ABSTRACT
Introduction: The use of cell-based therapies in the management of sports injuries of the upper limb is increasingly popular despite the limited scientific evidence available for their use. We aim to evaluate the evidence for the use of cell-based therapies in these injuries and recommend areas for further research.
Areas covered: In accordance with a published protocol (PROSPERO; Registration No. CRD42020193258), a comprehensive search of the literature was performed using the MEDLINE and EMBASE databases from inception to June 2020. All human studies reporting on the clinical, histological, or radiological outcomes following the use of cell-based therapies in the management of epicondylitis or rotator cuff pathology were included in this study. This resulted in 22 studies being included in this review, all of which underwent risk of bias assessments.
Expert opinion: The evidence for the use of cell-based therapies in upper limb sports injuries is limited and generally of low quality. Given the heterogeneity in the cell types used, their harvesting methods and cell amounts, future research should be targeted at developing standardization of the reporting of these studies and more direct comparative studies looking at the efficacy of the different cell types.
Graphical Abstract
1. Introduction
Injuries of the upper limb are debilitating to athletes, with tendinopathies of the shoulder and elbow being among the most prevalent [Citation1]. Rotator cuff pathology represents the most common shoulder pathology, for which patients seek medical attention [Citation2], and epicondylitis represents a prevalent cause of patients presenting with elbow pain [Citation3]. Despite the prevalence of these conditions, the success of the currently available treatment options is variable, with the rate of re-tear following rotator cuff repair reported to range from 20% to as high as 94% [Citation4,Citation5], and recurrence rates of up to 25% in non-operatively treated epicondylitis [Citation6].
One of the reasons for the difficulty in treating these injuries is the complex organization of the area of insertion of tendons into the bone, the enthesis. The enthesis is comprised of four zones: tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone [Citation7]. Rotator cuff and epicondylar tendinopathy share common histological features [Citation8]. Often, following surgical repair, fibrovascular scar tissue forms between tendon and bone, resulting in a weakened construct, and subsequent failure of repair [Citation9]. This inability to restore the normal biology of the tendon has meant that, despite technical advances in surgical treatment, failure rates are still high. However, cell-based therapies have shown promise in their ability to restore the natural biology of damaged tendons [Citation10].
Cell-based therapies include a multitude of treatment modalities using cells at various stages of differentiation. Given their potential applications in musculoskeletal medicine [Citation11,Citation12], it is not surprising that their popularity continues to increase [Citation13]. At the heart of these advancements are mesenchymal stem cells (MSC) and tenocytes [Citation14,Citation15].
Mesenchymal stem cells are multipotent cells able to differentiate into any type of cell of mesodermal lineage in vitro; these include chondrocytes, osteocytes, and adipocytes [Citation16]. However, in vivo, this has not yet been demonstrated [Citation17–19]. Tenocytes also have some limited potential as a progenitor cell [Citation20]. These unique abilities render cell-based preparations a potentially invaluable tool in the treatment of musculoskeletal sports injuries, and may offer a quicker return to sport [Citation21–23]. In vivo, the therapeutic effects of MSC are likely resulting from their trophic, paracrine and immunomodulatory functions as opposed to proliferation and differentiation [Citation24–26]. Much like MSC, tenocytes stimulate growth factors and other immunomodulatory cells to promote a healing response [Citation27].
The term MSC was coined by Caplan in 1991 [Citation28]. Despite their name, MSC are not stem cells. The National Institutes of Health (NIH) defined stem cell as a cell from the embryo, fetus, or adult that has, under certain conditions, the ability to reproduce itself for long periods (long-term self-renewal), remain unspecialized or differentiate to specialized cells [Citation29]. Neither MSC nor tenocytes have this ability, and so cannot be called stem cells. As a result, the International Society for Cellular referred to these cells as mesenchymal stromal cells [Citation30]. Indeed, Caplan himself advocated a change in nomenclature from MSC to ‘medicinal signaling cells’ to better reflect their in vivo secretory function [Citation31]. However, the term MSC has persisted, and there are currently several clinicians overstating the capabilities of these cell-based therapies, with a rapid rise in the number of rogue ‘stem cell’ clinics [Citation32].
The most common sources of MSC are bone marrow and adipose tissue [Citation33,Citation34]. They are found infrequently in the bone marrow, making up less than 0.01% of mono-nucleated cells [Citation16,Citation35,Citation36] or a few hundred cells per milliliter [Citation37]. In contrast, adipose tissue contains roughly 400,000 MSC per milliliter of lipoaspirate [Citation38]. To increase the concentration of these cells further, processing is performed; the concentrate produced is, thus, referred to as bone marrow concentrate (BMC) for bone marrow or the stromal vascular fraction (SVF) in the case of lipoaspirate [Citation39,Citation40]. Given the heterogeneity of the sources of MSC, one could be forgiven for failing to see the common link. Recent studies have identified the pericyte as the origin of most, if not all, MSC [Citation41]. Pericytes are ubiquitous and found in all vascularized tissues, and as such, MSC can be isolated from all vascularized tissues [Citation25]. This means that, in the event of vessel damage, the released pericytes, activated by the injury, become MSC engendering a regenerative environment to promote healing of the injured tissue [Citation17].
Cell-based therapies for the treatment of sports related injuries are being increasingly used given the relative safety of their use, quicker return to sport and ability to treat tissues that are slow or difficult to heal [Citation42]. Despite their clear potential, cell-based therapies have little scientific evidence for their use. This review aims to address two central questions: what the available evidence for the use of cell-based therapies in the treatment of rotator cuff and epicondylar injuries is, and where should future research be directed.
2. Methods
2.1. Search strategy
This study was performed in line with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [Citation43]. It was prospectively registered on the International Prospective Register of Systematic Reviews (PROSPERO; Registration No. CRD42020193258)
A preliminary search of the literature on regenerative cell use in common sports injuries was performed. Several articles were found encompassing both lower limb and upper limb injuries. A separate review investigating the pathologies associated with lower limb injuries was performed [Citation44]. Given the difficulties associated with enthesopathies, a decision was made to focus on the two most common and difficult to treat upper limb enthesopathies, namely, rotator cuff and epicondylar injuries.
A sensitive search strategy for multiple databases was devised by one author (NK). A comprehensive search of the literature was performed using the MEDLINE and EMBASE databases from inception to June 2020. Article titles were searched for the following terms, limited to humans, as well as their corresponding or related MeSH terms: ‘mesenchymal stem cell’ OR ‘stem cell’ OR ‘stromal vascular fraction’ OR ‘bone marrow’ OR ‘tenocyte’ in two separate searches where they were combined with the following:
Search 1, MSC use in rotator cuff pathology, all fields limited to ‘Human’:
AND ‘rotator cuff’ [KEYWORD] OR ‘rotator cuff injury’ [KEYWORD] OR related MeSH terms.
Search 2, MSC use in epicondylar tendinopathy, all fields limited to ‘Human’:
AND ‘epicondylitis’ [KEYWORD] OR ‘tennis elbow’ [KEYWORD] OR related MeSH terms.
To search the gray literature, each trial registry was searched for ‘mesenchymal stem cell’ and ‘regenerative cell’ on 06.06.2020 using the following databases to capture any results from finished trials with unpublished results: CENTRAL trials registry of the Cochrane Collaboration, WHO International Clinical Trials Registry Platform (ICTRP), EU Clinical Trials Register and ClinicalTrials.gov.
