ABSTRACT
Introduction: Survivin (SVN) is a member of the inhibitor of apoptosis (IAP) protein family that promotes cellular proliferation and inhibits apoptosis. Overexpression of SVN is associated with autoimmune disease, hyperplasia, and tumors and can be used as a biomarker in these diseases. SVN is widely recognized as a tumor-associated antigen (TAA) and has become an important target for cancer diagnosis and treatment.
Areas covered: We reviewed SVN research progress from the PubMed and clinical trials focused on SVN from https://clinicaltrials.gov since 2000 and anticipate future developments in the field. The trials reviewed cover various modalities including diagnostics for early detection and disease progression, small molecule inhibitors of the SVN pathway and immunotherapy targeting SVN epitopes.
Expert opinion: The most promising developments involve anti-SVN immunotherapy, with several therapeutic SVN vaccines under evaluation in phase I/II trials. SVN is an important new immune-oncology target that expands the repertoire of individualized combination treatments for cancer.
1. Introduction
1.1. What is SVN?
SVN is the smallest member of the inhibitor of apoptosis (IAP) protein family. These proteins play a key role in regulating cell mitosis and inhibiting apoptosis. In 1997, Ambrosini cloned the gene for SVN which is located on human chromosome 17 [Citation1]. The wild-type human SVN gene, called BIRC5, spans 147 kb and consists of four exons and three introns. Human SVN is a 16.5-kDa protein with 142 amino acids. SVN contains two functional domains: a baculoviral IAP repeat (BIR) at the N-terminus (100 aa) and an α-helix in the C-terminus (42 aa). Both the BIR and the α-helical regions are involved in regulation of mitosis. In contrast, the apoptosis regulation region only involves the BIR. SVN is found in the nucleus, cytoplasm, mitochondria, exosomes, the outer surface of the cell membrane and in the extracellular matrix. The multiple functions of SVN are affected by its subcellular localization, reversible dimerization, and extensive posttranslational modification including acetylation, ubiquitination, and especially phosphorylation [Citation2].
1.1.1. Nuclear SVN is primarily involved in cell mitosis
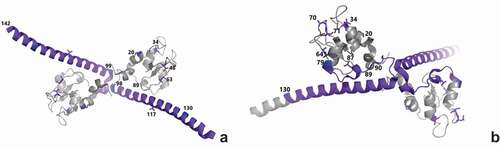
SVN-activated cell mitosis happens in the nucleus and is dependent on the phosphorylation of SVN. The phosphorylation of nuclear SVN at Ser20, Thr34, and Thr48 is important for accurate chromosome alignment and is therefore required for mitosis, cell proliferation, and cell viability [Citation3] (). SVN phosphorylated at Thr117 along with Aurora-B kinase, inner centromere protein (INCENP), and Borealin () regulates chromosomal/microtubule attachment, the spindle checkpoint, and cytokinesis [Citation4,Citation5]. Residues 89–98 bind to the nuclear export protein CRM1 (chromosome region maintenance 1). Acetylation of residues 89–130 promotes SVN dimerization and nuclear translocation. Residues 99–142 interact with microtubules [Citation3]. Ubiquitination at Lys63 regulates chromosome alignment by controlling the dynamic association of SVN with centromeres [Citation6].
(A) Amino acid residues associated with mitosis. Ser20, Thr34, Thr48, and Lys63 regulate chromosome alignment. Thr117 regulates the chromosome/microtubule attachment. Residues 89–98 bind to CRM1. Residues 89–130 localize SVN in the nucleus. Residues 99–142 interact with microtubules. (B) Amino acid residues associated with apoptosis. Ser20 binds to XIAP. Thr34 antagonizes the release of cytochrome C (Cyt-C). Leu64,87 and Asp70,71 suppress the release of SMAC/DIABLO. Residues 79–90 bind with HSP90 and HSP60. Residues 89–130 are involved with nuclear export. (PDB:1e31)
There are several mechanisms for tumorigenesis involving nuclear SVN. A large number of studies have demonstrated that nuclear SVN promotes the repair of DNA double-strand breaks in cancer cells through interaction with the catalytic subunit of DNA-dependent protein kinase (DNA-PKCs) [Citation7] and Ku70 (the DNA repair subunit protein) [Citation8], and reduce DNA damage associated with poly ADP-ribose polymerase (PARP) activation [Citation9]. As a consequence, chromosomal aberrations are increased, and cells overexpressing SVN develop into tumors with shortened latency and decreased apoptosis, promoting adaptive tumor evolution [Citation10].
Alternative splicing of SVN pre-mRNA may result in several different SVN forms exhibiting different characteristics in mitotic control and tumorigenesis [Citation11,Citation12]. In addition, post-transcriptional phosphorylation of SVN plays an important role in its function. Cyclin dependent kinase 1 (CDK1) promotes phosphorylation of SVN at Thr34 during mitosis [Citation13]. Polo-like kinase 1 (PLK1) catalyzes the phosphorylation of SVN at Ser20, and phosphorylated SVN activates Aurora B kinase activity, resulting in the abnormal cytokinesis and chromosomal segregation that are the basis of many solid tumors [Citation14].
1.1.2. Mitochondrial SVN contributes to inhibition of apoptosis in two main ways
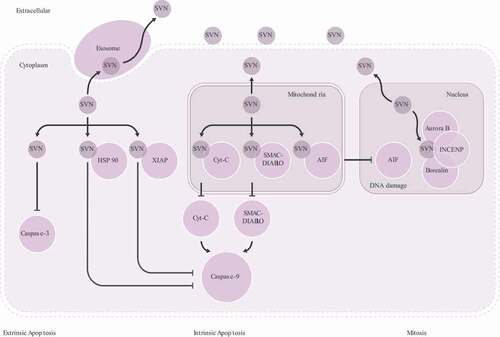
Mitochondrial SVN inhibits apoptosis by binding to apoptosis inducing proteins ().
Two apoptosis inducing proteins, Cyt-C and SMAC (second mitochondria-derived activator of caspase – also known as direct inhibitor of apoptosis-binding protein with low pI, DIABLO) in mitochondria – can be released into the cytoplasm where they participate in formation of the apoptosome complex [Citation15]. This activates pro-caspase-9 leading to induction of apoptosis [Citation16] (). Phosphorylation of mitochondrial SVN at Thr34 antagonizes this release of Cyt-C. Additionally, following phosphorylation at Leu64,87 and Asp70,71, SVN forms a complex with SMAC/DIABLO that suppresses its release from the mitochondria and further inhibits apoptosome formation [Citation2–4,Citation17].
The second mechanism of SVN-mediated suppression of apoptosis involves apoptosis-inducing factor (AIF). AIF is transferred from the mitochondria intermembrane space into the nucleus in response to apoptotic stimuli where it leads to DNA fragmentation. SVN binding with AIF in the mitochondria prevents apoptosis by this caspase-independent pathway [Citation3] ().
1.1.3. Cytoplasmic SVN is derived from both the nucleus and mitochondria and acts to prevent apoptosis
SVN also exists in the cytoplasm where it inhibits apoptosis through directly binding to caspase 3 or by inhibition of caspase 9 through HSP60 and XIAP (). Deacetylation of residues 89–130 in SVN is necessary for formation of monomeric SVN which is the form required for nuclear export and inhibition of apoptosis [Citation2]. When released from mitochondria, SVN lacking phosphorylation at Ser20 can bind with the X-linked inhibitor of apoptosis (XIAP). This SVN-XIAP complex increases the stability of XIAP, leading to increased inhibition of caspase-9-dependent apoptosis [Citation4,Citation18]. Phosphorylation at Thr48 is also critical to the anti-apoptotic roles of SVN [Citation3]. HSP90 and HSP60 bind with SVN at residues 79–90 and act as molecular chaperones, increasing SVN stability and its inhibition of apoptosis () [Citation4,Citation19,Citation20].
1.1.3. SVN is distributed in regenerative tissues and organs
SVN is widely expressed in embryonic and fetal organs where it promotes mitosis and cellular proliferation [Citation21]. In adult organisms, SVN participates in the generation of bone marrow stem cells, thymocytes [Citation22] and the differentiation of erythrocytes [Citation23]. SVN is also required for the differentiation of leukocytes and the survival and maturation of dendritic cell (DC) progenitors [Citation24,Citation25]. SVN plays an essential role in organ regeneration after resection and promotes epithelial cell proliferation [Citation26].
