ABSTRACT
Background: Information is limited from real-life studies evaluating the efficacy and safety of brodalumab.Research design and methods: In this real-life study, we retrospectively examined a database of 90 patients with moderate-to-severe psoriasis treated with brodalumab (210 mg, s.c.) and followed for 1 year. Disease severity and treatment response were assessed by the Psoriasis Area and Severity Index (PASI) at baseline and after 4, 12, 24, 36, and 48 weeks. Predictors of a PASI response were evaluated by logistic regression.Results: After 48 weeks, 92.2% of patients (mean age 50.2 ± 15 years) treated with brodalumab achieved a PASI score of <3. PASI score decreased from 17.4 ± 10.3 at baseline to 1.7 ± 3.9 and 1.4 ± 3.7 at 12 and 24 weeks, and PASI 75, 90, and 100 response was achieved in 87.3%, 81.8%, and 72.7% of patients, respectively, at 48 weeks.
Univariate regression revealed that previous exposure to anti-IL17A treatment was associated with poorer PASI response between 36 and 48 weeks. In difficult-to-treat cases previously having failed with other biologics, brodalumab significantly improved outcome, leading to complete remission.Conclusion: Brodalumab was observed to be effective and safe in patients with moderate-to-severe chronic psoriasis in a real-world setting.
1. Introduction
Psoriasis is a chronic inflammatory immune skin disease affecting around 2–3% worldwide [Citation1,Citation2].
Biological treatments such as tumor necrosis factor (TNF) and interleukin (IL) 12/23 and IL-17 inhibitors have revolutionized the therapeutic management of moderate-to-severe psoriasis, permitting a significant number of patients to attain clear skin [Citation3–10]. However, many patients still remain untreated or do not respond to or experience treatment-related toxicities [Citation11].
Brodalumab is a fully human monoclonal immunoglobulin G2 antibody and the third approved anti-IL-17 agent following secukinumab and ixekizumab with a unique broad mechanism of action based on the IL-17-receptor blockade [Citation12]. It selectively binds to human IL-17RA, thereby inhibiting the biological activity of several cytokines, including IL-17A, IL-17 F, IL-17 C, IL-17A/F heterodimer, and IL-25.
The rapid onset of action and high level of efficacy of brodalumab for the treatment of moderate-to severe plaque psoriasis was demonstrated in three phase 3 randomized double-blind trials (RCTs): AMAGINE-1, AMAGINE-2, and AMAGINE-3 [Citation12–15]. At week 12, PASI 75 improvement was obtained in a significantly higher proportion of patients treated with brodalumab at 210 mg and 140 mg doses vs. placebo (86% and 67%, respectively, vs. 8% [AMAGINE-2] and 85% and 69%, respectively, vs. 6% [AMAGINE-3]; p < 0.001). The rates of static physician’s global assessment (sPGA) scores of 0 or 1 were also significantly higher for brodalumab (p < 0.001). PASI 100 response rates at 12 weeks were also significantly higher with 210 mg of brodalumab compared to ustekinumab (44% vs. 22% [AMAGINE-2] and 37% vs. 19% [AMAGINE-3], P < 0.001). The PASI 100 response rates with 140 mg of brodalumab were 26% in AMAGINE-2 (P = 0.08 vs. ustekinumab) and 27% in AMAGINE-3 (P = 0.007).
The clinical results with brodalumab achieved during these phase 3 trials were compared with placebo and ustekinumab and followed-up until week 52 for the two different dosages (140 mg and 210 mg) used [Citation16]. Previous biologic treatments did not influence the efficacy results with brodalumab in AMAGINE-2 and AMAGINE-3 trials. In patients who had failed treatment with previous biologics, brodalumab was found to be three times more effective than ustekinumab based on PASI 100 [Citation17]. Furthermore, results from three phase 3 trials have highlighted the effect of brodalumab for the rapid and sustained treatment of scalp psoriasis [Citation18]. At week 12, significantly more patients receiving brodalumab achieved 75% and 100% improvement rates from baseline psoriasis scalp severity index (PSSI) compared with placebo. After 1 year, 63.8% of patients receiving brodalumab achieved a nail psoriasis severity index (NAPSI) of 0 compared to 39.1% treated with ustekinumab. However, clinical trials cannot be performed in all patient types; thus, a complete assessment of the therapies, especially in the long-term, requires real-world evidence on unselected patient populations [Citation19].