The references of the included studies were also searched manually for further relevant studies.
2.2. Eligibility criteria
Studies were included if the following inclusion criteria (PICOS) were met:
Population. Male and female humans who have either epicondylitis or rotator cuff pathology.
Interventions. The use of cell-based therapies includes mesenchymal stem cells, tenocytes, tenocyte-like cells, and any processed or concentrated cell preparations thought to contain mesenchymal stem cells, such as stromal vascular fraction and bone marrow concentrate.
Control. Patients with epicondylitis or rotator cuff pathology treated without the use of cell-based therapies.
Outcomes. Studies had to contain clinical outcomes such as patient reported outcome measures (PROMs), histological analysis of tendon tissue pre- and post-intervention or imaging outcomes such as an evaluation of tear size or tendon healing following the use of cell-based therapies.
Study designs. Randomized controlled trials (RCT), controlled clinical trials (CT), retrospective studies, non-randomized studies, cohort studies, case series, and case reports.
2.3. Data collection
Titles and abstracts were screened by two authors (NK and KB) for relevance. Following this, selection criteria were applied independently by two authors (NK and KB). If unclear from the review of the title and abstract whether a study was appropriate for inclusion, full texts were examined. Consensus was used to resolve any disagreements between reviewers, referring to a third, senior reviewer (VA) if consensus was not reached.
2.4. Data extraction
The following data were extracted: patient demographics (age, sex), nature of injury, intervention performed, biologic used and the source, cell count of biologic, functional outcomes (PROMs), radiological outcomes and length of follow up.
For all studies containing data presented as means with standard deviations or standard errors, values of all relevant outcome measures for pre- and post-intervention were extracted in order to calculate the effect sizes.
2.5. Quality assessment of included studies
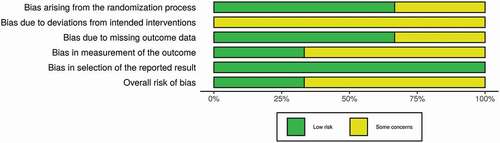
A risk of bias assessment was completed using the Cochrane Risk of Bias 2 (RoB2) tool for RCTs. This tool provides an algorithm by which an overall risk of bias judgment is reached per each study. Five domains were evaluated for risk of bias (the randomization procedure, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result) by two authors (KB and NK) independently. Each domain was labeled as either high risk of bias, low risk of bias, or some concerns. If a study had all five domains as low risk, the overall risk of bias would be low. However, if any of the five domains were labeled high risk or some concern, their overall risk of bias would also be high risk or some concern, respectively. Any disagreements that arose were resolved by a third author (VA).
Case series, case-control studies and cohort studies were assessed using the Agency for healthcare research and quality (AHRQ) tool [Citation45]. Using this tool cohort, studies had a maximum of 13 stars, case-control studies had a maximum of 11 stars, and case series had a maximum of 9 stars, where the greater the number of stars, the lower the risk of bias.
2.6. Data analysis
To provide an overall summary of the data extracted, for all studies containing data presented as means with standard deviations or standard errors, effect sizes were calculated for available outcomes. For RCTs, the mean difference between pre- and post-surgery was calculated for each outcome in both the intervention and control groups. Effect sizes were then calculated between the mean differences in the intervention and control groups. For non-RCTs, effect sizes were calculated from the pre- and post-intervention data. Given the typically small sample size in each study, effect sizes were bias corrected using the Hedge’s G method [Citation46]. All calculated effect sizes were defined as small (0.2), medium (0.5), large (0.8), or very large (1.3) [Citation47], and were presented in effect size plots with 95% confidence interval error bars.
Outcomes were grouped according to the injury they reflect: RC or epicondylitis. Injury patterns were further divided into RCT and non-RCT studies. Given the heterogeneity of the study protocols, a full meta-analysis was not possible.
3. Results
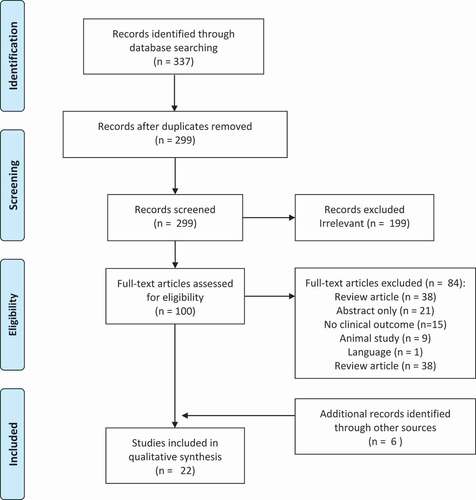
The primary search yielded 337 results (see PRISMA flowchart – ).
After reviewing full texts, both authors (NK, KB) agreed that 16 studies were eligible for inclusion. After searching through the references of eligible studies and all relevant review articles manually, five further cited studies were found to be eligible for inclusion. One additional study with available data was found by searching the gray literature.
Of the 22 studies that met the inclusion criteria, 16 were relating to rotator cuff pathology and 6 to epicondylitis. A summary of the main study characteristics can be found in .
Table 1. Study characteristics and levels of evidence
3.1. Risk of bias assessment
The three included RCTs were assessed for risk of bias using the Cochrane RoB2 tool with all three displaying some concerns for bias (see and [Citation48]). Sixteen studies were assessed using the AHRQ tool (see ). The seven case series [Citation49–55] had risk of bias assessment between 4 and 6 out of nine stars. The two case-control studies [Citation56,Citation57] had risk of bias assessments between 6 and 9 out of 11 stars. The seven cohort studies [Citation58–64] had risk of bias assessments between 6 and 10 out of 13 stars. The three case reports [Citation27,Citation65,Citation66] were assumed to be at a high risk of bias, and thus not assessed further.
Table 2. Risk of bias domains
Table 3. Risk of Bias assessments using AHRQ tool
3.2. Shoulder: Rotator Cuff Injury
Sixteen studies involving the use of cell-based therapies in rotator cuff disease were found. Seven studies assessed augmentation of RC repair surgery, and nine evaluated the use of intra-articular, or intra-tendinous, injections.
3.2.1. Bone Marrow Concentrate
Three studies investigated the use of BMC alone. Two studies [Citation55,Citation56] involved BMC augmented surgical repair of the RC and demonstrated maintained tendon integrity on MRI in 87% of patients at 10 years [Citation56] and 100% of patients at 12 months [Citation55]. The remaining study [Citation62] demonstrated improved pain and function scores following BMC injection.
Ellera Gomes et al. [Citation55] reported on 14 patients with complete RC tears. All patients underwent an augmented mini-open RC repair by a single surgeon and BMC was injected into the tendon and the bony footprint. This was followed by 4 weeks immobilization in a sling and physiotherapy thereafter. The UCLA shoulder score improved from 12 ± 3 preoperatively to 31 ± 3.2 at a minimum of 12 months follow up. Magnetic resonance imaging follow-up was available in all patients at 12 months and all 14 patients demonstrated tendon integrity.
