ABSTRACT
Introduction: Current guidelines recommend prophylactic treatment of hemophilia B with the missing coagulation factor IX, either with standard half-life or extended half-life products. Extended half-life products have half-lives three to six times longer than the former, allowing a reduction in the number of weekly injections and therefore, potentially impacting on treatment adherence and quality of life. Albutrepenonacog alfa is an extended half-life fusion protein of coagulation factor IX with recombinant human albumin, indicated for both on-demand and prophylactic treatment for bleeding in patients with hemophilia B of all ages.
Areas covered: The authors review the clinical and pharmacokinetic characteristics of albutrepenonacog alfa, as well as the available information regarding trough levels and real-world evidence. Given the availability of other factor IX products in the market, indirect comparisons of clinical and pharmacokinetic characteristics are presented.
Expert opinion: The authors exhibit their expert opinion on which patient profiles are candidates for prophylactic treatment with albutrepenonacog alfa, and on the management of patients in terms of dosing, regimens of administration and protocols for switching the treatment.
1. Introduction
Hemophilia B is a congenital bleeding disorder caused by a deficiency of coagulation factor IX (FIX), manifested by either spontaneous bleeding or bleeding after trauma or surgery [Citation1,Citation2]. According to the World Federation of Hemophilia, hemophilia B affects approximately 3.75 per 100,000 males, and rates vary considerably between different countries [Citation3,Citation4]. The severity of hemophilia depends on the blood levels of clotting factor – levels of 5–40% have been correlated with mild disease; 1–5%, with moderate disease; and less than 1%, with severe disease [Citation5].
According to current guidelines, prophylaxis with FIX concentrates should be the goal of therapy to preserve normal musculoskeletal function, since it prevents bleeding and joint destruction, and it is a cost-effective intervention. Both plasma-derived (pd-FIX) and recombinant FIX (rFIX) concentrates are recommended options for replacement therapy [Citation2]. However, rFIX concentrates are the preferred choice in some countries, due to a negligible risk of pathogen contamination [Citation6–8].
Current prophylactic treatment options include either standard half-life FIX (SHL-FIX) or extended half-life FIX (EHL-FIX) products (). SHL-FIX products typically need to be administered two or three times per week. On the other hand, EHL-FIX have half-lives three to six times longer than SHL-FIX, and therefore entail less injections and a potential improvement in adherence [Citation1,Citation2,Citation9,Citation10].
Table 1. Current recombinant prophylactic treatment options for hemophilia B approved by the EMA
The clinical development program of albutrepenonacog alfa (recombinant fusion protein linking FIX with albumin -rIX-FP-), initiated in 2010, has provided promising clinical data and has brought up new prophylactic regimens for patients with hemophilia B.
The authors of this publication held a virtual meeting to review the available evidence and provide expert opinion on the new scenario of prophylaxis of bleeding in patients with hemophilia B treated with rIX-FP.
2. Characteristics of albutrepenonacog alfa (recombinant fusion protein linking FIX with albumin, rIX-FP)
The rIX-FP molecule was designed to improve the biological characteristics of rFIX in vivo. To that purpose, rFIX was genetically fused to albumin via a peptide linker that undergoes cleavage in parallel with FIX activation. Therefore, the molecule remains intact until activation and cleavage of albumin, which makes the circulation time of the product longer than that of SHL-FIX [Citation11,Citation12]. Albumin is a convenient fusion protein, since its half-life is around 20 days, it lacks enzymatic and immune activity, it is a natural carrier of other proteins and peptides, it is already abundant in plasma, and it is a very stable molecule [Citation13]. Besides, albumin recycles via the neonatal Fc receptor pathway, which is an additional mechanism responsible for prolonging the half-life of rIX-FP in vivo [Citation14].
rIX-FP is approved for long-term prophylaxis to prevent bleeding in patients with severe hemophilia B, at a weekly dose of 35–50 IU/kg of body weight. Adult patients who are well-controlled on a once-weekly regimen might be treated with up to 75 IU/kg on an interval of 10 or 14 days. When control is also achieved with these regimens, further extension of the treatment interval to 21 day with a dose of 100 IU/kg may be considered in some adult patients [Citation15].