The current retrospective analysis evaluated the real-world experience of the Lazio region of Italy with patients treated with brodalumab with particular focus on cases that highlight critical aspects of the disease and their management in mainstream practice.
2. Materials and Methods
2.1. Study design and patient population
This cross-sectional ‘snapshot’ study included patients who were treated for at least 12 weeks with brodalumab from four large Tertiary Care Hospitals in Central Italy in the Lazio Region (i.e. coined the ‘Lazio Experience’). Patients were treated at the following centers: The Dermatology Unit of ‘Tor Vergata’ University of Rome, at Institute of Dermatology, Catholic University of the Sacred Heart in Rome, at Division of Dermatology ‘Daniele Innocenzi,’ University of Rome ‘La Sapienza,’ Polo Pontino and The Dermatology Unit of Istituto Dermopatico dell’Immacolata – IRCCS, Rome, from 29 May 2019 to 18 January 2021. Patients started treatment at different times, so these data represent only a cross-sectional ‘snapshot’ of our experience up to 18 January 2021.
All patients were ≥18 years old and may have been naïve or previously been treated with other biologics. Patients with generalized or palmoplantar pustular psoriasis or who had started treatment within a clinical trial were excluded. Brodalumab was administered at a dose of 210 mg (s.c.) at 0, 1 and 2 weeks and then every 2 weeks as per product information leaflet [Citation20]. No change in dose or frequency and no concomitant therapies were allowed. For each patient, demographic and clinical data (age, sex, body mass index; BMI, age of onset and duration of psoriasis, comorbidities, and smoking habits), previous therapeutic history and special-site involvement (palmo-plantar, scalp psoriasis, genital, and nails) and PASI score at the moment of enrollment were collected using a dedicated database. All patients gave written informed consent for their participation prior to enrollment. This study complied with the ethical standards laid down in the 1975 Declaration of Helsinki.
2.2. Outcome measures
Clinical efficacy was evaluated using PASI 75, 90 and 100 (represented by a 75%, 90%, 100% reduction in PASI score) at the following time points: 4, 12, 24, 36, and 48 weeks. Primary inefficacy (failure to achieve PASI 75 after 16 weeks of treatment) or secondary inefficacy (loss of PASI 75 after 16 weeks of treatment) were also assessed. Treatment duration and reasons for any drug withdrawal were recorded. Only cases with completed data were collected for the present study.
2.3. Statistical analysis
Data are presented as mean±standard deviation for continuous variables and number and percentage for categorical variables. Simple univariate and multivariate logistic regression was assessed to evaluate the association between dependent variables (e.g. sex, age, age at onset of disease, disease duration, body weight, BMI, PASI at baseline, number of comorbidities, number of previous biological therapies, presence of psoriatic arthritis, involvement of difficult to treat areas such as the scalp, palm-plantar, nails, and genital involvement and previous therapy with IL-17A inhibitors) on achievement of PASI 75, 90, and 100 at 4, 12, 24, 36, and 48 weeks and presented as odds ratio (OR) and 95% confidence intervals (CI). Effectiveness data were analyzed using a last observation carried forward (LOCF) method as previously described [Citation21]. A p-value of <0.05 was considered statistically significant. All analysis was performed using STATA version 11.2 software (Statacorp LP Inc., College Station, TX, USA.).
3. Results
3.1. Baseline clinical characteristics
In this real-life retrospective longitudinal study, 90 patients with moderate-to-severe plaque psoriasis were treated with brodalumab and followed over a period of 12 months. Of the 90 patients who started treatment, 90 (100%) patients were followed for 4 and 12 weeks, 82 (91.1%) for 24 weeks, 63 (70%) for 36 weeks and 55 (61.1%) for 48 weeks. Baseline characteristics of patients are presented in . The majority of patients were male (67.8%) aged 50.2 ± 15 years and with a long history of psoriasis (mean disease duration of 20.8 ± 12.3 years). Psoriasis lesions were frequently localized in difficult-to-treat locations, such as the scalp (N = 44, 48.9%) and genital areas (N = 25, 27.8%). Comorbidities were present in 78 (86.6%) patients, hypertension and obesity being the most frequent observed (26.7% and 23.3%, respectively). Most patients were previously treated with a biological drug (N = 47, 52.2%) and 19 (40.4%) of these were anti-IL17A.