Hernigou et al. [Citation56] reported on 45 patients with supraspinatus tears matched for tear size and location, shoulder dominance, sex, and age with an equivalent group. All procedures were undertaken using arthroscopic techniques. In the experimental group, BMC were injected at the tendon bone junction and the bony footprint. In both groups, the first week post-operatively involved an arm sling and passive forward flexion. Active range of motion exercises were commenced at 6 weeks, and active resistance exercises at 8 weeks. Light daily activities were allowed from 2 months, and heavy manual work or sporting activities allowed after 6 months. At the 10-year follow-up MRI, 39 of 45 patients (87%) in the experimental group had intact RC compared to 20 of 45 patients (44%) in the control group (p < 0.05).
Darrow et al. [Citation62] retrospectively evaluated the use of either BMC or whole bone marrow, injected into the joints and tendons of 18 subjects with RC tears. Participants were advised to perform daily shoulder stretches as part of their rehabilitation. They reported improved functional outcomes and pain scores for both groups but did not provide separate data or information on the size of tears and specific site of injection.
3.2.2. Bone Marrow Concentrate (BMC) and Adjunctive therapies
Five studies reported on the use of BMC and other therapies. One study [Citation64] reported on the use of BMC and adjunctive therapies in revision RC surgery and found functional scores that trended toward improvement, although for the most part these were not significant. Four studies [Citation57–59,Citation67] reported on the results of BMC and adjunctive therapies injected into RC tendon. All four studies reported improvements in functional scores following the injection of BMC and adjunctive therapies, two of which were compared with a control group [Citation57,Citation67]. No significant improvement in tear size was demonstrated when compared to baseline [Citation67] or control groups [Citation57].
Muench et al. [Citation64] reported on the use of a biologically augmented patch saturated with a BMC/Platelet Rich Plasma (PRP) mix in 22 patients who required revision rotator cuff surgery. Patients were placed in a 30 degree abduction sling for at least 6 weeks. From day 28 postoperatively, patients were allowed 60 degrees of active assisted external rotation, 30 degrees of abduction and forward flexion from 30 to 180 degrees during physical therapy. Subsequently, patients were allowed to start active assisted range of motion in external rotation and forward flexion without limitations up to 12 weeks postoperatively. This was followed by RC strengthening until 18 weeks. The Simple Shoulder Test (SST) scores improved from 2.6 ± 3.0 pre-operatively to 5.2 ± 4.2 post-operatively (p < 0.05). The American Shoulder and Elbow Surgeon (ASES) score increased from 40.2 ± 21.6 pre-operatively to 53.9 ± 31.4 post-operatively; however, statistical significance was not reached. The Visual Analogue Scale (VAS) pain scores decreased from 5.6 ± 2.5 pre-operatively to 4.2 ± 3.4, again this did not reach statistical significance.
Centeno et al. [Citation58] reported on two distinct groups of patients, one with osteoarthritis and one with RC disorders. Improvements in the functional scores of patients who received intra-articular/intra-tendinous injections of a combination of BMC, PRP and Plasma Lysate (PL) were reported. No details regarding rehabilitation were available. The Disabilities of the Arm, Shoulder and Hand score (DASH) improved from 36.1 pre-treatment to 17.1 post-treatment at an average follow-up of 7.1 months (p < 0.001). Similarly, the numeric pain scale (NPS) improved from 4.3 pre-treatment to 2.4 post-treatment at a mean follow-up of 8.3 months (p < 0.001). However, the results were not separated between the RC group and the osteoarthritis group. When the two groups were compared, uni and multivariate analysis showed no differences in outcomes.
Kim et al. [Citation57,Citation59] published two studies reporting on 12 patients who had sub-acute partial rotator cuff tears. The initial study reported on 12 patients who had a BMC-PRP mixture injected into the site of their tear. In the second study, the patients from the initial group were compared to a control group who received physiotherapy alone. The experimental group had no rehabilitation, while the control group were shown RC exercises to perform for 3 months. After 3 months, the treatment group showed greater functional outcome improvements compared to the control group (VAS 1.9 ± 0.7 and 3.7 ± 1.8, respectively (p < 0.05) and ASES 74.1 ± 8.5 and 62.2 ± 12.2, respectively (p < 0.05)). There were no significant changes in tear size between the two groups.
Centeno et al. [Citation67] performed a prospective RCT crossover trial with 14 patients in the treatment group receiving a BMC injection and a second group receiving exercise therapy. Patients were instructed to limit lifting and pushing and to perform passive range of motion exercises thrice daily up to day three post-procedure. From day three to week four, patients were encouraged to continue thrice daily range of motion exercises with the addition of pendulum, pulley exercises, and shoulder girdle strengthening exercises. From weeks five to 11, patients were advised to start resistance training, and with no restrictions after week 12. They found significant improvements in the Disabilities of the Arm, Shoulder and Hand (DASH) Score, Numerical Pain Scale and Single Assessment Numeric Evaluation (SANE) at all time points prior to crossover in favor of the BMC-only group. At 24 months however there were no statistically significant differences in these variables between the BMC-only group and the crossover group (exercise therapy with the opportunity to cross over at 3 months). Magnetic resonance imaging assessment of tears, by three blinded assessors, showed a mean decrease in size of 26% in the BMC group, which was not statistically significant.
3.2.3. Mesenchymal Stem Cells
Four studies evaluated the use of MSC. Three [Citation60,Citation61,Citation63] studies reported on the injection of MSC. Functional scores increased in all studies, however significance was limited to a high dose MSC [Citation61,Citation63] and was not reached compared to a control group of arthroscopic repair alone [Citation60]. There was also a significant decrease in re-tears in the MSC-treated group compared to the control group [Citation60]. In the remaining study [Citation68], MSCs were used to augment RC repair and, while functional scores increased in the treatment group, so too did the re-rupture rate.
Kim et al. [Citation60] reported on 35 patients treated with adipose-derived MSC/fibrin glue injection compared with a control group of 35 patients undergoing surgical repair alone. Passive exercises were permitted from the day after surgery, while active exercises were not permitted until at least week six. Active assisted exercises were initiated 6 weeks after surgery alongside muscle-strengthening exercises. Manual labor or recreational activities were delayed for 6 months. The Constant score in the injection group improved from 65.2 ± 14.6 pre-operatively to 78.3 ± 14.9 postoperatively (P < 0.001). The UCLA score increased from 26.5 ± 5.2 pre-operatively to 29.8 ± 5.1 post-operatively. This improvement did not reach statistical significance. There were no statistically significant differences between the UCLA scores and Constant score between both groups during the final follow-up. The VAS scores at rest and during motion also saw statistically significant improvements in both groups, with no difference between the groups. The structural outcomes of the injection group, as assessed by MRI at a mean of 13.9 months (12–21 months), showed 30 tears with complete healing, with a statistically significant reduction in the number of re-tears (5 vs 10 p < 0.001). There was a statistically significant reduction in re-tears in patients who had full thickness tears (3 vs 9 p < 0.001).
Lamas et al. [Citation68] reported on eight patients using a xenogeneic scaffold augmented with bone marrow derived from cultured MSC compared to a control group of five patients who received the scaffold alone. Post-operatively, patients were placed in an abduction sling for a non-specified duration. The trial was stopped early because of a high complication rate. Of those who completed the study, five patients (62.5%) sustained a re-rupture in the MSC group compared to three patients (60%) in the control group. The Constant score improved from a mean of 44.5 (30–63) pre-operatively to 72 (43–87) post-operatively and the VAS improved from 8.1 (7–9) pre-operatively to 2.9 (1–8) postoperatively in the MSC group.