SVN undergoes regulated partitioning between the nucleus, mitochondria, cytoplasm, exosome and extracellular matrix. Nuclear SVN binding with Aurora-B, INCENP, and Borealin regulates mitosis. Mitochondrial SVN binding with Cyt-C or SMAC/DIABLO and cytoplasm SVN binding with HSP 90 or XIAP inhibits apoptosis through binding to pro-caspase-9 (intrinsic apoptosis). Mitochondrial SVN binds to AIF resulting in inhibition of DNA damage. Cytoplasm SVN inhibits apoptosis by binding to caspase-3 (extrinsic apoptosis). Cytoplasmic SVN can be released to extracellular matrix via exosome trafficking.
1.2. Why is SVN important?
SVN is important as it is specifically expressed in tumor tissues where it upregulates mitosis and cell survival while downregulating apoptosis and cell death. Since SVN promotes cell proliferation and inhibits apoptosis, it is thought that the unlimited growth of cancer cells may to some extent result from these functions of SVN. In 1998, during the Cancer Drug Screening Program at the National Cancer Institute (USA), an anti-SVN monoclonal antibody test found SVN expression in 60 human tumor cell lines [Citation27]. Clinical trials in the last 20 years have detected high expression of SVN in most cancer patients including melanoma [Citation28–30], pancreatic cancer [Citation31,Citation32], colon cancer [Citation33,Citation34], cervical cancer [Citation35,Citation36], lung cancer [Citation37,Citation38], bladder cancer [Citation39,Citation40], diffuse large B-cell lymphoma [Citation41,Citation42], and acute myeloid leukemia [Citation43,Citation44] among others. This is in marked contrast to the absence or low expression of SVN in healthy, differentiated cells.
This high correlation between cancer and SVN expression highlights SVN as a promising cancer biomarker, both for diagnosis/prognosis and treatment.
2. SVN as a biomarker for diagnosis/prognosis
2.1. SVN as biomarker for tumor progression
Upregulated SVN is correlated with cancer progression and poor prognosis in patients across a wide range of tumor types including glioma [Citation45], breast cancer [Citation46,Citation47], cervical squamous cell carcinoma (CSCC) [Citation48], acute myeloid leukemia (AML) [Citation49], gallbladder cancers (GBC) [Citation50], renal cell carcinoma (RCC) [Citation51], diffuse astrocytic tumors [Citation52], and diffuse large B-cell lymphoma [Citation42].
Across these studies, SVN overexpression (mRNA and/or protein) was significantly associated with advanced tumor stage, poor tumor differentiation, and bad overall survival. Moreover, the subcellular localization of SVN is correlated with the classification of tumor tissue subtypes.
The diagnostic use of SVN has been assessed in a number of clinical trials. For example in NCT00315653, urinary SVN mRNA was evaluated as a diagnostic marker for bladder cancer (tinyurl.com/yd7tedd7). In NCT02016833, SVN, together with WT-1 and HPV16 E7 were used for the assessment of specific immune responses (tinyurl.com/y8luvv8v).
As overexpression of SVN induces abnormal cell proliferation, SVN expression has proved to be a useful marker of hyperplasia in conditions such as benign prostatic hyperplasia [Citation53], endometrial polyps [Citation54], colon polyps [Citation55], and mesothelial hyperplasia [Citation56].
2.2. SVN as a biomarker for metastasis
SVN is expressed in almost all cancers. During cancer development and progression, high levels of SVN expression result in many unfavorable consequences including the stimulation of cancer cell proliferation, inhibition of apoptosis, induction of tumor stromal angiogenesis and reduction in the sensitivity of cancer cells to radiotherapy and chemotherapy. Collectively, these effects drive tumor metastasis. SVN expression levels are associated with lymph node metastasis in cervical cancer [Citation57] and research has shown that breast cancer stem cells carrying an SVN gene knockout lost their metastatic properties and underwent apoptosis [Citation58]. Furthermore, knocking down SVN by siRNA significantly decreases clonogenicity, migration, and invasion, suppressing tumor growth and lymph node metastasis in a mouse model of cervical cancer [Citation59], while high expression of SVN was found in the metastatic lymph node tissues in thyroid papillary carcinoma [Citation60].
Thus, there is compelling evidence over many studies of different tumor types for strong positive correlation between expression of SVN and tumor metastasis, suggesting that SVN might be a suitable biomarker to monitor progression of malignancy.
2.3. SVN as a biomarker for autoimmune and inflammatory diseases
In addition to cancer, SVN is overexpressed in autoimmune and inflammatory diseases. In autoimmune disease, SVN expression prevents apoptosis in autoreactive lymphocytes and increases the production of autoantibodies and cytokines. High SVN levels have been detected in many autoimmune and inflammatory diseases including rheumatoid arthritis (RA) [Citation61,Citation62], myasthenia gravis (MG) [Citation63], multiple sclerosis (MS) [Citation64], systemic lupus erythematosus (SLE) [Citation65], inflammatory bowel disease (IBD) [Citation66], and systemic sclerosis (SSc) [Citation67]. In NCT03444623, SVN was assessed both as a biomarker for predicting the risk of developing rheumatoid arthritis (RA) and as a prognostic marker for joint destruction in established RA (tinyurl.com/y8fgj8zc). The results show that, along with other clinical symptoms and serological measurements, testing serum SVN is helpful to identify those patients who will progress from arthralgia to full RA [Citation68]. In NCT03440892, serum SVN was tested as a possible companion diagnostic to assess therapeutic response in RA (tinyurl.com/ycetvvnk).
2.4. Combination of SVN test with other biomarkers
Other biomolecules besides SVN are already used extensively as biomarkers for cancer prediction or prognosis. Well-known examples include EGFR in molecular apocrine breast cancer (MABC) [Citation69], VEGF in colorectal cancer [Citation70] and oncogenic BRAF mutation [Citation71] and KRAS mutations in pancreatic cancer [Citation72]. Since SVN is also upregulated in autoimmunity and inflammation, for tumor diagnosis, it is necessary to combine an SVN test with other biomarkers. For example, determination of the levels for SVN and VEGF contributes to higher accuracy of noninvasive diagnosis [Citation73]; dual over-expression of SVN and VEGF in peripheral blood is a valuable prognostic marker in patients with squamous carcinoma of the larynx [Citation74]; and expression of both SVN and EGFR is useful in the diagnosis of triple-negative breast cancer (TNBC) [Citation75]. Combined expression of Aldh1, SVN and EpCAM is a prognostic factor for survival and tumor recurrence in colon cancer patients [Citation76]. Finally, SVN is likely to be used as a biomarker alongside p53 and cMyc in a combined test for autoantibodies characteristic of cancer in general [Citation77] and small-cell lung cancer in particular [Citation78].
In conclusion, SVN is a potentially useful marker for cancer and inflammatory disease, indicative of stage or the risk of progression. It is likely to be used in combination with other markers to improve the sensitivity and specificity of such tests.
3. SVN as a target for cancer treatment
Although the number of publications on SVN has declined slightly since a peak in 2015, the field remains extremely active with a total of nearly 9,000 publications to date. This extensive body of fundamental research has laid the foundation for clinical studies on cancer treatment targeting SVN. However, as SVN was only discovered in 1997 all ongoing clinical studies are still at a relatively early stage (phase I or phase II). We retrieved a list of ongoing SVN-related clinical trials from ClinicalTrials.gov and these are summarized in .
Table 1. Ongoing clinical trials on SVN as therapeutic interventions in patients (Source: ClinicalTrials.gov)
The clinical studies mainly fall into two treatment categories: (1) small molecule-based inhibitors aimed at direct inhibition of SVN or SVN expression and (2) immunotherapy-targeting tumor cells expressing SVN.
3.1. Small molecule inhibitors of SVN
Here, the general goal of an effective treatment targeting SVN is to reduce its expression or activity. A common approach is to suppress transcription or translation of SVN and its functionally related molecules including HIF-1α, Sp1/Sp3, PI3K/Akt, KLF5, NF-κB, STAT3, E2F, β-catenin, and IL-4 [Citation37,Citation79]. These molecules either interact with SVN or upregulate SVN expression. Other important regulatory proteins such as p53, YY1, Egr-1, and Rb downregulate SVN transcription and prevent SVN expression [Citation80].