3. Pharmacokinetics
rIX-FP was first studied in a phase I, multicenter, dose-escalation trial that assessed pharmacokinetics (PK) and safety of the molecule in 25 adult patients with hemophilia B. rIX-FP produced an increase of FIX activity levels, compared to the same dose of rFIX or pd-FIX. The mean terminal half-life and the mean area under the curve (AUC) were higher with rIX-FP than with rFIX – 5.3- and 7-fold increase, respectively. Other PK parameters, such as incremental recovery and clearance, were also improved with rIX-FP [Citation16,Citation17]. In the phase III study, a 14-day PK evaluation was also performed, and rIX-FP displayed a better PK profile when compared to SHL-FIX products; the mean terminal half-life was 4.3 times longer than with the previous FIX treatment [Citation18]. In the pediatric population, when compared to the previous FIX treatment, the mean terminal half-life of rIX-FP was 4.3 times longer, and the clearance was 6.4 times slower [Citation19].
4. Trough levels
In patients with hemophilia B, maintaining appropriate trough levels of the clotting factor helps to reduce the incidence of bleeds, and consequently, to reduce joint disease and improve quality of life and life expectancy [Citation2,Citation20]. However, due to phenotype variability among patients, current recommendations tend to individualized prophylaxis approaches, rather than considering specific trough levels for all patients. Besides, the time that a patient remains above certain levels can be as important as the trough level itself [Citation20,Citation21]. Achieving high trough levels is particularly important in patients with bleeding episodes, with arthropathies, with high physical activity, and those undergoing surgery or invasive procedures [Citation21].
Trough levels higher than 5 IU/dL can be maintained 7 days after the administration of 25 IU/kg of rIX-FP, or 14 days after 50 IU/kg in adults [Citation17], and 10 days after 50 IU/kg in children [Citation19]. In contrast, the mean FIX activity is around 5 IU/dL 48 hours after the administration of 50 IU/kg of either rFIX or pd-FIX, both in adults and pediatrics [Citation17,Citation19]. For the adult and adolescent population, the mean steady‐state trough FIX activity level was 20.9% when receiving 35‐50 IU/kg every 7 days, and 12.76% when receiving 50‐75 IU/kg every 14 days. In pediatrics treated with 35‐50 IU/kg every 7 days, it was 12.8% [Citation22]. In the extension study that evaluated the efficacy of rIX-FP in adult patients previously enrolled in the phase III study or who initiated prophylaxis following surgery, mean steady-state trough FIX activity was 22.0%, 19.8%, 13.6%, and 7.6% with 7-, 10-, 14-, and 21-day regimens, respectively [Citation23].
5. Clinical efficacy of rIX-FP
A phase III study evaluated the efficacy and safety of rIX-FP in 63 previously treated adolescent and adult patients with severe hemophilia B. The median annualized spontaneous bleeding rate (AsBR) was reduced from 15.43 to 0.00 after switching from on-demand to weekly prophylaxis treatment (p < 0.0001). Median AsBR was also 0.00 on 10- and 14-day regimens. The annualized bleeding rate (ABR) was 0.00 in patients on 7- and 10-day regimens, and 1.08 in patients with 14-day regimens. The annualized joint bleeding rate (AjBR) was 0.00 on all prophylaxis regimens [Citation18]. In the extension study, median AsBR was 0.00, 0.28, 0.37 and 0.00 for the 7-, 10-, 14- and 21-day regimens, respectively [Citation23], meaning that 14- and 21-day regimens have comparable efficacy to the 7-day regimen.
In children under 12 who were treated with rIX-FP on a prophylaxis weekly regimen, median AsBR turned out to be 0.00 [Citation19]. The extension study for the pediatric population showed a very low median AsBR on any regimen: 0.00 for the 7- and 10-day regimens, and 1.10 for the 14-day regimen [Citation24].