Jo et al. [Citation61] divided 19 participants receiving an intra-tendinous injection of cultured adipose derived MSCs with saline alone into three dosage groups: low, medium and high. Post-injection, patients were immobilized for four weeks
in an abduction brace. Shrugging, protraction, and retraction of shoulder girdles; intermittent exercise of the elbow,
wrist, and hand; and external rotation of the arm to neutral with the brace were encouraged as tolerated. Gradual weaning from the abduction brace at 4 weeks with passive and active assisted range of motion exercises was commenced and strengthening exercises thereafter. The 2-year follow-up results were published separately [Citation63]: eight bursal-side defects in the high-dose group had a 100% improvement in the defect size at 2 years. There was no statistically significant improvement in the four articular-side defects in the high-dose group nor any of the bursal-side, articular-side or intra-tendinous defect sizes in the medium and low-dose groups. Functional scores improved in all groups during the final follow-up, but these were mostly statistically significant in the high-dose group only.
3.2.4. Mesenchymal Stem Cells and adjunctive therapies
A single study evaluated the use of MSC in conjunction with other therapies.
Protzman et al. [Citation65] reported the findings of a case of recurrent RC tears where a dermal allograft combined with MSCs and PRP was used for surgical repair. The post-operative rehabilitation regime was not described. A biopsy taken 8 months post-operatively showed that the graft had become fully incorporated and had undergone tissue remodeling.
3.2.5. Tenocytes
The use of tenocytes was reported in two studies. Both studies [Citation27,Citation51] report improved tear size following the injection of tenocytes.
Wang et al. [Citation66] theorized that the use of tenocyte-containing preparations may promote healing ‘through replenishing the depleted tenocyte population seen in end-stage tendinopathy.’ They reported a single patient in whom autologous tenocytes were harvested from the patellar tendon and injected into a partial thickness tear. Following injection, the patient was rested from all training for 4 weeks. This was followed by light training and at 12 weeks the patient was allowed to return to full training. Two independent radiologists reported that the partial thickness supraspinatus tear was no longer detectable on MRI scan at 10 months post procedure. However, tendinopathy (as defined by tendon thickening with persistent focal signal increase) persisted at 10-month follow-up.
Schwab et al. [Citation27] reported a patient in whom the subscapularis tendon was injected with tenocytes obtained from autologous palmaris longus tendon. Post procedure, there was a period of 3 days of complete rest. This was followed by modified training in the pool at two and a half weeks, and full training over three to 4 weeks. Three blinded radiologists reported improvements in both tear size (judged by Walton criteria [Citation69]) and tendinopathy.
3.2.6. Stromal Vascular Fraction
Only one study reported the use of SVF.
Hurd et al. [Citation70] used adipose tissue from the abdomen, flank or inner thigh to obtain SVF for injection into the tendons of 12 patients. A control group of six patients received a corticosteroid injection into the subacromial space. Patients were advised to avoid overhead activities for the first 2 days, and continue with any home treatment program already instigated. No further specific restrictions were placed. The American Shoulder and Elbow Surgeons scores improved from a baseline of 58.7 ± 5.8 to 89.4 ± 4.9 at 52 weeks post treatment in the SVF group compared to the steroid group, which changed from 50.6 ± 6.7 at baseline to 68.4 ± 4.4at 52 weeks post treatment (p < 0.05). In the Short Form Survey 36 (SF-36), VAS and MRI appearances showed no statistically significant difference between the groups.
3.2.7. Clinical outcomes
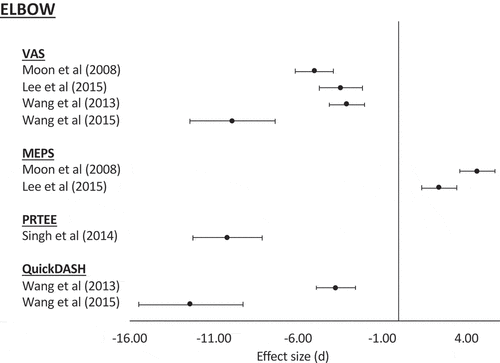
The main clinical outcome scores used were the UCLA shoulder score, VAS pain score, ASES score, and Constant score. The effect sizes are represented in and for relevant studies excluding case reports. Five studies [Citation57,Citation59,Citation60,Citation68,Citation70] reported on both pre- and post-intervention VAS scores. The average pre-intervention VAS was 4.8. This improved to 1.8 at a mean follow up of 11.7 months (3–28.3 months). Four studies [Citation57,Citation59,Citation64,Citation70] reported on the ASES score with an average score pre-intervention of 44.4. This improved post-intervention to an average of 72.3 at a mean follow-up of 12 months (3–30 months). Two studies reported on the UCLA shoulder score [Citation55,Citation60] and Constant score [Citation60,Citation68], respectively. The mean UCLA score pre-intervention was 19.3. This increased post-intervention to 30.4 at a mean follow up of 20.2 months (12–28.3 months). The Constant score was a mean of 54.9 pre-intervention increase to 75.2 post-intervention at a mean of 20.2 months (12–28.3 months).
3.2.8. Imaging outcomes
Most of the studies reporting imaging outcomes used MRI as their imaging modality of choice. Five studies [Citation57,Citation59,Citation61,Citation67,Citation70] reported a decrease in tear size at follow-up (two using ultrasound and three using MRI). Four studies [Citation27,Citation60,Citation63,Citation66] reported healing in 85.7% to 100% of patients. Two studies reported on tendon integrity, with Ellera-Gomes et al. [Citation55] reporting integrity in 100% of patients at 12 months and Heringou et al. [Citation56] reporting integrity in 87% of patients at 10-year follow-up.
3.2.9. Complications
Among the complications detailed in the studies, Heringou et al. [Citation56] report 6 re-tears between 2 and 4 years. The RCT by Lamas et al. [Citation68] was stopped early because of a high complication rate. In the treatment group, three patients developed lesions requiring further surgery. Additionally, the re-tear rate was 62.5% in the treatment group, another factor which hastened the cessation of the trial. Five patients required revision procedures at an average of 1.9 years in the study by Muench et al. [Citation64]. Additionally, one patient required excision of painful suture material and one patient sustained a deep infection.
3.3. Elbow: Epicondylitis
Six studies concerning the use of cell-based therapies for epicondylitis were identified. One involved augmenting surgical treatment, and theremaining reported results following intra-tendinous injections of cell-based preparations into the lateral epicondyle.
3.3.1. Bone Marrow Concentrate
Two studies reported on the use of BMC. Both studies report improved functional scores following surgical debridement [Citation49] or in isolation [Citation52].
Moon et al. [Citation49] investigated the use of autologous BMC injections in 26 elbows with epicondylitis (24 patients). The patients received a dose of BMCs with bupivacaine immediately following arthroscopic debridement of degenerative tissue within the common extensor origin. The elbow was immobilized in a splint for 2 days, active resistance exercises started at 6 weeks postoperatively, and more vigorous exercise allowed after two to 3 months. At 6-month follow-up, patients had significant improvements in VAS (7 at baseline improving to 1.7) and Mayo Elbow Performance Score (MEPS) (52 ± 7.6 at baseline improving to 89 ± 7.9, p < 0.001).
Singh et al. [Citation52] examined the use of BMC injection without operative treatment in 26 patients with lateral epicondylitis. Post-injection, patients were advised to rest and modify their activities. Patient-rated Tennis Elbow Evaluation (PRTEE) scores improved from 72.8 ± 7.0 at baseline to 14.86 ± 3.5 at 3 months (p < 0.0001).