Overexpression of interleukin enhancer-binding factor 3 (ILF3/NF110) enhances the promoter for the SVN gene(BIRC5). This effect makes ILF3 an additional SVN target. Small molecules targeting the above-mentioned genes and their products have been studied both in vitro and in clinical trials. Sepantronium bromide (YM155), a small molecule inhibitor of SVN, exerts its effect by direct binding to ILF3/NF110, attenuating SVN gene transcription in a concentration-dependent manner [Citation81]. YM155 downregulates SVN expression and induces apoptosis in human hormone-refractory prostate cancer (HRPC) cell lines [Citation82] and non-small-cell lung cancer (NSCLC) cell lines in nude mice [Citation83]. The safety and efficacy of YM155 when combined with carboplatin (C) and paclitaxel (P) was examined in patients with advanced NSCLC in a phase I/II study (NCT01100931). The triple combination exhibited a favorable safety profile but failed to demonstrate an improvement in the response rate [Citation84]. Another phase I study was conducted to evaluate the safety and pharmacokinetics of YM155 in combination with the tyrosine kinase inhibitor erlotinib in patients with EGFR TKI refractory advanced NSCLC. The results suggest that inhibiting SVN is a potential therapeutic target in this defined group of patients [Citation85].
EZN-3042, an SVN mRNA antagonist developed by Enzon Pharmaceuticals Inc., is a modified RNA oligonucleotide in which the ribose moieties are ‘locked’ in the 3ʹ endo conformation with a methylene bridge [Citation86,Citation87]. Hybridization of EZN-3042 to SVN mRNA blocks translation and promotes tumor cell apoptosis by inhibiting SVN-dependent anti-apoptotic activity. An initial phase I study (NCT01186328) investigated EZN-3042 dose escalation and safety in children with relapsed acute lymphoblastic leukemia (ALL). Although EZN-3042 was well tolerated after single application, dose-related toxicity was observed on introduction of the combined chemotherapy, resulting in termination of the study [Citation88].
Both YM155 and EZN-3042 demonstrated inhibition of SVN in cancer cell lines. Although YM155 was safe and well tolerated in a phase I clinical trial, the efficacy of the compound has not yet been confirmed in advanced clinical trials. In contrast, EN-3042 has shown signs of efficacy, but the sample size of the clinical trial was small and there was overt toxicity when combined with other chemotherapies.
The other effective treatment to reduce the expression or activity of SVN is the use of antisense oligonucleotide (ASO) technology. LY2181308, an antisense molecule able to inhibit or block SVN expression, demonstrated tolerable toxicity and therapeutic responses when used as a single agent in early-phase clinical trials [Citation89,Citation90]. A further trial (NCT00620321) using LY2181308 as a single agent in patients with AML also showed that it was well tolerated. Used in combination with cytarabine and idarubicin, LY2181308 has shown a good safety profile and some clinical benefit [Citation91]. However, the combination of LY2181308 with docetaxel did not show significant differences in efficacy over docetaxel alone in patients with NSCLC (NCT01107444), despite a concomitant increase in side effects including neutropenia, anemia, thrombocytopenia, and sensory neuropathy [Citation92].
Tolfenamic acid (TA) has been shown anticancer activity in several cancer models, inhibiting both tumor growth and angiogenesis. As a small molecule inhibitor, it induces the degradation of specificity proteins (Sps), leading to a decrease in expression of SVN in various in vitro and in vivo tumor settings such as pancreatic cancer cells [Citation93,Citation94], human medulloblastoma (MB) cell lines, a mouse xenograft model [Citation95], Ewing sarcoma (ES) cells [Citation96], and colon cancer cells [Citation97]. Though these findings point to the use of TA as a potential cancer treatment, a clinical trial of TA in pancreatic cancer in 2014 was withdrawn without a published reason (NCT02159248).
Mithramycin A (MIT-A), a DNA and RNA polymerase inhibitor, was shown to reduce the expression of SVN in in vitro and in vivo studies in Drosophila and human cells [Citation98], ES cells [Citation99] and an xenograft model of cervical cancer [Citation100].
A 2017 clinical trial of MIT-A in ES patients showed no clinical response and the trial was terminated early (NCT01610570). Subsequent analysis showed that the average plasma concentration of MIT-A after administration at the maximum safe dose was substantially below the concentration required for on-target effect [Citation101].
In summary, the performance of small molecules of anti-SVN drugs has fallen short of expectations based on cell line data and animal models. None have performed satisfactorily in phase I/II clinical trials, displaying either lack of significant clinical benefits (LY2181308, YM55), or emergence of drug resistance mutations coupled with side effects and off-target toxicity (EZN-3042, mithramycin, anti-EGFR) [Citation102,Citation103].
There are a number of possible reasons for the failure of the small molecule strategy:
First, most anti-SVN small molecules are dependent on the inhibition of the SVN signaling pathway. These effects could be bypassed by compensatory up-regulation of other pathways involving IAP family proteins or the induction of different isoforms of SVN resulting from alternative splicing. Second, small molecule drugs can cause toxicity in humans due to on-target or off-target toxicity. The anti-SVN small molecules produced so far are perhaps not specific enough to discriminate between these two possibilities.
Going forward, the strategy should therefore be to design more specific ‘purpose-built’ inhibitors of SVN, and carry out more research on the compensatory pathways to identify synergistic combination treatments or dual inhibitors. In this way, it might be possible to identify an agent that kills SVN expressing cells directly, reducing the potential for the emergence of escape mutations.
3.2. Immunotherapies targeting SVN
Immunotherapies for cancer has shown great promise in recent years. For targeting a TAA like SVN, any strategy should include at least T cell-based immunotherapy, along with antibody-based immunotherapy if the TAA (SVN) is expressed on the surface of tumor cells. The modes of SVN immunotherapy used so far in clinical trials include cancer vaccines; adoptive dendritic cell (DCs, antigen presenting cells) based vaccines and combinations of different immunotherapies. Although several reports describe the expression of SVN on tumor cell surface, antibody-based anti-SVN immunotherapies (including CAR-T therapy) have not as yet translated into clinical trials.
The following subsections review the T cell–based vaccines against SVN, DC-based vaccines, and combination therapies.
3.2.1. Vaccines targeting SVN peptides
In cancer cells, overexpressed SVN can be degraded into peptides by cytoplasmic proteases. Antigenic SVN peptides then associate with major histocompatibility complex I (MHCI) in the cytoplasm. This SVN-MHCI complex is subsequently presented on the outer surface of tumor cells via the exosome pathway () [Citation104,Citation105]. This SVN-MHCI complex can also be present and/or cross-presented by antigen presenting cells (APC) allowing recognition by CD4+ and/or CD8 + T cell receptors (TCR). This leads to activation of naïve T cells bearing TCRs with the appropriate specificity, stimulating their clonal expansion and differentiation into effector cells that can kill tumor cells displaying the SVN-MHC complex.
Elevated SVN expression by tumor cells is an adaptation that allows them to avoid apoptosis. Although this might be expected to expose cancer cells to normal immune surveillance as described above, there is considerable evidence that the immune system in cancer patients is inhibited from recognizing SVN epitopes, allowing tumor cells to escape killing. However, the overexpression of SVN in tumors compared to normal tissue makes it a good target for immunotherapy aiming to break this tolerance. Vaccines targeting SVN might therefore be an efficient strategy to activate lymphocytes and kill the tumor cells with high SVN expression.
DPX-Survivac, developed by IMV, is a T cell activating vaccine comprising SVN peptides in a proprietary oil-based delivery system (DPX). The vaccine induces naïve T cells to differentiate into polyfunctional antigen-specific CD4+ T helper and CD8+ cytotoxic T lymphocytes (CTLs). The responsive immune cells can then target endogenously produced SVN epitopes presented by human leukocyte antigen (HLA) class I [Citation106]. DPX-Survivac creates a depot at the injection site from which the SVN peptides and adjuvant are released, provoking a sustained immune response against cancer cells expressing SVN, resulting in reduced tumor cell proliferation and an increase in tumor cell death.