Table 1. Baseline clinical characteristics of psoriasis patients
3.2. Switching from previous anti-IL17A treatments to brodalumab
A total of 19 patients were previously treated with anti-IL17A biologics, 12 with secukinumab and 7 with ixekizumab. Reasons for switching to brodalumab are summarized in . Loss of efficacy was the most frequent reason, applicable to all patients previously treated with secukinumab and half those previously treated with ixekizumab (). One-quarter of patients previously treated with ixekizumab were also switched to brodalumab due to the development of eczematous lesions and the same proportion due to other adverse events. Additional information including the outcome of each patient switched from secukinumab or ixekizumab to brodalumab is provided in . While treatment was stopped in two patients previously treated with ixekizumab (due to worsening of atopic dermatitis and inefficacy on the articular component), complete remission (PASI 100) was maintained in 8/12 (66.7%) of patients and 4/7 (57.1%) in those previously treated with secukinumab.
Table 2. Reason for switching from an anti-IL17A to brodalumab
Table 3. Reason for switching from an anti-IL17A to brodalumab and outcome
3.3. PASI response
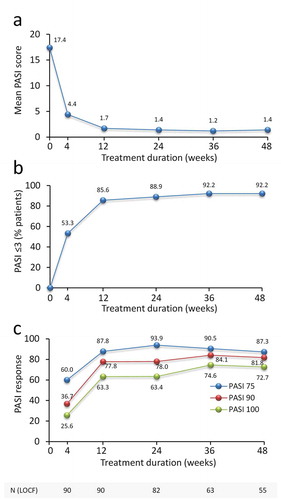
Brodalumab treatment decreased mean PASI score from 17 ± 10.3 at baseline to 1.7 ± 3.9 and 1.4 ± 3.7 at 12 and 24 weeks (). At 12 weeks, 85.6% of patients achieved a PASI score of ≤3 and this was maintained up to 48 weeks in 92.2% of patients (). At 12 weeks, PASI 75, 90 and 100 responses were achieved in 87.8%, 77.8%, and 63.3% (). At 48 weeks, PASI 75, 90 and 100 responses were achieved in 87.3%, 81.8%, and 72.7% of patients, respectively.
Figure 1. Effect of brodalumab in psoriatic patients on PASI score and achievement of PASI 75, 90 and 100 response over 48 weeks. (a) PASI is presented as mean values. (b) % patients achieving a PASI score ≤3 and (c) % patients achieving PASI 75, 90 and 100 response. Mean PASI score, % patients achieving PASI score ≤3 and % patients achieving PASI 75, 90 and 100 response are presented for each time point. The number of patients at each time point is shown. LOCF = last observation carried forward (LOCF) method
3.4. Predictors of improved PASI response
Univariate logistic regression revealed that patients having less previous exposure to anti-IL17A treatment such as secukinumab or ixekizumab were associated with a significant improvement in PASI response. For PASI 90 and PASI 100 responses at Week 36 the improvement in PASI response was 5-fold higher compared to patients with a greater extent of previous exposure to the anti-IL17A agents (OR:5, p-value = 0.03, CI:1.16–20 and OR:4.35, p-value = 0.02, CI:1.25–16.67, respectively) and PASI 75 and PASI 90 responses at week 48 (OR 5.88, p-value = 0.04, CI:1.10–33.33, and OR 4.55, p-value = 0.04, CI:1.08–20, respectively). No other significant associations emerged from analysis either by univariate or multivariate logistic regression.
3.5. Treatment interruption/suspension
Treatment with brodalumab was interrupted in 10 (11.1%) patients, and the suspension was temporary in two of them. The reasons for treatment interruption were: cerebral ischemia (1 patient) (the therapy was re-started three months after the acute event with fast response); alcohol abuse and transaminase increase (three times major the normal value) (one patient) (the therapy was re-started after one month of suspension and after correcting the lifestyle with an optimal response; the second treatment cycle did not require a second induction); primary inefficacy, defined as failure to achieve PASI 75 after 16 weeks of treatment (1 patient); loss of efficacy, defined as loss of PASI 75 after 16 weeks of treatment (2 patients); lacking control of the articular component (1 patient); recurrent candidiasis (2 patients); lower limb myalgia (1 patient); and worsening of atopic dermatitis (1 patient).