3.3.2. Mesenchymal Stem Cells
One study reported the use of MSC.
Lee et al. [Citation53] investigated injection of allogenic adipose-derived cultured MSCs and fibrin glue into the common extensor origin. Twelve participants were split into two groups, a low dose group and a high dose group. No specific rehabilitation was instigated post injection. The VAS improved from a baseline of 66.8 ± 14.5 to 14.8 ± 13.1 at 1 year. Similarly, the MEPS improved from a baseline of 64 ± 13.5 to 90.6 ± 5.8 at 52 weeks. The appearances of tendinous defect on ultrasound were also found to have significantly decreased at 52 weeks.
3.3.3. Tenocytes
Three studies reported on the use of tenocytes. All studies [Citation50,Citation51,Citation54] demonstrated improved functional scores and appearances on imaging following injection of tenocytes.
Wang et al. reported two studies [Citation51,Citation54], the first of which included 16 patients injected with patellar tendon-derived tenocytes. Patients were advised to rest for 2 days post injection and then perform only light activities for 4 weeks. Additionally, advice regarding four times daily forearm extensor muscle stretches was given. Visual Analogue Scale scores improved from 5.9 ± 2.2 at baseline to 0.8 (no standard deviation provided) at 12 months. The QuickDASH score improved from 45.88 ± 15.2 at baseline to 2.88 ± 0.7 at final follow-up (P < 0.001). The MRI appearances of the tendon were also significantly improved at 12 months. In the second study, the same cohort was followed up to 4.5 years. The QuickDASH was 6.61 ± 1.9 at final follow up and VAS was 1.21 ± 0.3 at final follow up.
Connell et al. [Citation50] performed a similar study using tenocyte-like cells derived from skin fibroblasts. In this study, cells were injected into the lateral epicondyle of 12 patients. Patients were advised to limit the use of the injected arm for 24 hours after which normal activity could resume bar heavy lifting. The PRTEE score improved from a baseline of 78 (71–88) to a median of 12 (0–25) at 6 months (p < 0.05). Assessment of tendons using ultrasonography at 6 months showed improvement in appearance (p < 0.05).
3.3.4. Clinical outcomes
The PRTEE, VAS and MEPS were the most commonly used functional scores. The effect sizes are represented in for relevant studies excluding case reports. Three studies [Citation49,Citation53,Citation71] reported on the VAS; at baseline, the mean VAS was 6.5 ± 0.5, improving to 1.5 ± 0.2 at an average 24 months (6–54.1 months) follow-up. Two studies [Citation49,Citation53] reported on the PRTEE score. At baseline, the mean score was 75.4 ± 2.6, which improved to 13.4 ± 1.4 at an average of 4.5 months (3–6 months) follow up. Two studies [Citation49,Citation53] reported on the MEPS, with a baseline of 58 improving to 89.8 at a mean follow-up of 9 months (6–12 months).
3.3.5. Imaging outcomes
Four studies reported on imaging outcomes post intervention. Two studies [Citation50,Citation53] reported improved defect size and appearances tending toward normality at 6 months and 12 months, respectively. Two studies from Wang et al. [Citation51,Citation71] reported on the MRI appearance of the tendon. Both showed improved appearances at 12 months, and these improvements were maintained at 5 years.
3.3.6. Complications
Of the studies reported, there was only one reported complication. In the pilot study by Wang et al. [Citation51], one patient exhibited worsening of the appearances of the tear post intervention. The deterioration resulted in the need for surgical intervention. No further complications were reported in any of the studies.
4. Conclusion
Within the limitations of this study, tenocytes have shown the most promise in the management of epicondylar tendinopathy. In the studies that used tenocytes, both clinical and imaging scores were improved, with imaging improvements maintained at up to 5-year follow-up. In the management of rotator cuff pathology, BMC showed the most promising results when used in isolation or as an adjunct to surgical repair. The studies using BMC evidenced improvements in functional scores and fewer complications.
In the non-randomized studies, the magnitude of the effect of intervention on clinical scores was mostly large, suggesting that patient scores are likely to show meaningful improvements following treatment with cell-based therapies. However, in the RCTs, the effect magnitude of cell-based therapies was only small to medium. This highlights the need for further high-quality randomized studies to establish whether the use of cell-based therapies truly results in improved patient outcomes.
Whilst there are many promising findings reported in the included studies, a lack of standardization in methods, culture and cell type make firm conclusions difficult to draw. We suggest that future studies should focus on establishing techniques to reliably identify cell type and number. This would lay the foundations for greater comparability of studies, and enable direct comparison of outcome measures. It is also imperative that the outcomes measured focus on patient pain, function and quality of life. To this end, we suggest that PROMs should be the primary outcome measure for future studies. Whilst imaging evidence of tendon integrity is a useful metric of the regenerative abilities of cell-based therapies, if this is not accompanied by improvements in a patient’s function, pain or quality of life, the usefulness of these therapies as a suitable treatment would understandably be questioned.
5. Expert Opinion
Twenty-two studies were included in this systematic review, of which only three were of level 1 evidence [Citation72]. Three of the studies were RCTs, all of which pertained to the treatment of rotator cuff injuries. There were mixed results with regard to clinical outcomes, with some showing no significant difference between treatment and control groups. However, most functional scores were significantly better in the treatment groups compared to the control group. None of the studies reported serious adverse effects as a result of the cell-based treatment. However, Lamas et al. [Citation68] terminated their study early due to a number of complications in both treatment and control groups which they attributed to the scaffold used.
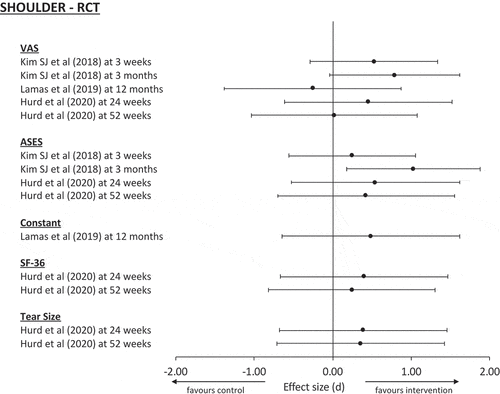
It was not possible to conduct a meta-analysis of the available data in any of the sections due to the heterogeneity between studies. Studies varied greatly in the type of cell-based therapy used, in their functional and imaging outcomes and their intervals of measurement/follow up (). The wide variety in the reporting of methods and in the cell amounts make replication and standardization of studies difficult to achieve [Citation73]. However, assessment of effect sizes was possible (,,). In the three RCTs [Citation57,Citation68,Citation70] included in this study, with the exception of VAS score in the study by Lamas et al. [Citation68], the effect sizes favored the intervention group. However, with the exception of the ASES score in Kim et al. [Citation57] at 3 months, which showed a large effect, the remaining studies showed at best a medium effect. For these studies, the confidence intervals suggest that, while the majority of patients might see a favorable response following surgery, there will also be a proportion of patients who do not respond as well, or indeed, at all ().