6. Comparison to other EHL-FIX
A systematic review conducted by Davis and collaborators compared, through indirect statistical comparison, the efficacy of rIX-FP to that of other SHL-FIX and EHL-FIX for prophylaxis treatment of adult patients [Citation9]. On a 7-day regimen, the mean ABR was significantly lower with 35–50 IU/kg of rIX-FP than with 50 IU/kg of rFIX-Fc (1.2 [1.8] vs 3.1 [2.9], p < 0.001). Mean AsBR and AjBR values were not provided in the rFIX-Fc pivotal study, so an indirect comparison was not possible. However, and although data come from different studies, the median AsBR and AjBR values were lower with rIX-FP than with rFIX-Fc (median AsBR was 0.00 with all regimens of rIX-FP vs 1.0 for weekly and 0.9 for individualized administration of rFIX-Fc; the median AjBR was 0.00 with all regimens of rIX-FP vs 1.1 for weekly and 0.4 for individualized administration of rFIX-Fc) [Citation18,Citation25]. No indirect comparison has been performed in pediatric patients, since mean bleeding rates were not reported in the pivotal study of rFIX-Fc [Citation26].
An indirect comparison with N9-GP was neither possible, since mean ABR, AsBR, and AjBR were not provided in the pivotal study [Citation27]; however, rIX-FP achieved a lower median ABR than N9-GP, on 7-day regimen (0.00 vs 1.04). rIX-FP also achieved lower mean ABR values than the SHL-FIX products encompassed in the systematic review [Citation9,Citation28,Citation29].
7. Real-world evidence using rIX-FP
A retrospective study collected data from preexisting medical records of patients treated with rIX-FP for 8 weeks or more in Germany, and compared its efficacy with the patient’s prior FIX product. The mean ABR decreased from 2.6 (2.9) with the prior product to 0.3 (0.6) with rIX-FP, and the proportion of patients with zero bleeds increased from 24% to 81%. The mean factor consumption was nearly half with rIX-FP than with prior prophylaxis (44.2 vs 82.3 IU/kg/week) [Citation30].
A similar study was performed with patients from Italy, Belgium and the United Kingdom. Mean ABR reductions after the switch ranged from 67.7% to 94.3%, depending on the doses administered in each country. The administration of rIX-FP increased the proportion of patients experiencing zero spontaneous bleeds from 41% to 88%. Dosing and frequency of administration were also reduced after the switch [Citation31].
In the United States, 145 patients treated with rIX-FP were included in a study to evaluate utilization and bleeding rates. The mean ABR changed from 8.9 to 0.7 when switching from rFIX-Fc to rIX-FP, and from 4.5 to 1.5 when switching from rFIX to rIX-FP. Mean and median consumption was also diminished with rIX-FP, when compared to previous FIX, and specifically to rFIX or rIX-Fc [Citation32,Citation33].
Some case series and case reports from patients receiving prophylactic treatment with rIX-FP in Spain have been recently presented. In a case series of six patients treated for two years, the median ABR was 0.50, the median AsBR was 0.25, and patients reported good quality of life [Citation34]. A case report of a patient with severe hemophilia B treated with rFIX from the age of 15 months to 7 years with poor venous access and repetition infections, using rFIX and then switching to rIX-FP, showed a reduction of 64.8% in FIX consumption, and no hospitalizations were observed after 6 months with rIX-FP [Citation35]. Another patient, a 3-year-old with moderate hemophilia B, was switched from rFIX to rIX-FP due to difficult venous access and mobility reasons (health emergency secondary to COVID-19): the efficacy was maintained, the reported quality of life was improved, and the number of injections administered was reduced [Citation36]. A recent case series has described the efficacy and safety of rIX-FP in two previously untreated pediatric patients in Italy [Citation37].
A few recent reported real world experiences have associated prophylaxis with rIX-FP with spontaneous bleeding and poor response [Citation38–41]. In a single center case series, the recommendations of the European Summary of Product Characteristics (SmPC) to perform the switches to rIX-FP were not followed in none of the three patients reported with poor bleed control. All of them started with a 2-week regimen, without ensuring well-control in the previous weekly regimen, as recommended in the SmPC, which may explain the clinical outcome in these three patients [Citation38]. On the other hand, the authors reported that 25 other patients had been switched to rIX-FP since its licensure, with similar dosing to the three reported cases and had positive outcomes. In another report, key information was missing: no data on the dose or dose regimens were provided, neither, for example, regarding the patients’ baseline bleeding rate, physical activity levels and joint health. Furthermore, patients ranged in age from a few months to 23 years. The mean or median ages of patients on each product were not provided and, as children typically have higher bleeding rates than adults, this could have had an impact on the findings [Citation39,Citation40]. The authors thereof speculated that a poor distribution of rIX-FP to the extravascular space could be the key contributor for these results, although it has been published that extravascular distribution of rIX-FP is comparable to that of natural FIX [Citation42,Citation43]. Moreover, evidence to ensure that biodistribution of FIX is related to bleeding rates is still lacking, and clinical efficacy is still the most relevant outcome to assess treatment success [Citation44,Citation45]. In a third report, 3 out of the 7 patients treated with rIX-FP reported unexpected bleeding episodes. However, mean doses used were lower than those currently recommended in the product label [Citation41].