A phase I/II trial was carried out to determine the safety and immunogenicity of DPX-Survivac when co-administered with low dose oral cyclophosphamide in 30 patients with ovarian cancer (NCT01416038). DPX-Survivac was well tolerated and led to a sustained and high immune response against tumor cells. The phase II DeCidE1 stage (NCT02785250) treated 19 patients with advanced recurrent ovarian cancer. Of these, 79% of patients achieved disease control, 53% experienced tumor regressions and 37% achieved durable clinical benefit lasting ≥ 6 months. These results demonstrate that DPX-Survivac is a well-tolerated and effective treatment with limited side effects [Citation107]. Additional clinical studies investigated the safety and efficacy of DPX Survivac in combination with immune adjuvants. These studies mainly focused on ovarian, fallopian tube cancer, peritoneal cancer and B-cell lymphoma (DLBCL). DPX-Survivac was granted fast track application by the FDA as a maintenance therapy in advanced ovarian, fallopian tube and peritoneal cancers. The vaccine also received orphan drug status from the FDA and the European Medicines Agency as a treatment for all stages of ovarian cancer.
SurVaxM (SVN53-67/M57-KLH) is a 15 amino acid peptide encompassing human SVN amino acid residues 53–67 carrying a substitution of cysteine to methionine at position 57. This modification significantly increases the affinity of the peptide for MHC on DCs. Keyhole limpet hemocyanin (KLH), a vaccine adjuvant, is conjugated to SVN53-67/M57 by maleimide coupling to the N-terminal cysteine [Citation108]. This extended peptide encompasses seven T cell epitopes, providing for effective presentation by at least four HLA-A alleles and at least one B cell epitope allowing assessment of humoral responses to the administered antigen. Studies of murine glioma in vivo and human glioma cells ex vivo have demonstrated that SurVaxM stimulates an effective antitumor immune response by activating SVN-specific CD8 + T cells, stimulating SVN-specific CD4+ T cell proliferation and providing tumor-specific CD4+ helper support [Citation109].
NCT02334865 was a phase I safety study of SurVaxM administered in incomplete Freund’s adjuvant with GM-CSF in patients with newly diagnosed multiple myeloma receiving lenalidomide maintenance therapy. In NCT01250470, SurVaxM was evaluated in patients with histologically confirmed SVN-positive malignant gliomas that had recurred or progressed after therapy. The vaccine was administered with sargramostim (recombinant human GMCSF) and Montanide ISA51 as adjuvants. The combination was well tolerated, and six of eight patients developed humoral and cellular responses, with three patients exhibiting a partial response or stable disease for over 6 months [Citation109].
Other clinical trials of SurVaxM investigating combinations with a range of adjuvants such as sargramostim, temozolomide, ISA-51 or Sandostatin LAR include NCT02455557 (sargramostim) and NCT03879694 (ISA-51, sargramostim, and Sandostatin LAR).
Single or pooled peptides also show promise as the basis of cancer vaccines. However, they have certain drawbacks, such as quality control during the manufacturing process and regulatory uncertainty over whether pooled peptides will be regarded as a single agent. The development of an ROP-SVN protein-based vaccine is a variation on the use of pool peptides that should overcome the problem. The approach involves identifying a set of overlapping peptides covering the whole sequence of a target protein and linking them to generate a recombinant overlapping peptide (ROP) containing multiple T cell epitopes [Citation110,Citation111]. Furthermore, ROP-SVN is an artificial protein without a native conformational, and should therefore be devoid of SVN function. Thus, in contrast to native SVN, ROP-SVN will not inhibit apoptosis. We have shown that ROPs stimulate stronger CD4+, CD8+ T cell responses than the native antigen [Citation112]. A phase I clinical trial with ROP-SVN is planned.
3.2.2. Dendritic cell (DC) vaccines targeting SVN
DCs are the most important antigen-presenting cells (APC) in the mammalian immune system. For the most part, DCs exist in an immature state, both in the circulation or resident in tissue. They only mature and become activated after phagocytosis and processing of antigens. DCs exhibit high expression of the MHC molecules which present processed antigens to T cells in the form of an antigen-MHC complex. This complex can activate naïve T cells through antigen-specific engagement with the T cell receptor. Activated T cells then differentiate to become specific CD4+ T helper cells or specific CD8+ cytotoxic T cells (CTL) that suppress infection or tumor growth. The significance of this pathway is reflected in the fact that over 60% of immunotherapy clinical trials using SVN have focused on a DC strategy to stimulate strong and specific anti-tumor T cell responses ().
CVD908ssb is an attenuated Salmonella strain commonly used as a bacterial vector to deliver TAAs into the cytosol of APCs in situ. Xin Xu et al. constructed a CVD908ssb vector encoding SVN [Citation113]. They found that CVD908ssb-TXSVN infected DCs induced antigen-specific CD8+ T cell responses in vitro and in vivo. Vaccination with CVD908ssb-TXSVN induced potent antitumor activity in murine models of neuroblastoma and lymphoma. This finding is being clinically evaluated in, a first-in-human study of the CVD908ssb-TXSVN vaccine (NCT03762291). This phase I trial, in multiple myeloma patients, started in 2019 and is scheduled to complete in 2022.
Established tumors can evade immune-detection of SVN expression by limiting SVN availability to DCs – for example by reducing MHC class I expression and causing more generalized immunosuppression – by secreting factors like IL10 and VEGF that inhibit DC maturation or recruit myeloid-derived suppressor cells (MDSC). Together, these mechanisms prevent the generation of SVN antigenic peptide-MHC complexes and stimulation of SVN specific T-cells. An innovative strategy to bypass this evasion is to derive DCs from the monocytes of cancer patients which can then be loaded ex vivo with TAAs such as SVN. The mature DCs are then infused back into patients, where the SVN-MHC complex is presented to naïve T cells, eliciting CD8+ CTL and CD4+ T helper responses to recognize and kill the tumor cells. This ex vivo targeting of DCs is more effective than relying on in vivo immunization.
The introduction of TAA genes into a bacterial or viral vector has been widely explored as a way to create vaccines. This approach is commonly used in a prime-boost strategy where different vectors encoding the same target TAA gene are used separately for priming and boosting.
Adenovirus is a non-enveloped double stranded DNA virus. Its high infection efficiency, episomal nature, and ability to infect a broad range of both replicating and nonreplicating cells mean that it has been widely used as a vector for gene therapy applications and for recombinant vaccines. The first use of an adenoviral vector carrying the SVN gene as an immunotherapeutic vaccine targeting DCs was in prostate cancer. DCs were collected from prostate cancer patients and transduced with an adenoviral vector directing expression of a full-length SVN gene. The T-cell response against SVN peptides was tested after three rounds of ex vivo stimulation. The results demonstrated that DCs transduced with SVN can induce SVN-specific CTL responses and kill tumor cells [Citation114].
NCT02851056 is an early phase I trial evaluating the safety, efficacy and biological activity of a vaccine called Dendritic Cell Survivin Vaccine (DC: AdmS) in multiple myeloma patients undergoing autologous hematopoietic cell transplants (HCT). The vaccine is made using DCs isolated from the patient’s own blood transfected with a replication deficient adenoviral vector carrying the SVN gene. Between 7 and 30 days after the first vaccination with DC: AdmS, participants are given granulocyte colony-stimulating factor (G-CSF) to mobilize progenitors from the bone marrow. CTLs and stem cells are then collected by apheresis. Post-chemotherapy, T cells and CD34 progenitor cells are returned to the patient. Patients receive a second round of DC: AdmS vaccination 20–34 days after HCT.
3.2.3. Combining DC therapy with other vaccines
The epithelial-expressed glycoprotein mucin 1 (MUC1) is another important tumor-associated antigen. A recombinant adenovirus vaccine expressing both MUC1 and SVN (Ad-MS) was evaluated in a mouse model of melanoma [Citation115]. The results showed that Ad-MS immunization enhanced CTL responses to a combined SVN/MUC1 DNA vaccine nearly two-fold. This was further enhanced by nearly 60% when vaccination was combined with IL-2 as an adjuvant to promote T cell proliferation. The authors proposed that the combined strategy may be effective in breaking through immune tolerance in melanoma.
NCT02688673, NCT02693236, and NCT02688686 were phase I/II immunotherapy studies in patients with small-cell lung cancer, esophageal cancer, and advanced non-small-cell lung cancer, respectively. The intervention in all cases involved treatment with DC cells infected with an adenoviral vector carrying SVN and MUC1 genes combined with cytokine induced killer cells. The current status of these Chinese studies is unclear, with no published results.