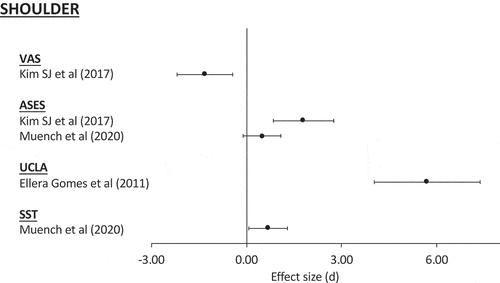
In the non-randomized studies of rotator cuff injuries, UCLA and VAS scores showed large effect sizes, with the confidence intervals indicating all patients could expect to experience improvement as a result of intervention. However, while Kim et al. [Citation59] demonstrated a large effect for the ASES score, Muench et al. [Citation64] demonstrated only a small effect. Considering both these studies, the data would suggest that the majority of patients would experience a meaningful improvement in this outcome following intervention, but some may not respond so well. In the non-randomized studies of epicondylitis, large effect sizes were demonstrated for all clinical scores, with confidence intervals suggesting that all patients should achieve a meaningful response to intervention (). This would indicate that cell-based therapies used for the management of epicondylitis would result in improvement in clinical scores for all patients. Whilst these results are promising, given the lack of randomization, they should be interpreted with a degree of caution.
The use of effect sizes in this study has highlighted the issue of responders and non-responders to treatments. This issue in any therapy, not just a biological therapy, is very much to the forefront in musculoskeletal medicine [Citation74–85], and in these days of personalized medicine, this is an issue which needs to be taken into account. However, to our knowledge, this approach, though desirable and scientifically valid, has not been taken when planning investigations in this field. It is extremely likely that, although it would make sense to stratify patients according to their intrinsic capability and propensity to respond to a given therapeutic intervention, the practicalities and costs of such an approach would be prohibitive.
Serious complications related to the use of cell-based therapies are rare [Citation40], with the majority of complications reported being limited to pain related to route of administration [Citation86]. Among the studies included in this review, there was a low rate of complications, with none being directly attributed to the use of cell-based therapies.
The standardization of cell-based therapies would enable greater comparability of studies, and also allow the utilization of demonstrably successful techniques to improve patient care. Unfortunately, given the complexity in the heterogeneity of the cells and the variability in their procurement, this is unlikely to happen in the near future [Citation87]. The American Academy of Orthopedic Surgeons (AAOS) have suggested methods for achieving greater standardization which include everything from nomenclature to the source and preparation of MSC [Citation73,Citation88]. However, until a global consensus is reached, it is unlikely that advances in the field will be reproducible on a large scale. One recently developed reporting tool, which reached a consensus using a modified Delphi method, is the DOSES tool [Citation89]. This tool implores the researcher to report the Donor, Origin tissue, Separation method, Exhibited cell characteristics, and Site of delivery of the cell-based therapy used. By utilizing reporting tools like DOSES, it is hoped that the transparency and standardization of cell-based therapies can be achieved. This would allow for a greater understanding of the preparations and, once their efficacy was established, allow greater reproducibility in their clinical applications.
The potential for cell-based therapies in the management of sports injuries is limitless. In the future, in select cases, cell-based therapies may eliminate the need for the surgical management of common sports injuries, thus removing an element of risk. With a move toward standardization of reporting and greater regulation of cell-based therapies, it is likely that they will become more widely available and, with this, the ability to conduct high quality and more readily reproducible studies will also increase.
Article highlights
Of the 22 studies included (16 rotator cuff pathology and 6 epicondylitis) 3 were level 1 evidence, 3 were level 2 evidence with the remaining 16 being level 3 or below
In the non-randomized studies, the magnitude of effect of intervention on clinical scores was mostly large. In the randomized controlled trials, the effect magnitude of cell-based therapies was only small to medium
Within the limitations of the included studies, tenocytes showed the most promising results for the treatment of epicondylitis, while bone marrow concentrate demonstrated the most promising results for the treatment of rotator cuff pathology
The rate of complications in the included studies was low, with none of the complications reported being directly attributable to the use of cell-based therapies
Future research should focus on standardization of cell-based therapies to allow for the reproducibility of treatments that are shown to be effective
This box summarizes key points contained in the article.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Availability of data and material
The authors declare that all data supporting the findings of this study are available within the article
Additional information
Funding
References
- Kaux J-F, Forthomme B, L. goff C, et al. Current opinions on tendinopathy. J Sports Sci Med, Internet. Asist Group. 2011. 10: 238–253. Available from. https://pubmed.ncbi.nlm.nih.gov/24149868.
- Valencia Mora M, Ruiz Ibán MA, Díaz Heredia J, et al., Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells, Internet. Baishideng Publishing Group Inc. 2015. 7: 691–699. Available from: https://pubmed.ncbi.nlm.nih.gov/26029341 4
- Vaquero-Picado A, Barco R, Antuña SA. Lateral epicondylitis of the elbow. Internet. EFORT Open Rev. 2016 Available from;1(11):391–397. The British Editorial Society of Bone & Joint Surgery.
- Lädermann A, Denard PJ, Burkhart SS. Management of failed rotator cuff repair: a systematic review. Journal of ISAKOS : Joint Disorders & Orthopaedic Sports Medicine. 2016;1(1):32–37.
- Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96(1):9–16.
- Faro F, Wolf JM, Lateral epicondylitis: review and current concepts. J Hand Surg Am. 2007;32:1271–1279. Available from: http://www.sciencedirect.com/science/article/pii/S0363502307006491
- Montgomery SR, Petrigliano FA, Gamradt SC. Failed rotator cuff surgery, evaluation and decision making. Internet. Clin Sports Med. 2012;31(4):693–712. Elsevier.
- Chard MD, Cawston TE, Riley GP, et al., Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 1994;53(1):30– 34. Available from: http://ard.bmj.com/content/53/1/30.abstract
- Edwards SL, Lynch ST, Saltzman MD, et al. Biologic and pharmacologic augmentation of rotator cuff repairs. J Am Acad Orthop Surg [Internet]. 2011;19. Available from: https://journals.lww.com/jaaos/Fulltext/2011/10000/Biologic_and_Pharmacologic_Augmentation_of_Rotator.2.aspx
- MacLean S, Khan WS, Malik AA, et al. Tendon regeneration and repair with stem cells. Stem Cells Int Internet]. 2011/10/27.Hindawi Publishing Corporation; 2012;2012:316281. Available from: https://pubmed.ncbi.nlm.nih.gov/25098364.
- Andia I, Maffulli N. Biological therapies in regenerative sports medicine. Sport Med. 2017;47(5):807–828.
- Dunn A, Talovic M, Patel K, et al. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. Internet. J Orthop Res. 2019;37(6):1246–1262. John Wiley & Sons, Ltd.
- Ryan KA, Sanders AN, Wang DD, et al. Tracking the rise of stem cell tourism. Internet. Regen Med. 2009 Available from;5(1):27–33. Future Medicine.
- Sutter WW Autologous Cell-Based Therapy for Tendon and Ligament Injuries. Clin Tech Equine Pract [Internet]. 2007;6:198–208. Available from: http://www.sciencedirect.com/science/article/pii/S1534751607000352
- Heathman TRJ, Nienow AW, McCall MJ, et al. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Internet. Regen Med. 2015;10(1):49–64. Future Medicine.