8. Safety
rIX-FP was well tolerated by adult and pediatric patients included in the clinical trials of the development program. Of the reported adverse events (AEs), most were mild or moderate in severity [Citation16,Citation18,Citation19,Citation46]. As described in the SmPC, the common (≥1/100 to <1/10) mild/moderate AEs are headache, dizziness and injections site reactions. Other adverse reactions as rash, hypersensitivity or eczema are uncommon (uncommon (≥1/1,000 to <1/100) [Citation15]. In the pivotal study, the most frequently reported treatment-emergent AEs were nasopharyngitis, headache, arthralgia, and influenza; only two patients experienced serious AEs (SAEs), and were classified as not related to rIX-FP treatment [Citation18]. In the extension study, the majority of treatment-emergent AEs were also mild to moderate. About 17% of adults experienced SAEs, and only one was considered to be related to the treatment: arterial thrombosis after undergoing knee replacement surgery, followed by persistent postoperative complications, although the patient’s history was also considered a contributing factor. 29.1% of pediatric patients experienced SAEs, but none was related to treatment [Citation23,Citation24]. The safety profile of the 21-day regimen was similar to that of the 14-day regimen [Citation23]. No inhibitors or antibodies were detected in any patient participating in these studies. However, an isolated case of a young patient with hemophiliac arthropathy, mainly due to a delay in the initiation of an adequate prophylactic treatment, treated with prophylactic rIX-FP and developing a low-titer inhibitor has been reported [Citation47]. This patient started with an off-label prophylactic regimen (50 IU/kg every 14 days).
9. Conclusions
rIX-FP has an excellent efficacy in the prophylactic treatment of patients with hemophilia B, with very low median values of ABR, AsBR and AjBR, which can positively impact joint protection, preservation of physical activity and quality of life, as observed in real-world data. Clinical trials have also demonstrated that over 95% of treated patients can achieve a steady-state FIX activity trough level higher than 5%, and a good bleeding control under 7-, 14- and even 21-day regimens; all this data translates in a decrease in the burden of treatment administration, less factor consumption and higher adherence rates.
In summary, rIX-FP is an excellent choice for the prophylactic treatment of any patient with hemophilia B, providing benefits in terms of efficacy, adherence and quality of life.
10. Expert opinion
According to the authors’ opinion, and based on its clinical efficacy, rIX-FP is a convenient option for prophylactic treatment of hemophilia B in all patients, in any age group. Its PK profile allows an extension of the dosing interval up to 14 or even 21 days, and a reduction in the number of intravenous injections, which may have a positive impact on patient’s quality of life. In fact, substantial improvements have been observed in the quality of life of pediatric patients, as well as in the satisfaction of their caregivers [Citation48]. Extended dosing intervals can also have a positive impact on adherence, which in turn can lead to improved clinical outcomes. Excellent adherence rates have been observed in both clinical trials and clinical practice with rIX-FP [Citation49].
Specifically, rIX-FP can be a good option in pediatric and teenage patients, since they usually have higher physical activity, more difficult venous access, higher number of traumatic bleeds and higher FIX clearance. Additionally, the fact of receiving intravenous injections can be more unpleasant and burdensome in pediatric and young patients, so reducing their frequency may be advisable. Likewise, adult patients who have poor venous access or a high level of physical activity can obtain an extra benefit from rIX-FP administration.