Telomerase (TERT) is a ribonucleoprotein complex that adds short repeat sequences to the end of chromosomes, protecting them from damage and allowing replication to extend to the end of the unique sequences carried by the chromosome. Human TERT (hTERT) is active in stem cells and most cancer cells, but absent from most somatic cells. The lack of TERT in somatic cells accounts for the progressive loss of telomere ends at each round of cell division. As around 85% of human cancers express hTERT, it is an attractive target for cancer immunotherapy. Jointly targeting SVN and hTERT may therefore minimize immune escape due to antigen loss by cancer cells.
An early clinical study evaluated vaccination with DCs pulsed with p53, SVN, and hTERT peptides in patients with malignant melanoma. The results showed that the vaccination was feasible and safe, and led to stabilization of disease in 24% of patients [Citation116]. Following this study, a phase 1/2 trial in patients with malignant melanoma (NCT00197912) used DCs pulsed with p53, SVN, and hTERT as a vaccine in combination with IL-2, cyclophosphamide and the NSAID celecoxib to dampen immunosuppressive mechanisms. The combined treatment was safe and well tolerated. The fraction of patients with stable disease more than doubled and 6-month survival significantly increased compared to the previous trial [Citation116]. Clinical trials like NCT00573495, NCT00961844, NCT01456065, NCT01334047 are examples of other autologous DC vaccines targeting hTERT/SVN with different adjuvants in various types of cancer. These four phase I/II studies have no results posted.
Transfecting DCs with RNA is another effective way of achieving antigen presentation. NCT00074230 was a phase I/II trial evaluating vaccination with autologous DCs transfected with RNAs encoding TAAs for Melan-A, MAGE-3, and SVN antigen in patients with stage IV cutaneous melanoma. Pulsing the DCs with KLH to enhance antigen presentation was tested in a subset of patients. In this trial, some of the patients achieved full remission and/or survived for more than 10 years, though two patients eventually developed asymptomatic sarcoidosis following a durable response to therapy [Citation117].
In summary, immunotherapies targeting SVN is still at an early stage. The advantage of immunotherapy over small molecule-based therapies is that they directly induce tumor cell death rather than indirectly through signal pathway dysregulation. This is reflected in the more prosmising results shown by immunotherapies in clinical trials, though there is still much that needs to be done to optimize SVN immunotherapy.
4. Conclusions
SVN plays an important role in cell proliferation and mitosis throughout embryonic development and in adult stem cells. The fact that most tumors overexpress this molecule to support their growth and metastasis makes it a promising biomarker for diagnosis and therapy. In keeping with this, several clinical trials are underway to test its value as a diagnostic or prognostic marker of tumor progression and metastasis. In addition to its role in cancer diagnosis, SVN is also being tested as diagnostic/prognostic marker for autoimmune and proinflammatory diseases. Note that because SVN is a marker for both cancer and autoimmunity, it will be important to use it in combination with other diagnostic markers to distinguish malignancy from inflammatory disease.
SVN is a promising target for cancer therapy because, while it is effectively absent from normal tissues, it is overexpressed in most tumor types. Despite this promising observation, the development of anti-SVN therapies for cancer has been slow, though there has been some progress. Various modes of treatment have been tested, ranging from small molecule inhibitors to immunotherapies. The small molecule approach is hindered by low efficacy and high toxicity. However, SVN immunotherapies have shown more promise, with significant efficacy in cancer cell lines and in mouse models. Although clinical trials with anti-SVN agents are at an early stage, there are already some preliminary indications of efficacy with meaningful clinical benefit to patients.
Given the distinct role in cancer biology played by SVN, there is still room for optimism that anti-SVN immunotherapy will contribute to useful therapies. However, this is most likely to be as part of a combination approach rather than as a stand-alone therapy. Such combinations are likely to comprise multiple TAA and delivery technologies, additional immunotherapies (such as immune checkpoint inhibitors) and small molecule drugs such as kinase inhibitors targeted to tumor specific characteristics. Such a multi-agent approach should create synergies that lead to dose reductions, minimize toxicity, and allow therapy to be optimized for both tumor and patient.
A key issue is that many of the approaches discussed here are complex interventions that involve ex vivo interventions and/or autologous cell isolation, treatment and infusion. Such treatments are likely to be difficult to operate at scale and expensive. In this context, more straightforward immunization strategies based on peptides or recombinant proteins – such as ROP-SVN – stand out as more likely to be robust and practical approaches.
4.1. Expert opinion: survivin as a biomarker for diagnosis and therapy
Survivin (SVN) is both an attractive biological marker for diagnosis and a compelling target for therapy in both autoimmune diseases and cancer.
This is particularly true for cancer, where SVN expression is associated with advanced tumor stage, poor tumor differentiation and low overall survival. These findings strongly support the use of SVN both as a prognostic biomarker of cancer progression and the basis of a companion diagnostic to monitor anti-SVN therapy.
| 1. | Survivin as a biological marker for diagnosis | ||||
Measurement of SVN will allow prognosis of cancer severity and metastasis. Since SVN is expressed in most tumor types, it will serve as a versatile diagnostic platform for use in multiple tumor indications. However, one caveat is that other biomarkers need to be used alongside SVN to exclude the possibility that high SVN expression is due to autoimmune disease.
| 2. | Survivin as a biological target for treatment | ||||
SVN is also an attractive biomarker for treatment. At the moment, the strategies targeting SVN focus on (1) small molecules that inhibit or trigger degradation of SVN, (2) immunotherapy such as DC therapy targeting SVN epitopes, (3) cancer vaccines using SVN or SVN-derived peptides. Representatives of all three approaches have shown initial evidence of efficacy in clinical trials.
Given the interest in SVN, it is surprising that there is still uncertainty over its subcellular location. While most studies find SVN is located predominantly intracellularly and in the nucleus, it has also been reported that SVN can be found on the cell surface. If this is the case, it will enable a new class of SVN diagnostics and open the way to antibody and CAR-T-based therapies.
| 2. | One safety issue and its solution | ||||
There is a theoretic but important safety consideration relating to SVN-based therapies, particularly subunit vaccines and/or SVN expressed vector-based vaccines. In both cases, artificially elevating cellular SVN levels might inhibit cell death and enhance cell division, leading to hyperplasia and increasing the risk of developing new primary tumors.
One possible way to avoid this is to use a nonfunctional version of SVN, such as a recombinant overlapping peptide (ROP) as the immunogen instead of the wild-type protein. In an ROP construct, a synthetic gene is created that encodes a complete set of overlapping peptides derived from the native wild-type protein sequence. Each component peptide overlaps its adjacent sequences by 10–20 amino acids, linked by a short recognition sequence for a lysosomal protease. The resultant ROP therefore encodes all T cell epitope information from the wild-type protein, along with some B cell epitopes. However, the wild-type conformation will be comprehensively disrupted, and the ROP will lack the functions of the native protein. Once an ROP enters antigen presenting cells, either through phagocytosis (subunit vaccines) or expressed exogenously after delivery by a vector (vector-based vaccines), the ROP will be cleaved into fragments and presented to stimulate T and B cells. Thus, ROPs represent a powerful immunogenic vaccine platform that lacks the safety concerns associated with expression of native antigens – such as SVN – with intrinsic physiological functions.
| 2. | Future perspective | ||||
The development of immune-oncology approaches has moved us closer to the day when cancer will be viewed as a manageable disease. However, the multiple routes of cancer development and progression mean that no single therapeutic approach will be sufficient to achieve control. Even if tumor growth is inhibited, cancer cells will always evolve to escape monotherapy. Ideally, cancer treatment will need simultaneously to target multiple pathways driving the proliferation, survival and spread of cancer cells. To be effective, therefore, anti-SVN therapy will need to be combined with agents that target other established cancer biomarkers such as EGFR and HER-2. Responses should be augmented with immune-oncological agents such as check point inhibitors or agonists of co-stimulatory receptors such as 4-1BB. Finally, agents such as angiokinase inhibitors that target tumor (lymph)angiogenesis and the tumor microenvironment will potentiate cancer cell killing and reduce the opportunity for tumor escape. Nonetheless, the identification of SVN has provided another important therapeutic target that can be exploited by the rapidly expanding arsenal of targeted immune-oncology modalities.
Article highlights
SVN is found in multiple cellular compartments and in the extracellular matrix. Through activation of mitosis and inhibition of apoptosis, SVN promotes cell proliferation and survival.