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143– 147. Available from: http://science.sciencemag.org/content/284/5411/143.abstract
- Caplan AI, Correa D, The MSC: an injury drugstore. Cell Stem Cell. Internet]. 2011;9:11–15. Available from: http://www.sciencedirect.com/science/article/pii/S1934590911002943
- Crisan M, Corselli M, Chen C-W, et al. Multilineage stem cells in the adult. Internet. Organogenesis. 2011;7(2):101–104. Taylor & Francis.
- Guimarães-Camboa N, Cattaneo P, Sun Y, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–359.e5. Available from: http://www.sciencedirect.com/science/article/pii/S1934590916304623
- Lee KJ, Clegg PD, Comerford EJ, et al. A comparison of the stem cell characteristics of murine tenocytes and tendon-derived stem cells. BMC Musculoskelet Disord. 2018; BioMed Central Ltd.19(1). https://doi.org/10.1186/s12891-018-2038-2.
- Millett PJ, Gobezie R, Boykin RE. Shoulder osteoarthritis: diagnosis and management. Am Fam Physician. 2008;78(5):605–611.
- Spahn G, Lipfert JU, Maurer C, et al. Risk factors for cartilage damage and osteoarthritis of the elbow joint: case-control study and systematic literature review. Arch Orthop Trauma Surg. 2017;137(4):557–566.
- Albano D, Messina C, Usuelli FG, et al. Magnetic resonance and ultrasound in achilles tendinopathy: predictive role and response assessment to platelet-rich plasma and adipose-derived stromal vascular fraction injection. Eur J Radiol Internet]. 2017;95:130–135. Available from: http://www.sciencedirect.com/science/article/pii/S0720048X17303261
- Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11):e54–e54.
- Caplan AI. Mesenchymal Stem Cells: time to Change the Name! Internet. Stem Cells Transl Med. 2017;6(6):1445–1451. John Wiley & Sons, Ltd.
- Phinney DG, Sensebé L, Mesenchymal stromal cells: misconceptions and evolving concepts. Cytotherapy. Internet]. 2013;15:140–145. Available from: http://www.sciencedirect.com/science/article/pii/S1465324912000345
- Schwab LM, Blanch P, Young M. Autologous tenocyte implantation into shoulder tendon pathology in an elite swimmer. Phys Ther Sport. 2018;29:19–25. Churchill Livingstone
- Caplan AI. Mesenchymal stem cells. Internet. J Orthop Res. 1991;9(5):641–650. John Wiley & Sons, Ltd.
- Bethesda MD: National Institutes of Health USD of H and HS. In Stem Cell Information [Internet]. NIH Stem Cell Inf. Home Page. 2001 [cited 2020 Jun 30]. p. stemcells.nih.gov/info/2001report/chapter4.htm. Available from: https://stemcells.nih.gov/info/2001report/chapter4.htm
- Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. 2005;7(5):393–395. Elsevier
- Caplan AI. What’s in a Name? Internet. Tissue Eng Part A. 201016(8):2415–2417. Mary Ann Liebert, Inc., publishers.
- Murray IR, Chahla J, Frank RM, et al. Rogue stem cell clinics. Bone Joint J [ Internet]. 2020;102-B(2):148–154. The British Editorial Society of Bone & Joint Surgery.
- Le Blanc K, Pittenger MF, Mesenchymal stem cells: progress toward promise. Cytotherapy, Internet. Taylor & Francis. 2005. 7: 36–45. Available from: .
- Gimble JM. Adipose tissue-derived therapeutics. Internet. Expert Opin Biol Ther. 2003;3(5):705–713. Taylor & Francis.
- Muschler GF, Nitto H, Boehm CA, et al. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. Internet. J Orthop Res. 2001 Available from;19(1):117–125. John Wiley & Sons, Ltd.
- Hernigou P, Poignard A, Beaujean F, et al. Percutaneous autologous bone-marrow grafting for nonunions: influence of the number and concentration of progenitor cells. JBJS [ Available from:]. 2005;87. Internet: https://journals.lww.com/jbjsjournal/Fulltext/2005/07000/Percutaneous_Autologous_Bone_Marrow_Grafting_for.3.aspx.
- Fraser JK, Wulur I, Alfonso Z, et al., Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. Internet]. 2006;24:150–154. Available from: http://www.sciencedirect.com/science/article/pii/S016777990600028X
- Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. Internet]. 2004;6:7–14. Available from: http://www.sciencedirect.com/science/article/pii/S1465324904707197.
- Centeno C, Pitts J, Al-Sayegh H, et al., Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Zustin J, editor. Biomed Res Int, Internet. Hindawi Publishing Corporation. 2014. 2014:370621. Available from.
- Usuelli FG, D’Ambrosi R, Maccario C, et al. Adipose-derived stem cells in orthopaedic pathologies. Br Med Bull. 2017;124(1):31–54. Oxford University Press
- Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. Elsevier
- Looi QH, Eng SP, Liau LL, et al. Mesenchymal stem cell therapy for sports injuries - from research to clinical practice. Sains Malaysiana. Internet]. 2020;49:825–838. Available from: http://www.ukm.my/jsm/pdf_files/SM-PDF-49-4-2020/12.pdf.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Kader N, Asopa V, Baryeh K, et al., Cell-based therapy in soft tissue sports injuries of the knee: a systematic review. Expert Opin Biol Ther, Internet. Taylor & Francis. 2021. 1–13. Available from https://doi.org/10.1080/14712598.2021.1872538
- Viswanathan M, Patnode CD, Berkman ND, et al. Assessing the risk of bias in systematic reviews of health care interventions. Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US). 2008; Available from: http://www.ncbi.nlm.nih.gov/pubmed/30125066
- Ellis PD. Effect sizes and the interpretation of research results in international business. J Int Bus Stud. 2010. 41(9):1581–1588.
- Rosenthal JA Qualitative descriptors of strength of association and effect size. J Soc Serv Res. 1996;21:37–59.
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods, Internet. John Wiley & Sons, Ltd. 2020. 121: 55–61
- Moon YL, Jo S-H, Song CH, et al. Autologous bone marrow plasma injection after arthroscopic debridement for elbow tendinosis. Ann Acad Med Singap. 2008;37(7):559–563.
- Connell D, Datir A, Alyas F, et al. Treatment of lateral epicondylitis using skin-derived tenocyte-like cells. Br J Sports Med. 2009;43(4):293–298.
- Wang A, Breidahl W, Mackie KE, et al. Autologous tenocyte injection for the treatment of severe, chronic resistant lateral epicondylitis: a pilot study. Am J Sports Med. 2013;41(12):2925–2932.
- Singh A, Gangwar DS, Singh S. Bone marrow injection: a novel treatment for tennis elbow. J Nat Sci Biol Med. 2014;5(2):389–391. Medknow Publications
- Lee SY, Kim W, Lim C, et al. Treatment of lateral epicondylosis by using allogeneic adipose-derived mesenchymal stem cells: a pilot study. Stem Cells. 2015;33(10):2995–3005. Wiley-Blackwell
- Wang A, Mackie K, Breidahl W, et al. Evidence for the durability of autologous tenocyte injection for treatment of chronic resistant lateral epicondylitis: mean 4.5-year clinical follow-up. Am J Sports Med. 2015;43(7):1775–1783.
- Ellera Gomes JL, Da Silva RC, Silla LMR, et al. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):373–377
- Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811–1818.
- Kim SJ, Kim EK, Kim SJ, et al. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res. 2018;13(1):1–7.