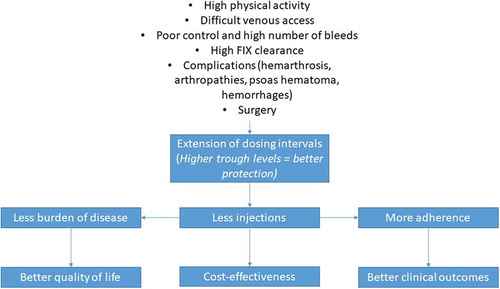
Adult patients who require a high number of infusions or those poorly controlled with the former treatment can also especially benefit from rIX-FP, since the reduction in the number of injections can entail an even larger increase in quality of life, and it could be a cost-effective option. Actually, a recent pharmacoeconomic model assessed the budget impact of treating severe hemophilia B with reimbursed rIX-FP over 3 years in Italy, concluding that rIX-FP is expected to decrease pharmaceutical costs [Citation50]. A recent real world study found that the cost per success (defined as patients experiencing no spontaneous bleeding) in patients switching from nonacog alfa to rIX-FP was reduced by 29% in Italy, 55% in Belgium, and 50% in the United Kingdom. The reduction continued to be true even when the price of nonacog alfa or when the efficacy of rIX-FP were reduced by 20% [Citation31]. Patients with complications, such as arthropathies, severe muscle hematomas, and life-threatening hemorrhages, or those who are to undergo surgery, are also good candidates for prophylaxis with rIX-FP ().
Figure 1. Characteristics of patients who may especially benefit from prophylactic treatment with rIX-FP and potential advantages
•Optimal dosing of FIX products relies on accurately monitoring plasma factor levels [Citation51]. The authors agreed that trough levels need to be individualized according to each patient’s characteristics. Higher trough levels should be considered in patients with joint concerns, uncontrolled patients, patients undergoing surgery or on secondary prophylaxis after muscle hematomas or life-threatening hemorrhages. Likewise, children and adolescents with a high clearance and high physical activity would also be candidates for higher trough levels. Current recommendations include factor target levels of 5–15% for children and adults performing high-risk activity, 15–30% for patients performing intensive sport activity, and 50–80% for patients performing very high impact physical activity [Citation21]. Schedules involving weekly dosing or dosing every two weeks are feasible with rIX-FP, and it is a good option for patients requiring high trough levels.
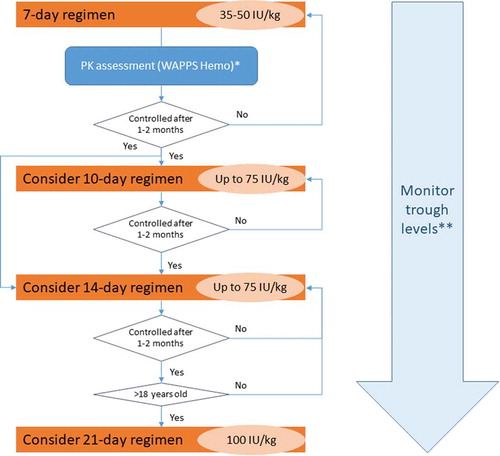
As stated in the summary of product characteristics, the usual dose of rIX-FP for long-term prophylaxis to prevent bleeding is 35–50 IU/kg once a week, and well-controlled adolescent and adult patients on a once-weekly regimen can receive up to 75 IU/kg dose every 10 or 14 days. In 18-year-old or older patients, a greater extension of the treatment interval can be considered [Citation15]. In order to help practitioners to switch patients from other FIX products to rIX-FP, the authors designed an algorithm, where decisions are prompted by hemostatic efficacy after a period under a specific regimen (). According to the opinion of the experts, and based on recent recommendations [Citation20,Citation52], a PK assessment can be done before switching to estimate the dose and frequency of infusion for maintaining appropriate trough levels; and/or two weeks after switching, to assess whether the dose and frequency of infusion are adequate. The Web Accessible Population Pharmacokinetic Service for Hemophilia (WAPPS-Hemo) is a free tool to make PK evaluations easier [Citation53]. The authors also suggest to assess trough levels at the discretion of the treating clinician, and perform further PK studies when changes in trough levels are identified. The one-stage (OS) assay is useful and reliable to confirm that adequate rIX-FP levels have been achieved and maintained. However, it should be considered that some activated partial thromboplastin time (aPTT) reagents, such as actin FS or kaolin-based aPTT reagents could lead to an underestimation of the activity of up to 50% [Citation15,Citation54]. Therefore, FIX activity was measured in clinical trials evaluating rFIX‐FP with silica activator aPTT reagent [Citation55,Citation56].