SVN is highly expressed in tumor tissues where it upregulates mitosis and enhances cell survival by downregulating apoptosis and cell death.
SVN is a biomarker for tumor metastasis and progression and several clinical trials have demonstrated its value in cancer diagnosis and prognosis of tumor progression and metastasis.
Clinical trials on small molecules to target SVN have not proved successful. One possible reason for this is that inhibition of SVN may lead to compensation mechanisms involving signaling by other IAP family members.
In contrast, immunotherapies targeting SVN are starting to show significant potential. Strategies under evaluation include peptide vaccines, a recombinant overlapping peptide vaccine, dendritic cell-based vaccines and combination therapies.
To avoid selection of tumor progression through expansion of SVN non-expressing clones, future treatments should target multiple tumor biomarkers in addition to SVN.
Declaration of interest
Dr Shisong Jiang receives grants from Oxford Vacmedix UK Ltd and CBI who are spin-outs from University of Oxford and develop cancer vaccines against survivin. Dr Mark Edwards reports personal fees from Oxford Vacmedix UK Ltd, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Ambrosini G, Adida C, Altieri DC., A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921.
- Altieri DC. Survivin - The inconvenient IAP. Semin Cell Dev Biol. 2015;39:91–96.
- Nogueira-Ferreira R, Vitorino R, J Ferreira-Pinto M, et al. Exploring the role of post-translational modifications on protein-protein interactions with survivin. Arch Biochem Biophys. 2013;538(2):64–70.
- Hu F, P.d., Zheng W, et al. Elucidating respective functions of two domains BIR and C-helix of human IAP survivin for precise targeted regulating mitotic cycle, apoptosis and autophagy of cancer cells. Oncotarget. 2017;8(69):113687–113700.
- Silke J, Vaux DL. IAP gene deletion and conditional knockout models. Semin Cell Dev Biol. 2015;39:97–105.
- Qp V. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 2005;310(5753):1499–1504.
- Gullulu O, Hehlgans S, E Mayer B, et al. A spatial and functional interaction of a heterotetramer Survivin-DNA-PKcs complex in DNA damage response. Cancer Res. 2021. DOI:https://doi.org/10.1158/0008-5472.CAN-20-2931.
- Hu S, Qu Y, Xu X, et al. Nuclear survivin and its relationship to DNA damage repair genes in non-small cell lung cancer investigated using tissue array. PLoS One. 2013;8(9):e74161.
- Ju L, Zhang X, Deng Y, et al. Enhanced expression of Survivin has distinct roles in adipocyte homeostasis. Cell Death Dis. 2017;8(1):e2533.
- Conde M, Susanne Michen, Ralf Wiedemuth, et al. Chromosomal instability induced by increased BIRC5/Survivin levels affects tumorigenicity of glioma cells. BMC Cancer. 2017;17(1):889.
- Adamopoulos PG, Tsiakanikas P, Adam EE, et al. Unraveling novel survivin mRNA transcripts in cancer cells using an in-house developed targeted high-throughput sequencing approach. Genomics. 2021;113(1 Pt 2):573–581.
- Zhang M, Yang J, Li F. Transcriptional and post-transcriptional controls of survivin in cancer cells: novel approaches for cancer treatment. J Exp Clin Cancer Res. 2006;25(3):391–402.
- Chen X, Duan N, Zhang C, et al. Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J Cancer. 2016;7(3):314–323.
- Chu Y, Yao PY, Wang W, et al. Aurora B kinase activation requires survivin priming phosphorylation by PLK1. J Mol Cell Biol. 2011;3(4):260–267.
- Paul A, Krelin Y, Arif T, et al. A new role for the mitochondrial pro-apoptotic PROTEIN SMAC/diablo in phospholipid synthesis associated with tumorigenesis. Mol Ther. 2018;26(3):680–694.
- Du C, M.f., Li Y, et al. Smac, a mitochondrial protein that promotes cytochrome c–dependent caspase activation by eliminating IAP Inhibition. Cell. 2000;102(1):33–42.
- Khan Z, Khan AA, Yadav H, et al. Survivin, a molecular target for therapeutic interventions in squamous cell carcinoma. Cell Mol Biol Lett. 2017;22(1):8.
- Dharmapatni AA, Smith MD, Findlay DM, et al. Elevated expression of caspase-3 inhibitors, survivin and xIAP correlates with low levels of apoptosis in active rheumatoid synovium. Arthritis Res Ther. 2009;11(1):R13.
- Kelly RJ, Lopez-Chavez A, Citrin D, et al. Impacting tumor cell-fate by targeting the inhibitor of apoptosis protein survivin. Mol Cancer. 2011;10(1):35.
- Venkatesan N, Kanwar JR, Deepa PR, et al. Targeting HSP90/Survivin using a cell permeable structure based peptido-mimetic shepherdin in retinoblastoma. Chem Biol Interact. 2016;252:141–149.
- Khan S, Aspe JR, Asumen MG, et al. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009;100(7):1073–1086.
- Fukuda S, L.m.p. Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34+ cells by hematopoietic growth factors implication of survivin expression in normal hematopoiesis. Blood. 2001;98(7):2091–2100.
- Leung CG, Xu Y, Mularski B, et al. Requirements for survivin in terminal differentiation of erythroid cells and maintenance of hematopoietic stem and progenitor cells. J Exp Med. 2007;204(7):1603–1611.
- Andersson SE, N D Svensson M, C Erlandsson M, et al. Activation of Fms-like tyrosine kinase 3 signaling enhances survivin expression in a mouse model of rheumatoid arthritis. PLoS One. 2012;7(10):e47668.
- Pratibha Singh JH, Peirong H, Speth JM, et al. Blockade of prostaglandin E2 signaling through EP1 and EP3 receptors attenuates Flt3L-dependent dendritic cell development from hematopoietic progenitor cells. Blood. 2012;119(7)(7):1671–1682.
- Cohran V, Managlia E, M Bradford E, et al. Epithelial PIK3R1 (p85) and TP53 regulate survivin expression during adaptation to ileocecal resection. Am J Pathol. 2016;186(7):1837–1846.
- Tamm I, Y.w, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58(23):5315–5320.
- Xiaoyu J, Shanshan G, Yongbo Q, et al. Effects of poly(I:C) and MF59 co-adjuvants on immunogenicity and efficacy of survivin polypeptide immunogen against melanoma. J Cell Physiol. 2018;233(6):4926–4934.
- Mishra H, Kumar Mishra P, Ekielski A, et al. Functionalized nanoliposomes loaded with anti survivin and anti angiogenic agents to enhance the activity of chemotherapy against melanoma by 4-pronged action. Med Hypotheses. 2018;116:141–146.
- Michael Samarkos GP, Athanasoula K, Benopoulou Ο, et al. Significance of survivin mRNA blood levels in patients with melanoma. J BUON. 2018;23(7):96–103.
- Bastea LI, Hollant LMA, Döppler HR, et al. Sangivamycin and its derivatives inhibit Haspin-Histone H3-survivin signaling and induce pancreatic cancer cell death. Sci Rep. 2019;9(1):16588.
- Brown M, Zhang W, Yan D, et al. The role of survivin in the progression of pancreatic ductal adenocarcinoma (PDAC) and a novel survivin-targeted therapeutic for PDAC. PLoS One. 2020;15(1):e0226917.
- Heidari Z, Sagheb HM, Hakimi A, et al. Evaluation of immunohistochemical expression of survivin and its correlation with −31G/C gene polymorphism in colorectal cancer. Med Mol Morphol. 2019;52(2):82–89.
- Qianwen Shao JX, Deng R, Wei W, et al. The expressions of YAP1, β-catenin and survivin in colon cancer tissues and their clinical significance. Int J Clin Exp Pathol. 2018;11(12):6032–6038.
- Tianyi Y, Hongwen Y, Yang X, et al. Role of Smac, survivin, XIAP, and Omi/HtrA2 proteins in determining the chemotherapeutic response of patients with cervical cancer treated with neoadjuvant chemotherapy. Cancer Biomark. 2019;26(3):249–259.
- Yu M, Xu B, Yang H, et al. <p>MicroRNA-218 regulates the chemo-sensitivity of cervical cancer cells through targeting survivin. Cancer Manag Res. 2019;11:6511–6519.