3.6. Use of brodalumab in selected ‘difficult to treat’ patients
3.6.1. Patient with high burden of disease
3.6.1.1. Patient #1
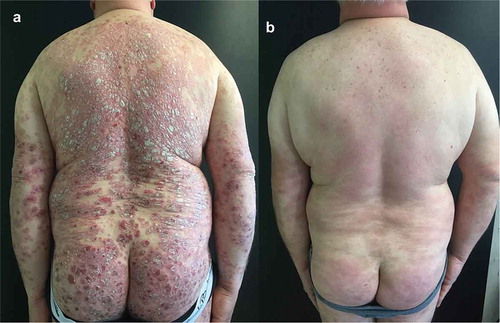
A 50-year-old man was examined for the presence of severe chronic plaque psoriasis. His medical history showed that he had suffered from psoriasis since the age of 38 and was initially treated with cyclosporine, etanercept, and ustekinumab with partial clinical benefit. Hypercholesterolemia and hypertriglyceridemia were the other main records in his medical history. At the time of his first observation, he presented with psoriatic plaques involving the scalp, abdomen, upper, and lower extremities (PASI = 48) (), expressing a severe impact of this condition on his quality of life, social life, and work activity. Treatment with brodalumab at scheduled dosage was started and after 4 and 24 weeks the patient achieved a substantial improvement in psoriatic lesions (PASI 15 and 8, respectively) () with a decreased burden of the disease on his life.
3.6.2. Erythrodermic psoriatic patients
3.6.2.1. Patient #2
A 52-year-old man presented with erythrodermic psoriasis. His medical history showed that he had suffered from recurrent similar episodes and he had already received treatment with etanercept and ustekinumab, both suspended for secondary loss of cutaneous efficacy. He also had a history of arterial hypertension and dyslipidaemia. The patient was admitted to hospital for severe, generalized and painful erythematous rash, involving about 90% of the body surface. Treatment with brodalumab at scheduled dosage was started in addition to liquids administered by intravenous injection, antibiotics, topical steroids, and moisturizing creams. After four weeks from the first injection, we detected a significant improvement (PASI 6) and the subjective symptomatology disappeared. Treatment was continued and complete remission was obtained at Week 16.
3.6.2.2. Patient #3
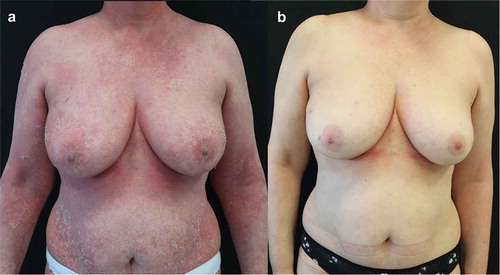
A 56-year-old woman was admitted to hospital for erythrodermic psoriasis. She was suffering from psoriasis for the past 6 years and she had already received two cycles of treatment with cyclosporine and treatment with methotrexate, suspended for fast relapse at the drug suspension and primary inefficacy, respectively. No comorbidities were recorded in her past medical history. In June 2020, the patient presented for the first time in her life with a diffuse erythroderma (), that was treated with systemic steroids (40 mg prednisone, then tapered and suspended over 3 weeks) and brodalumab at scheduled dosage. The patient experienced a rapid improvement, reaching clinical remission in 3 months ().
Figure 3. Representative images of outcome in the breast/abdomen region in Patient #2. A female patient aged 56 years with onset of psoriasis at 50 years of age treated for erythrodermic psoriasis. After presenting with diffuse erythroderma (A), that was treated with systemic steroids and brodalumab at scheduled dosage, she experienced a rapid improvement, reaching clinical remission in 3 months (B)
3.6.3. Patient with a previous history of eczematous reactions by an anti IL17A agent
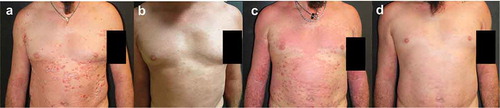
3.6.3.1. Patient #4
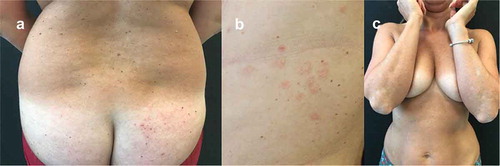
A 41-year-old woman was examined for the presence of severe chronic plaque psoriasis, already treated with narrow band UVB phototherapy, methotrexate, adalimumab, ustekinumab, izekizumab, and guselkumab. The patient had never obtained clinical remission during therapy with ixekizumab. However, this latter treatment was interrupted after 16 weeks due to the onset of a generalized eczema with diffuse intense itching symptoms (), a well-known side effect of anti-IL17A agents [Citation22]. After failure of treatment with guselkumab, in June 2020, the patient appeared with fine scaling and diffuse psoriatic lesions, mainly localized on the back and legs (PASI 9) (). She was then started on treatment with brodalumab at scheduled dosage and in October 2020 (after 4 months) she reached complete clinical remission (). To date, after 10 months from starting brodalumab treatment, psoriasis is still in remission and no eczematous lesions have appeared.