Figure 2. Algorithm for switching patients from FIX products to rIX-FP
Article highlights
Albutrepenonacog alfa is a fusion protein of recombinant FIX with human albumin, which extends the product circulation time.
Albutrepenonacog alfa is an effective and safe option for the prophylactic treatment of patients with hemophilia B of all ages.
Optimal dosing of FIX products relies on accurately monitoring plasma factor levels. High trough levels can be achieved with the administration of albutrepenonacog alfa, regardless of the administration regimen (7-, 10-, 14- or 21-day regimens), which represents an advantage for patients with bleeding episodes, arthropathies, high physical activity, and those undergoing surgery or invasive procedures.
This box summarizes key points contained in the article.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Declaration of interest
María Teresa Álvarez Román has participated as speaker, in advisory boards and sponsored symposia with Novo Nordisk, Bayer, Takeda, Roche, Pfizer, Octapharma, Amgen, Novartis, CSL Behring and Sobi. Olga Benítez has participated as consultant, speaker or has received medical grants from Bayer, CSL Behring, Pfizer, Sobi, Roche and Takeda. Francisco J. López Jaime has participated as consultant or speaker for CSL Behring, Novo Nordisk, Roche, Sobi and Takeda. José Mateo Arranz has participated as speaker or consultant for CSL-Behring, Bayer-AG, Sobi, Roche, Octapharma, Grifols, Takeda, Novo Nordisk. Ramiro Núñez has received fees from Novo Nordisk, Takeda, Grifols, Roche, Pfizer, Octapharma, CSL Behring and Sobi. Victor Jiménez Yuste has received grants, travel or research support from Pfizer, Grifols, Shire, Novo Nordisk, Sobi and Octapharma, and has participated in scientific advisory boards for CSL Behring, Sobi, Octapharma, Grifols, Pfizer, Shire, Novo Nordisk, Roche, Biomarin and Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The authors want to thank Ampersand Consulting and CSL Behring, respectively, for organizing and supporting the virtual meeting to review the available evidence.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040):187–197.
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(S6):1–158.
- Stonebraker JS, Bolton-Maggs PHB, Michael Soucie J, et al. A study of variations in the reported haemophilia B prevalence around the world. Haemophilia. 2012;18(3):e91–4.
- World Federation of Hemophilia. Report on the WFH Annual Global Survey 2019. 2020.
- White CG, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85(3):560.
- Australian Haemophilia Centre Directors’ Organisation. Guidelines for the management of haemophilia in Australia. 2016.
- National Hemophilia Foundation. MASAC Recommendations Concerning Products Licensed for the Treatment of Hemophilia and Other Bleeding Disorders. 2020.
- Keeling D, Tait C, Makris M. Guideline on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. Haemophilia. 2008;14(4):671–684.
- Davis J, Yan S, Matsushita T, et al. Systematic review and analysis of efficacy of recombinant factor IX products for prophylactic treatment of hemophilia B in comparison with rIX-FP. J Med Econ. 2019;22(10):1014–1021.
- Brennan Y, Parikh S, McRae S, et al. The Australian experience with switching to extended half-life factor VIII and IX concentrates: on behalf of the Australian Haemophilia Centre Directors’ Organisation. Haemophilia. 2020;26(3):529–535.
- Schulte S. Half-life extension through albumin fusion technologies. Thromb Res. 2009;124(Suppl):S6–8.
- Metzner HJ, Weimer T, Kronthaler U, et al. Genetic fusion to albumin improves the pharmacokinetic properties of factor IX. Thromb Haemost. 2009;102(10):634–644.
- Lyseng-Williamson KA. Coagulation Factor IX (Recombinant), Albumin Fusion Protein (Albutrepenonacog Alfa; Idelvion®): a review of its use in haemophilia B. Drugs. 2017;77(1):97–106.
- Chia J, Louber J, Glauser I, et al. Half-life– extended recombinant coagulation factor IX–albumin fusion protein is recycled via the FcRn-mediated pathway. J Biol Chem. 2018;293(17):6363–6373.
- Idelivon, Summary of product characteristics [Internet]. [cited 2021 Apr 21]. Available from: https://www.ema.europa.eu/en/documents/product-information/idelvion-epar-product-information_en.pdf
- Santagostino E. PROLONG-9FP clinical development program-phase i results of recombinant fusion protein linking coagulation factor IX with recombinant albumin (rIX-FP). Thromb Res. 2013;131:S7.