- Martinez-Garcia D, Pérez-Hernández M, Korrodi-Gregório L, et al. The natural-based antitumor compound T21 decreases survivin levels through potent STAT3 inhibition in lung cancer models. Biomolecules. 2019;9(8):361.
- Nitschkowski D, Marwitz S, Kotanidou SA, et al. Live and let die: epigenetic modifications of Survivin and Regucalcin in non-small cell lung cancer tissues contribute to malignancy. Clin Epigenetics. 2019;11(1):157.
- Krafft U, Tschirdewahn S, Hess J, et al. Validation of survivin and HMGA2 as biomarkers for cisplatin resistance in bladder cancer. Urol Oncol. 2019;37(11):810 e7–810 e15.
- Stec R, Cierniak S, Lubas A, et al. Intensity of nuclear staining for Ki-67, p53 and survivin as a new prognostic factor in non-muscle invasive bladder cancer. Pathol Oncol Res. 2020;26(2)(2):1211–1219.
- Zhang Y, Wang J, Sui X, et al. Prognostic and clinicopathological value of survivin in diffuse large b-cell lymphoma: a meta-analysis. Medicine (Baltimore). 2015;94(36):e1432.
- Hong JY, Chaehwa Park KJR, Hong M, et al. Clinical impact of serum survivin positivity and tissue expression of EBV-encoded RNA in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Oncotarget. 2017;8(8):13782–13791.
- Hong L, Yanning Tian CD, Xiang L, et al. Knockdown of Diacylglycerol Kinase Zeta (DGKZ) Induces Apoptosis and G2M Phase Arrest in Human Acute Myeloid Leukemia HL-60 Cells Through MAPKsurvivincaspase Pathway. Pharmazie. 2019;74:418–422.
- Huang J, Lyu H, Wang J, et al. Influence of survivin-targeted therapy on chemosensitivity in the treatment of acute myeloid leukemia. Cancer Lett. 2015;366(2):160–172.
- Zhang S, Zhang C, Song Y, et al. Prognostic role of survivin in patients with glioma. Medicine (Baltimore). 2018;97(17):e0571.
- Sha L, Yue Meng LW, Chang Y, et al. Increased levels of LAPTM4B, VEGF and survivin are correlated with tumor progression and poor prognosis in breast cancer patients. Oncotarget. 2017;8(25):41282–41293.
- Veiga GLD, Silva RDMD, Pereira EC, et al. The role of Survivin as a biomarker and potential prognostic factor for breast cancer. Rev Assoc Med Bras. 2019;65(6):893–901. 1992.
- Zhang Y, Yan H, Li R, et al. High expression of survivin predicts poor prognosis in cervical squamous cell carcinoma treated with paclitaxel and carboplatin. Medicine (Baltimore). 2019;98(20):e15607.
- Zareifar S, Ghorbani S, Monabbati A, et al. Expression of antiapoptotic proteins livin and survivin in pediatric AML patients, as prognostic markers. Pediatr Hematol Oncol. 2018;35(4):250–256.
- Salman T, Argon A, Kebat T, et al. The prognostic significance of survivin expression in gallbladder carcinoma. APMIS. 2016;124(8):633–638.
- Xiong C, Liu H, Chen Z, et al. Prognostic role of survivin in renal cell carcinoma: a system review and meta-analysis. Eur J Intern Med. 2016;33:102–107.
- Faccion RS, Bernardo PS, Faria de Lopes GP, et al. p53 expression and subcellular survivin localization improve the diagnosis and prognosis of patients with diffuse astrocytic tumors. Cell Oncol (Dordr). 2018;41(2):141–157.
- Morgia G, Micali A, Rinaldi M, et al. Survivin and NAIP in human benign prostatic hyperplasia: protective role of the association of serenoa repens, lycopene and selenium from the randomized clinical study. Int J Mol Sci. 2017;18(3):680.
- Gokmen Karasu AF, Sonmez FC, Aydin S, et al. Survivin expression in simple endometrial polyps and tamoxifen-associated endometrial polyps. Int J Gynecol Pathol. 2018;37(1):27–31.
- Jourabchin A, Mazoochi T, Haddad Kashani H, et al. Assessment of relationship between expression of survivin protein and histopathology diagnosis and malignancy severity in colon specimen. J Gastrointest Cancer. 2020;51(1):76–82.
- Kushitani K, Amatya VJ, Mawas AS, et al. Utility of Survivin, BAP1, and Ki-67 immunohistochemistry in distinguishing epithelioid mesothelioma from reactive mesothelial hyperplasia. Oncol Lett. 2018;15(3):3540–3547.
- Zhou XL, Wang M. Expression levels of survivin, Bcl-2, and KAI1 proteins in cervical cancer and their correlation with metastasis. Genet Mol Res. 2015;14(4):17059170.
- Siddharth S, Das S, Nayak A, et al. SURVIVIN as a marker for quiescent-breast cancer stem cells-An intermediate, adherent, pre-requisite phase of breast cancer metastasis. Clin Exp Metastasis. 2016;33(7):661–675.
- Kogo R, How C, Chaudary N, et al. The microRNA-218~Survivin axis regulates migration, invasion, and lymph node metastasis in cervical cancer. Oncotarget. 2015;6(2):1090–1100.
- Sonja Selemetjev SS, Paunovic I, Tatic S, et al. Concomitant high expression of survivin and vascular endothelial growth factor-C is strongly associated with metastatic status of lymph nodes in papillary thyroid carcinoma. J Cancer Res Ther. 2018;14(8):114–119.
- Ahn JK, Oh J-M, Lee J, et al. Increased extracellular survivin in the synovial fluid of rheumatoid arthritis patients: fibroblast-like synoviocytes as a potential source of extracellular survivin. Inflammation. 2010;33(6):381–388.
- Smith MD, Weedon H, Papangelis V, et al. Apoptosis in the rheumatoid arthritis synovial membrane: modulation by disease-modifying anti-rheumatic drug treatment. Rheumatology (Oxford). 2010;49(5):862–875.
- Kusner LL, Ciesielski MJ, Marx A, et al. Survivin as a potential mediator to support autoreactive cell survival in myasthenia gravis: a human and animal model study. PLoS One. 2014;9(7):e102231.
- Sharief MK, Y.k.s. Heightened expression of survivin in activated T lymphocytes from patients with multiple sclerosis. J Neuroimmunol. 2001;119(2):358–364.
- Legorreta-Haquet MV, Flores-Fernández R, Blanco-Favela F, et al. Prolactin levels correlate with abnormal B cell maturation in MRL and MRL/lpr mouse models of systemic lupus erythematosus-like disease. Clin Dev Immunol. 2013;2013:287469.
- Ebrahimiyan H, Aslani S, Rezaei N, et al. Survivin and autoimmunity; the ins and outs. Immunol Lett. 2018;193:14–24.
- Koike Y, Muroi E, Yoshizaki A, et al. Autoantibody against survivin in patients with systemic sclerosis. J Rheumatol. 2010;37(9):1864–1870.
- Erlandsson MC, Turkkila M, Pullerits R, et al. Survivin measurement improves clinical prediction of transition from arthralgia to RA-biomarkers to improve clinical sensitivity of transition from arthralgia to RA. Front Med (Lausanne). 2018;5:219.
- Xiaozhen Liu CF, Liu J, Jian L, et al. The importance of EGFR as a biomarker in molecular apocrine breast cancer. Hum Pathol. 2018;11:1–10.
- Morales-Gutierrez C, Abad-Barahona A, Moreno-González E, et al. Tumour VEGF/non tumour VEGF protein expression ratio as a biomarker for survival in colorectal cancer patients. Eur J Surg Oncol. 2011;37(6):526–531.
- Arrate Sevilla MCM, Ezkurra PA, Rasero J, et al. BRAF V600E mutational load as a prognosis biomarker in malignant melanoma. PLoS One. 2020;15(3):e0230136.
- Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17(3):153–168.
- Acimovic M, et al. Survivin and VEGF as novel biomarkers in diagnosis of endometriosis. J Med Biochem. 2016;35(1):63–68.
- Li YH, Vidakovic S, Milic N, et al. Elevated expressions of survivin and VEGF protein are strong independent predictors of survival in advanced nasopharyngeal carcinoma. J Transl Med. 2008;6(1):1.