Figure 4. Representative images of outcome at the lower back and gluteal region in Patient #4. A female patient aged 41 years treated for chronic plaque psoriasis. After presenting with severe chronic plaque psoriasis that was treated with UVB phototherapy, methotrexate and a range of other biologics, she complained of generalized eczema with diffuse intense itching symptoms during treatment with ixekizumab (A). Subsequent failure of treatment with guselkumab resulted in fine scaling and diffuse psoriatic lesions, mainly localized on the back and legs (B) that was subsequently treated with brodalumab at scheduled dosage where she experienced a rapid improvement, achieving clinical remission in 4 months (C)
3.6.4. Patients successfully re-treated
3.6.4.1. Patient #5
A 68-year-old man with a 29-year history of moderate-to-severe plaque psoriasis and recurrent episodes of erythrodermic psoriasis during his life. The patient was already treated with systemic steroids, cyclosporine, methotrexate, efalizumab, etanercept, and ustekinumab with partial clinical benefit. The patient was also affected by arterial hypertension and type-2 diabetes mellitus. He came to our observation in June 2019, with PASI 15 (), and he was started with brodalumab. After 2-weeks, the patient reported the disappearance of the plaques just after the second administration (PASI 0.5) (). In September 2019 (Week 12), the patient informed us he could not attend the control visit because he was admitted to the Stroke Unit of another hospital with a diagnosis of cerebral infarction and an endarterectomy of the right carotid. Therapy with brodalumab was suspended. In December 2019, after 3 months of brodalumab suspension, the patient presented a severe relapse of the disease (PASI 52) (), and a new cycle of brodalumab was started. Within two weeks, the PASI score decreased from 52 to 3 ().
Figure 5. Representative images of outcome at the back and gluteal region in Patient #5. A male patient aged 68 years with arterial hypertension and type-2 diabetes mellitus and onset of episodes of erythrodermic psoriasis during his life. At baseline, PASI was 15 (A). After 2 weeks of brodalumab treatment, PASI decreased to 0.5 (B). Brodalumab was suspended for 3 months as the patient was admitted to the Stroke Unit of another hospital with a diagnosis of cerebral infarction and an endarterectomy of the right carotid. Brodalumab was started again and after two weeks PASI score decreased from 52 to 3 (D)
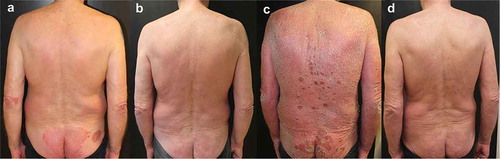
3.6.4.2. Patient #6
A 39-year-old man came to our observation for severe chronic plaque psoriasis from the age of 34 years. He had already received two cycles of treatment with cyclosporine (suspended for lack of efficacy) and treatment with methotrexate (suspended for side effects). He was also a habitual alcohol user (average 2–3 alcohol units/day, about 36 grams/day). He came to our observation in June 2019, with severe psoriasis involving a large part of the body surface (PASI 42) (); therefore, he was started on brodalumab at scheduled dosage, reaching complete clinical remission within a few weeks (). After 56 weeks of treatment, severe abnormalities in liver function tests were found due to abundant alcohol consumption linked to the layoff occurring during the SARS-Cov2 pandemic. His General practitioner suspended therapy with brodalumab and ultrasound showed a severe hepatic steatosis. After one month, the patient was erythrodermic (PASI 59) and with a compromised quality of life (). He was engaged to change his lifestyle and re-start the therapy. A new cycle of brodalumab (without induction phase) was started and after 8 weeks PASI 75 was again achieved (). The patient achieved a new complete remission after 16 weeks of treatment.