- Santagostino E, Negrier C, Klamroth R, et al. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patients. Blood. 2012;120(12):2405–2411.
- Santagostino E, Martinowitz U, Lissitchkov T, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood. 2016;127(14):1761–1769.
- Kenet G, Chambost H, Male C, et al. Long-acting recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in children: results of a phase 3 trial. Thromb Haemost. 2016;116(10):659–668.
- Iorio A, Blanchette V, Blatny J, et al. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15(12):2461–2465.
- Iorio A, Iserman E, Blanchette V, et al. Target plasma factor levels for personalized treatment in haemophilia: a Delphi consensus statement. Haemophilia. 2017;23(3):e170–e179.
- Gill JC, Roberts J, Li Y, et al. Sustained high trough factor IX activity levels with continued use of rIX-FP in adult and paediatric patients with haemophilia B. Haemophilia. Blackwell Publishing Ltd; 2019. p. e219–e222.
- Mancuso ME, Lubetsky A, Pan-Petesch B, et al. Long-term safety and efficacy of rIX-FP prophylaxis with extended dosing intervals up to 21 days in adults/adolescents with hemophilia B. J Thromb Haemost. 2020;18(5):1065–1074.
- Kenet G, Chambost H, Male C, et al. Long-Term safety and efficacy of recombinant coagulation factor IX Albumin Fusion Protein (rIX-FP) in Previously treated pediatric patients with hemophilia B: results from a phase 3b extension study. Thromb Haemost. 2020;120(4):599–606.
- Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369:2313–2323.
- Fischer K, Kulkarni R, Nolan B, et al. Recombinant factor IX Fc fusion protein in children with haemophilia B (Kids B-LONG): results from a multicentre, non-randomised phase 3 study. Lancet Haematol. 2017;4(2):e75–e82.
- Collins PW, Young G, Knobe K, et al. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124(26):3880–3886.
- Kavakli K, Smith L, Kuliczkowski K, et al. Once-weekly prophylactic treatment vs. on-demand treatment with nonacog alfa in patients with moderately severe to severe haemophilia B. Haemophilia. 2016;22(3):381–388.
- Lambert T, Recht M, Valentino LA, et al. Reformulated BeneFix®: efficacy and safety in previously treated patients with moderately severe to severe haemophilia B. Haemophilia. 2007;13(3):233–243.
- Oldenburg J, Yan S, Maro G, et al. Assessing bleeding rates, related clinical impact and factor utilization in German hemophilia B patients treated with extended half-life rIX-FP compared to prior drug therapy. Curr Med Res Opin. 2020;36(1):9–15.
- Hermans C, Marino R, Lambert C, et al. Real-World utilisation and bleed rates in patients with haemophilia B who switched to recombinant factor IX Fusion Protein (rIX-FP): a retrospective international analysis. Adv Ther. 2020;37:2988–2998.
- Escobar M, Leissinger C, Yan S, et al. Comparison of rFIX utilization and bleed rates in US hemophilia B patients on rIX- FP and their prior rFIX drug. Presented at: ISTH Congress; 2018 Jul 17–21; Dublin.
- Escobar M, Santagostino E, Lessinger C, et al. Prophylaxis with rIX-FP reduces consumption compared with previous FIX in both adult and pediatric patients. Presented at: ISTH Congress; 2017 Jul 8–13; Berlin.
- Álvarez-Román M, López M, Martínez M, et al. [Retrospective analysis of the use, effectiveness and quality of life in patients with hemophilia B in prophylaxis treatment with albumin-fused recombinant FIX (rIX-FP) in Spain]. Presented at: XXXVI SETH National Congress; 2020 Oct 26–30; online.
- Megías-Vericat J, Rodríguez López M, Poveda J, et al. Clinical, pharmacokinetic and economic analysis of the first switch to an extended half-life factor IX (albutrepenonacog alfa) in Spain. Presented at: ISTH Congress; 2019 Jul 6–10; Melbourne.
- Díaz Jordán B, Valverde Templado A, Cebanu T, et al. [Real-life experience using rIX-FP in a patient with hemophilia B on prophylaxis after 100 days of switch to extended life factor IX]. Presented at: XXXVI SETH National Congress; 2020 Oct 26–30; online.