- Zhang M, Zhang X, Zhao S, et al. Prognostic value of survivin and EGFR protein expression in triple-negative breast cancer (TNBC) patients. Target Oncol. 2014;9(4):349–357.
- Goossens-Beumer IJ, Zeestraten ECM, Benard A, et al. Clinical prognostic value of combined analysis of Aldh1, Survivin, and EpCAM expression in colorectal cancer. Br J Cancer. 2014;110(12):2935–2944.
- Megliorino R, Shi F-D, Peng -X-X, et al. Autoimmune response to anti-apoptotic protein survivin and its association with antibodies to p53 and c-myc in cancer detection. Cancer Detect Prev. 2005;29(3):241–248.
- Chen P, Zhu J, Liu D-Y, et al. Over-expression of survivin and VEGF in small-cell lung cancer may predict the poorer prognosis. Med Oncol. 2014;31(1):775.
- Boidot R, Vegran F, Lizard-Nacol S. Transcriptional regulation of the survivin gene. Mol Biol Rep. 2014;41(1):233–240.
- Galloway NR, Ball KF, Stiff T, et al. Yin Yang 1 (YY1): regulation of survivin and its role in invasion and metastasis. Crit Rev Oncog. 2017;22(1–2):23–36.
- Nakahara T, Takeuchi M, Kinoyama I, et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67(17):8014–8021.
- Nakamura N, Yamauchi T, Hiramoto M, et al. Interleukin enhancer-binding factor 3/NF110 is a target of YM155, a suppressant of survivin. Mol Cell Proteomics. 2012;11(7):M111 013243.
- Iwasa T, Okamoto I, Takezawa K, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103(1):36–42.
- Kelly RJ, Thomas A, Rajan A, et al. A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(10):2601–2606.
- Shimizu T, Nishio K, Sakai K, et al. Phase I safety and pharmacokinetic study of YM155, a potent selective survivin inhibitor, in combination with erlotinib in patients with EGFR TKI refractory advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2020;86(2):211–219.
- Hansen JB, Majken Westergaard NF, Kjaerulff LS, et al. SPC3042: a proapoptotic survivin inhibitor. Mol Cancer Ther. 2008;7(9):2736–2745.
- Eugene Park EJG, Hsieh Y-T, Schaefer P, et al. Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood. 2011;8(8):2191–2199.
- Raetz EA, Morrison D, Romanos-Sirakis E, et al. A phase I study of EZN-3042, a novel survivin messenger ribonucleic acid (mRNA) antagonist, administered in combination with chemotherapy in children with relapsed acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia and lymphoma (TACL) consortium. J Pediatr Hematol Oncol. 2014;36(6):458–463.
- Church DN, Talbot DC. Survivin in solid tumors: rationale for development of inhibitors. Curr Oncol Rep. 2012;14(2):120–128.
- Callies S, André V, Patel B, et al. Integrated analysis of preclinical data to support the design of the first in man study of LY2181308, a second generation antisense oligonucleotide. Br J Clin Pharmacol. 2011;71(3):416–428.
- Erba HP, Sayar H, Juckett M, et al. Safety and pharmacokinetics of the antisense oligonucleotide (ASO) LY2181308 as a single-agent or in combination with idarubicin and cytarabine in patients with refractory or relapsed acute myeloid leukemia (AML). Invest New Drugs. 2013;31(4):1023–1034.
- Ronald Natale FB, Kowalski D, Ramlau R, et al. Evaluation of antitumor activity using change in tumor size of the survivin antisense oligonucleotide LY2181308 in combination with docetaxel for second-line treatment of patients with non-small-cell lung cancer: a randomized open-label phase II study. J Thorac Oncol. 2014;9(11):1704–1708.
- Santhi Kondur JC, Baker CH, Safe S, et al. Tolfenamic acid enhances pancreatic cancer cell and tumor response to radiation therapy by inhibiting survivin protein expression. Mol Cancer Ther. 2009;8(3):533–542.
- Hurtado M, Sankpal U, Kaba A, et al. Novel survivin inhibitor for suppressing pancreatic cancer cells growth via downregulating Sp1 and Sp3 transcription factors. Cell Physiol Biochem. 2018;51(4):1894–1907.
- Eslin D, Lee C, Sankpal UT, et al. Anticancer activity of tolfenamic acid in medulloblastoma: a preclinical study. Tumour Biol. 2013;34(5):2781–2789.
- Shelake S, Sankpal UT, Eslin D, et al. Clotam enhances anti-proliferative effect of vincristine in Ewing sarcoma cells. Apoptosis. 2019;24(1–2):21–32.
- Pathi S, Li X, Safe S. Tolfenamic acid inhibits colon cancer cell and tumor growth and induces degradation of specificity protein (Sp) transcription factors. Mol Carcinog. 2014;53(Suppl S1):E53–61.
- Esteve PO, Chin HG, Pradhan S. Molecular mechanisms of transactivation and doxorubicin-mediated repression of survivin gene in cancer cells. J Biol Chem. 2007;282(4):2615–2625.
- Shelake S, Sankpal UT, Paul Bowman W, et al. Targeting specificity protein 1 transcription factor and survivin using tolfenamic acid for inhibiting Ewing sarcoma cell growth. Invest New Drugs. 2017;35(2):158–165.
- Shim JH, Shin JA, JungJY, et al. Chemopreventive effect of tolfenamic acid on KB human cervical cancer cells and tumor xenograft by downregulating specificity protein 1. Eur J Cancer Prev. 2011;20(2):102–111.
- Grohar PJ, Glod J, Peer CJ, et al. A phase I/II trial and pharmacokinetic study of mithramycin in children and adults with refractory Ewing sarcoma and EWS-FLI1 fusion transcript. Cancer Chemother Pharmacol. 2017;80(3):645–652.
- Annunziata MC, Stefano AD,Fabbrocini G, et al. Current recommendations and novel strategies for the management of skin toxicities related to anti-EGFR therapies in patients with metastatic colorectal cancer. Clin Drug Investig. 2019;39(9):825–834.
- Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2020 update. Pharmacol Res. 2020;152:104609.
- Becker A, Thakur BK, Weiss JM, et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848.
- Fenstermaker RA, Figel SA, Jingxin Q, et al. Survivin monoclonal antibodies detect survivin cell surface expression and inhibit tumor growth in vivo. Clin Cancer Res. 2018;24(11):2642–2652.
- Berinstein NL, Karkada M, Oza AM, et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology. 2015;4(8):e1026529.
- Inc., I., Announces Breakthrough Data from DeCidE1, its Ongoing Phase 2 Study of DPX-Survivac in Patients with Advanced Recurrent Ovarian Cancer. 2020.
- Ciesielski MJ, Ahluwalia MS, Munich SA, et al. Antitumor cytotoxic T-cell response induced by a survivin peptide mimic. Cancer Immunol Immunother. 2010;59(8):1211–1221.
- Fenstermaker RA, Ciesielski MJ, Jingxin Q, et al. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol Immunother. 2016;65(11):1339–1352.
- Jiang S, Ciesielski MJ, Jingxin Q, et al. Overlapping synthetic peptides as vaccines. Vaccine. 2006;24(37–39):6356–6365.
- Zhang H, Hai H, Demin L, et al. Comparing pooled peptides with intact protein for accessing cross-presentation pathways for protective CD8+ and CD4+ T cells. J Biol Chem. 2009;284(14):9184–9191.
- Lili Cai JZ, Zhu R, Shi W, et al. Protective cellular immunity generated by cross-presenting recombinant overlapping peptide proteins. Oncotarget. 2017;8(44):76516–76524.
- Xu X, Hegazy WAH, Linjie G, et al. Effective cancer vaccine platform based on attenuated salmonella and a type III secretion system. Cancer Res. 2014;74(21):6260–6270.
- Vladimir Pisarev BY, Salup R, Sherman S, et al. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9(17):6523–6533.
- Zhang H, Liu C, Zhang F, et al. MUC1 and survivin combination tumor gene vaccine generates specific immune responses and anti-tumor effects in a murine melanoma model. Vaccine. 2016;34(24):2648–2655.
- Trepiakas R, Berntsen A, Hadrup SR, et al. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. 2010;12(6):721–734.
- Uslu U, Erdmann M, Schliep S, et al. Sarcoidosis under dendritic cell vaccination immunotherapy in long-term responding patients with metastatic melanoma. Anticancer Res. 2017;37(6):3243–3248.