- Centeno CJ, Al-Sayegh H, Bashir J, et al. A prospective multi-Site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269–276.
- Kim SJ, Song DH, Park JW, et al. Effect of bone marrow aspirate concentrate-platelet-rich plasma on tendon-derived stem cells and rotator cuff tendon tear. Cell Transplant. 2017;26(5):867–878.
- Kim YS, Sung CH, Chung SH, et al. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. 2017;45(9):2010–2018.
- Jo CH, Chai JW, Jeong EC, et al. Intratendinous injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of rotator cuff disease: a first-in-human trial. Stem Cells. 2018;36(9):1441–1450.
- Darrow M, Shaw B, Schmidt N, et al., Treatment of shoulder osteoarthritis and rotator cuff tears with bone marrow concentrate and whole bone marrow injections. Schumacher U, editor. Cogent Med, Internet. Cogent OA. 2019. 6: 1628883. Available from. .
- Jo CH, Chai JW, Jeong EC, et al. Intratendinous Injection of Mesenchymal Stem Cells for the Treatment of Rotator Cuff Disease: a 2-Year Follow-Up Study. Internet. Arthrosc - J Arthrosc Relat Surg. 2020 Available from;36(4):971–980. The Arthroscopy Association of North America.
- Muench LN, Kia C, Jerliu A, et al. Clinical outcomes following biologically enhanced patch augmentation repair as a salvage procedure for revision massive rotator cuff tears. Arthrosc - J Arthrosc Relat Surg, Internet. Arthroscopy Association of North America. 2020. 1–10. Available from.https://doi.org/10.1016/j.arthro.2020.02.006
- Protzman NM, Stopyra GA, Hoffman JK. Biologically enhanced healing of the human rotator cuff: 8-month postoperative histological evaluation. Orthopedics. 2013;36(1):38–41.
- Wang AW, Bauer S, Goonatillake M, et al. Autologous tenocyte implantation, a novel treatment for partial-thickness rotator cuff tear and tendinopathy in an elite athlete. BMJ Case Rep. 2013;bcr2012007899.
- Centeno C, Fausel Z, Stemper I, et al. Trial of the treatment of rotator cuff tears with bone marrow concentrate and platelet products compared to exercise therapy: a midterm analysis. Stem Cells Int. 2020;2020:2020.
- Lamas JR, García-Fernández C, Tornero-Esteban P, et al. Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full-thickness rotator cuff tears. Trials. 2019;20(1):1–9.
- Walton MJ, Mackie K, Fallon M, et al. The reliability and validity of magnetic resonance imaging in the assessment of chronic lateral epicondylitis. J Hand Surg Am. Internet]. 2011;36:475–479. Available from: http://www.sciencedirect.com/science/article/pii/S0363502310014541.
- Hurd JL, Facile TR, Weiss J, et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study. J Orthop Surg Res. 2020;15(1):122.
- Wang A, Mackie K, Breidahl W, et al. Evidence for the durability of autologous tenocyte injection for treatment of chronic resistant lateral epicondylitis: mean 4.5-year clinical follow-up. Am J Sports Med. 2015;43(7):1775–1783. SAGE Publications Inc
- Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Jt Surg Am. 2015;97(1):1–3.
- Murray IR, Geeslin AG, Goudie EB, et al. Minimum information for studies evaluating biologics in orthopaedics (MIBO): platelet-rich plasma and mesenchymal stem cells. J Bone Jt Surg Am. 2017;99(10):809–819. Lippincott Williams and Wilkins
- Gulotta LV, Kovacevic D, Montgomery S, et al. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Internet. Am J Sports Med. 2010;38(7):1429–1437. SAGE Publications Inc STM.
- Degen R, Carbone A, Carballo C, et al. The effect of purified multi-potent human bone-marrow derived mesenchymal stem cells on rotator cuff tendon healing in an athymic rat. Orthop J Sport Med. 2016;4(7_suppl4):2325967116S0014. SAGE Publications
- Kroslak M, Murrell GAC. Surgical treatment of lateral epicondylitis: a prospective, randomized, double-blinded, placebo-controlled clinical trial. Internet. Am J Sports Med. 201846(5):1106–1113. SAGE Publications Inc STM.
- Saltzman BM, Leroux T, Meyer MA, et al. The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis: a meta-analysis of evidence level 1 studies. Am J Sports Med. 2016;45(11):2647–2653. SAGE Publications Inc STM
- Dragoo JL, Meadows MC, The use of biologics for the elbow: a critical analysis review. J Shoulder Elb Surg. Internet]. 2019;28:2053–2060. Available from: http://www.sciencedirect.com/science/article/pii/S105827461930521X
- Liu XN, Yang CJ, Kim JE, et al. Enhanced tendon-to-bone healing of chronic rotator cuff tears by bone marrow aspirate concentrate in a rabbit model. Clin Orthop Surg. Korean Orthopaedic Association. 2018;10(1):99–110.
- Yokoya S, Mochizuki Y, Natsu K, et al. Rotator cuff regeneration using a bioabsorbable material with bone marrow–derived mesenchymal stem cells in a rabbit model. Internet. Am J Sports Med. 2012 Available from;40(6):1259–1268. SAGE Publications Inc STM.
- McDougall RA, Canapp SO, Canapp DA. Ultrasonographic findings in 41 dogs treated with bone marrow aspirate concentrate and platelet-rich plasma for a supraspinatus tendinopathy: a retrospective study. Front Vet Sci 2018;5:98. Internet. Available from: .
- Lu L-Y, Kuang C-Y YF. Magnetic resonance imaging and biomechanical analysis of adipose-derived stromal vascular fraction applied on rotator cuff repair in rabbits. Chin Med J (Engl). Internet]. 2018;131. Available from: https://journals.lww.com/cmj/Fulltext/2018/01050/Magnetic_Resonance_Imaging_and_Biomechanical.11.aspx
- Chen JM, Willers C, Xu J, et al. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Internet. Tissue Eng. 2007 Available from;13(7):1479–1491. Mary Ann Liebert, Inc., publishers.
- Cao Y, Liu Y, Liu W, et al., Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast Reconstr Surg. Internet]. 2002;110(5). Available from: https://journals.lww.com/plasreconsurg/Fulltext/2002/10000/Bridging_Tendon_Defects_Using_Autologous_Tenocyte.11.aspx
- Andia I, Maffulli N. Some patients (and some of us) respond better to some biological therapies: the as yet unsolved conundrum. J Orthop Traumatol. 2018;19(1):1.
- Centeno CJ, Al-Sayegh H, Freeman MD, et al. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016;40(8):1755–1765. . Springer Verlag
- Brindley DA, French A, Suh J, et al. The implementation of novel collaborative structures for the identification and resolution of barriers to pluripotent stem cell translation. Stem Cells Dev. 2013;22(S1):63–72. .
- LaPrade RF, Geeslin AG, Murray IR, et al. Biologic treatments for sports injuries ii think Tank—Current concepts, future research, and barriers to advancement, part 1. Am J Sports Med. 2016;44(12):3270–3283. . SAGE Publications Inc
- Murray IR, Chahla J, Safran MR, et al. International expert consensus on a cell therapy communication tool: DOSES. JBJS Internet. 2019;101. Available from: https://journals.lww.com/jbjsjournal/Fulltext/2019/05150/International_Expert_Consensus_on_a_Cell_Therapy.6.aspx.