- Schiavulli M, Lupone MR, Pollio B, et al. Real-world experience of rIX-FP in two previously untreated paediatric patients with severe haemophilia B in Italy. Presented at: EAHAD Congress; 2021 Feb 3–5; online.
- Kleiboer B, Nielsen B, Ma AD, et al. Excessive breakthrough bleeding in haemophilia B patients on factor IX-albumin fusion protein prophylactic therapy: a single centre case series. Haemophilia. 2020;26(1):e23–e25.
- Malec LM, Croteau SE, Callaghan MU, et al. Spontaneous bleeding and poor bleeding response with extended half-life factor IX products: a survey of select US haemophilia treatment centres. Haemophilia. Blackwell Publishing Ltd; 2020. p. e128–e129.
- Malec LM, Croteau SE, Callaghan M, et al. Spontaneous bleeding and poor bleeding response with extended half-life factor IX products: a survey of select US hemophilia treatment centers. Res Pr Thromb Haemost. 2019;3.
- Rampotas A, Desborough MJR, Raza-Burton S, et al. A single centre retrospective study of low dose prophylaxis with extended half-life factor IX for severe haemophilia B. Haemophilia. 2020;26(2):278–281.
- Herrmann S, Doerr B, May F, et al. Tissue distribution of rIX‐FP after intravenous application to rodents. J Thromb Haemost. 2020;18(12):3194–3202.
- Herzog E, Harris S, Henson C, et al. Biodistribution of the recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in rats. Thromb Res. 2014;133(5):900–907.
- Walsh C, Coppens M, Escobar M, et al. Optimal trough levels in haemophilia B: raising expectations. Haemophilia. Blackwell Publishing Ltd; 2020. p. e334–e336.
- Lillicrap D. Evaluating the potential benefits of the extravascular pool of factor IX. Blood Coagul Fibrinolysis. 2021;32(1):68–69.
- Martinowitz U, Lubetsky A. Phase I/II, open-label, multicenter, safety, efficacy and PK study of a recombinant coagulation factor IX albumin fusion protein (rIX-FP) in subjects with hemophilia B. Thromb Res. 2013;131:S11–4.
- Zanon E, Pasca S, Simioni P. The sudden and unexpected appearance of inhibitors in a previously treated severe haemophilia B patient after the switch to albutrepenonacog alpha. Haemophilia. 2018;24(5):e372–e375.
- von Mackensen S, Shah J, Seifert W, et al. Health-related quality of life in paediatric haemophilia B patients treated with rIX-FP. Haemophilia. 2019;25(1):45–53.
- Mancuso ME, Oldenburg J, Boggio L, et al. High adherence to prophylaxis regimens in haemophilia B patients receiving rIX-FP: evidence from clinical trials and real-world practice. Haemophilia. 2020;26(4):637–642.
- Pradelli L, Villa S, Castaman G. Albutrepenonacog alfa (Idelvion®) for the treatment of Italian patients with hemophilia B: a budget impact model. Farmeconomia Heal Econ Ther Pathways. 2018;19:1–10.
- Lambert T, Benson G, Dolan G, et al. Practical aspects of extended half-life products for the treatment of haemophilia. Ther Adv Hematol. 2018;9(9):295–308.
- Iorio A. Using pharmacokinetics to individualize hemophilia therapy. Hematology. 2017;2017(1):595–604.
- Iorio A, Keepanasseril A, Foster G, et al. Development of a Web-Accessible Population Pharmacokinetic Service—Hemophilia (WAPPS-Hemo): study protocol. JMIR Res Protoc. 2016;5(4):e239.
- Ledger KT, Feussner A, Kalina U, et al. Performance of a recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in the one-stage assay. Presented at XXXII international congress of the WFH; 2016 Jul 24–26; Orlando.
- Kitchen S, Tiefenbacher S, Gosselin R. Factor activity assays for monitoring extended half-life FVIII and factor IX replacement therapies. Semin Thromb Hemost. 2017;43(3):331–337.
- Pouplard C, Galinat H, Ternisien C, et al. Multicentre evaluation of CK Prest® for assaying plasma levels of factor IX fused with albumin (Idelvion®). Haemophilia. 2019;25(5):e327–e330.
