending on IL23R_v5 and TYK2_v3 presence or TRAF3IP2_v1 allele absence. The functional significance of these associations could be related to the hyperactivation of IL-23- and IL-17 pathways in psoriatic skin, being IL-23 R, TYK2, and TRAF3IP2 all fundamental for type-17 T-cell responses and IL-17 signaling in target cells [Citation2,Citation46]. Since IL23R_v5 and TRAF3IP2_v1 SNPs map, respectively, within a regulatory region of IL23R and in a sequence generating the LOC107986521 lncRNA, they could influence IL-23R and TRAF3IP2 expression levels.
5. Conclusions
In this pharmacogenomic, real-life study, we identified a set of SNPs associating with optimal response to secukinumab in a cohort of 62 patients affected by psoriasis. Despite the limited sample size, our study is the first analyzing the simultaneous presence of a high number of genetic variants in patients undergone anti-IL-17A treatment. SNP panel included polymorphisms highly represented in the psoriatic population (i. e. HLA-C variants), as well as rare genetic variants (i. e. IL-36 G and CARD14) having a low frequency in psoriasis. To unveil their association with anti-IL-17A treatment, the latter genotypes need to be investigated in large cohorts of patients, as well as in different clinical subtypes characterized by specific haplotypes.
Among common genetic variants, HLA-Cw6 risk-allele strongly influenced response to the IL-17A blocker, confirming the relevance of this genetic variant for treatment efficacy observed with other biologics. We also identified HLA-C-related alleles, and MIC-DT, DDX58, and TYK2 SNPs significantly influencing response to the drug. In addition, we found that HLA-Cw6 status was associated with specific SNP patterns, mainly represented by polymorphisms of HLA-C genic and intergenic regions and PSORS1 locus.
Thus, the assessment of HLA-Cw6 genotype, together with other allelic variants of different genes involved in intersected pathogenic pathways, could be helpful to better classify patients and to predict treatment response to secukinumab.
Declaration of interest
L Bianchi has served as a speaker and as a consultant for Abbvie, Novartis, Janssen-Cilag, Pfizer, UCB, and Leo-Pharma outside the submitted work. G Girolomoni has been principal investigator in clinical trials sponsored by and/or and has received personal fees from AbbVie, Abiogen, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Meyers Squibb, Celgene, Celltrion, Eli-Lilly, Genzyme, Leo Pharma, Menlo therapeutics, Novartis, OM Pharma, Pfizer, Regeneron, Samsung, Sandoz, and UCB. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Acknowledgments
We acknowledge the precious contribution of Dr. Gabriele La Mesa of the Italian National Institute for Environmental Protection and Research (ISPRA, Rome, Italy) who performed CAP and produced relative statistical models.
Additional information
Funding
References
- Dand N, Mahil SK, Capon F, et al. Psoriasis and genetics. Acta Derm Venereol. 2020 Jan 30;100(3):adv00030.
- Girolomoni G, Strohal R, Puig L, et al. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol JEADV. 2017 Oct;31(10):1616–1626.
- Villanova F, Flutter B, Tosi I, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol. 2014 Apr;134(4):984–991.
- Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Iimmunol. 2011 Jul 1;187(1):490–500. (Baltimore, Md: 1950).
- Albanesi C, Madonna S, Gisondi P, et al. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunol. 2018;9:1549.
- Karle A, Spindeldreher S, Kolbinger F. Secukinumab, a novel anti-IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity. mAbs. 2016;8(3):536–550.
- Gisondi P, Altomare G, Ayala F, et al. Italian guidelines on the systemic treatments of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol JEADV. 2017 May;31(5):774–790.
- Galluzzo M, Talamonti M, De Simone C. Secukinumab in moderate-to-severe plaque psoriasis: a multi-center, retrospective, real-life study up to 52 weeks observation. Exp Opin Biol Ther. 2018 Jul;18(7):727–735.
- Ovejero-Benito MC, Prieto-Perez R, Llamas-Velasco M, et al. Polymorphisms associated with adalimumab and infliximab response in moderate-to-severe plaque psoriasis. Pharmacogenomics. 2018 Jan;19(1):7–16.
- Prieto-Pérez R, Solano-López G, Cabaleiro T, et al. New polymorphisms associated with response to anti-TNF drugs in patients with moderate-to-severe plaque psoriasis. Pharmacogenomics J. 2018 Jan;18(1):70–75.
- Talamonti M, Botti E, Galluzzo M, et al. Pharmacogenetics of psoriasis: HLA-Cw6 but not LCE3B/3C deletion nor TNFAIP3 polymorphism predisposes to clinical response to interleukin 12/23 blocker ustekinumab. Br J Dermatol. 2013 Aug;169(2):458–463.
- Prieto-Pérez R, Llamas-Velasco M, Cabaleiro T, et al. Pharmacogenetics of ustekinumab in patients with moderate-to-severe plaque psoriasis. Pharmacogenomics. 2017 Jan;18(2):157–164.
- Raposo I, Carvalho C, Bettencourt A, et al. Psoriasis pharmacogenetics: HLA-Cw*0602 as a marker of therapeutic response to ustekinumab. Eur J Dermatol. 2017 Oct 1;27(5):528–530.
- Masouri S, Stefanaki I, Ntritsos G, et al. A Pharmacogenetic study of psoriasis risk variants in a greek population and prediction of responses to anti-TNF-α and Anti-IL-12/23 agents. Mol Diagn Ther. 2016 Jun;20(3):221–225.
- Dand N, Duckworth M, Baudry D, et al. HLA-C*06:02 genotype is a predictive biomarker of biologic treatment response in psoriasis. J Allergy Clin Immunol. 2019 Jun;143(6):2120–2130.
- Galluzzo M, Boca AN, Botti E, et al. IL12B (p40) gene polymorphisms contribute to ustekinumab response prediction in psoriasis. Dermatology. 2016;232(2):230–236.
- Batalla A, Coto E, González-Fernández D, et al. The Cw6 and late-cornified envelope genotype plays a significant role in anti-tumor necrosis factor response among psoriatic patients. Pharmacogenet Genomics. 2015 Jun;25(6):313–316.
- van Vugt LJ, van den Reek J, Meulewaeter E, et al. Response to IL-17A inhibitors secukinumab and ixekizumab cannot be explained by genetic variation in the protein-coding and untranslated regions of the IL-17A gene: results from a multicentre study of four European psoriasis cohorts. J Eur Acad Dermatol Venereol JEADV. 2020 Jan;34(1):112–118.
- Costanzo A, Bianchi L, Flori ML, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: SUPREME study. Br J Dermatol. 2018 Nov;179(5):1072–1080.
- Ahmed H, Yusuf N. Genetic influences on pharmacological interventions in psoriasis. J Clin Exp Dermatol Res. 2017;8:392–406.
- Tsoi LC, Spain SL, Knight J, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012 Dec;44(12):1341–1348.
- Tsoi LC, Spain SL, Ellinghaus E, et al. Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nat Commun. 2015 May 5;6:7001.
- Tsoi LC, Stuart PE, Tian C, et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat Commun. 2017 May 24;8:15382.
- Ellinghaus E, Ellinghaus D, Stuart PE, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet. 2010 Nov;42(11):991–995.
- Nair RP, Duffin KC, Helms C, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009 Feb;41(2):199–204.
- Ryan C, Bowcock A, Menter A. Use of pharmacogenomics in psoriasis. Clin Invest. 2011;1:399.
- Stuart PE, Nair RP, Ellinghaus E, et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat Genet. 2010 Nov;42(11):1000–1004.
- Anderson MJ, Willis TJ. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology. 2003;84:511–525.
- Anderson MJ, Robinson J. Generalised discriminant analysis based on distances. Aust NZ J Stat. 2003;45:301–318.
- Kisiel B, Kisiel K, Szymański K, et al. The association between 38 previously reported polymorphisms and psoriasis in a Polish population: high predicative accuracy of a genetic risk score combining 16 loci. PloS One. 2017;12(6):e0179348.
- Feng BJ, Sun LD, Soltani-Arabshahi R, et al. Multiple Loci within the major histocompatibility complex confer risk of psoriasis. PLoS Genet. 2009 Aug;5(8):e1000606.
- Strange A, Capon F, Spencer CC, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010 Nov;42(11):985–990.
- Dand N, Mucha S, Tsoi LC, et al. Exome-wide association study reveals novel psoriasis susceptibility locus at TNFSF15 and rare protective alleles in genes contributing to type I IFN signalling. Hum Mol Genet. 2017 Nov 1;26(21):4301–4313.
- Capon F, Munro M, Barker J, et al. Searching for the major histocompatibility complex psoriasis susceptibility gene. J Invest Dermatol. 2002 May;118(5):745–751.
- Clop A, Bertoni A, Spain SL, et al. An in-depth characterization of the major psoriasis susceptibility locus identifies candidate susceptibility alleles within an HLA-C enhancer element. PloS One. 2013;8(8):e71690.
- Talamonti M, Galluzzo M. Role of the HLA-C*06 allele in clinical response to ustekinumab: evidence from real life in a large cohort of European patients. Br J Dermatol. 2017 Aug;177(2):489–496.
- Papini M, Cusano F, Romanelli M, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: results from extension phase of the SUPREME study. Br J Dermatol. 2019 Aug;181(2):413–414.
- Galluzzo M, D’Adamio S, Silvaggio D, et al. In which patients the best efficacy of secukinumab? Update of a real-life analysis after 136 weeks of treatment with secukinumab in moderate-to-severe plaque psoriasis. Exp Opin Biol Ther. 2020 Feb;20(2):173–182.
- Hundhausen C, Bertoni A, Mak RK, et al. Allele-specific cytokine responses at the HLA-C locus: implications for psoriasis. J Invest Dermatol. 2012 Mar;132(3 Pt 1):635–641.
- Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014 Feb;13(2):397–406.
- Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014 Dec 3;5:5621.
- Kimura K, Wakamatsu A, Suzuki Y, et al. Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes. Genome Res. 2006 Jan;16(1):55–65.
- Steinle A, Li P, Morris DL, et al. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001 May-Jun;53(4):279–287.
- Fania L, Morelli M, Scarponi C, et al. Paradoxical psoriasis induced by TNF-alpha blockade shows immunological features typical of the early phase of psoriasis development. J Pathol Clin Res. 2020 Jan;6(1):55–68.
- Zhu H, Lou F, Yin Q, et al. RIG-I antiviral signaling drives interleukin-23 production and psoriasis-like skin disease. EMBO Mol Med. 2017 May;9(5):589–604.
- Ishizaki M, Akimoto T, Muromoto R, et al. Involvement of tyrosine kinase-2 in both the IL-12/Th1 and IL-23/Th17 axes in vivo. J Iimmunol. 2011 Jul 1;187(1):181–189. (Baltimore, Md: 1950).
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018 Apr;178(4):854–862.