Figure 6. Representative images of outcome at the abdomen and chest region in Patient #6. A male patient aged 39 years with severe chronic plaque psoriasis. At baseline, PASI was 42 (A). After a few weeks with brodalumab, complete remission was achieved (B). After 56 weeks, treatment was suspended due to personal difficulties and excessive alcohol consumption and after 1 month the patient was erythrodermic (PASI 59) and with a compromised quality of life (C). He was engaged to change his lifestyle and also restarted brodalumab with a new cycle (without induction phase) and after 8 weeks PASI 75 was achieved with complete remission obtained after 16 weeks
4. Discussion
The results of this real-life study undertaken across the Lazio Region of Italy confirm the efficacy profile up to 1-year (48 weeks) that is also observed in the phase II and III RCTs of brodalumab. This efficacy profile was characterized by a rapid onset of action that was maintained over time.
In our experience, brodalumab was observed to be particularly suitable for severe/resistant psoriasis, such as that observed in cases presenting with a high burden of disease and where other biologic therapies have failed in terms of cutaneous efficacy. Among a range of potential predictor variables and risk factors of psoriasis severity included in logistic regression models, only prior exposure to IL17A biologics such as secukinumab and ixekizumab emerged as being negatively associated with an improvement in PASI response. Indeed, a reduced efficacy is recognized to occur also for other biologics, when switching between the same class [Citation23].
Switching among biologic drugs may be necessary in the real-world setting, as in the different cases we have described. However, switching from another anti-IL17 to brodalumab may influence the efficacy profile. The results of pivotal trials (AMAGINE-1/2/3) highlighted that previous treatment with anti-IL17 therapies showed no impact on the efficacy of brodalumab. In a real-life setting, a multicentre experience showed the efficacy of brodalumab based on PASI 75 response at week 12 in 11/23 (47.8%) patients with moderate-to-severe psoriasis previously treated with ixekizumab or secukinumab [Citation24]. In an open-label study on 39 patients with moderate-to-severe psoriasis who failed treatment with ixekizumab or secukinumab, achieved, at week 16, 76% of patients treated with brodalumab led to achieving PASI 75, 50% achieved PASI 90, 32% complete remission, and 71% an sPGA 0/1 (71%) [Citation25].
Pinter et al. (2019) described the efficacy and safety results of brodalumab in a case series of four patients with moderate-to-severe patients with different clinical features [Citation26]. Two of the four patients were biologic-naïve, and the other two failed previous treatment with biologics of different types. The efficacy in terms of rapid regression of plaques due to brodalumab treatment was assessed by PASI, BSA, and DLQI up to 14 months (Case 1), 10 months (Case 2), 8 months (Case 3), and 12 months (Case 4) [Citation26].
The situation is different when switching within the same class for patients who have experienced the onset of an eczematous reaction following treatment with secukinumab or ixekizumab. In fact, in these cases, brodalumab represents an excellent therapeutic alternative within the class of anti-IL17 biological drugs.
In patients with eczematous reaction induced by anti-IL17A, we found that switching to brodalumab, even in cases of eczema, led to significant improvement and then complete remission. In the UNCOVER-J phase III trial [Citation27], a 12% incidence of eczema was reported, also observed in case reports [Citation28] with ixekizumab, as well as other anti-IL17A [Citation29]. However, in the real-world setting, reports of eczematous eruption following anti-IL17A treatment are limited. After 3–4 months of treatment with secukinumab and ixekizumab, Caldarola et al. (2020) reported eczematous eruptions in 5.8% of 468 patients [Citation30].
For both treatments, the clinical phenotypes manifested as classical acute eczematous rash or atopic dermatitis-like rash or psoriasiform eruption, which showed some peculiar histopathological findings. Itching or burning symptoms in most of the cases were the reason for interrupting the treatments. The clinical presentation and the time of onset were similar to those already reported in the literature [Citation30]. In the Author’s opinion, the cause stemmed from the block of the only IL-17A isoform that may lead to overexpress other isoforms like IL-17 C. IL-17 C is known to trigger an autocrine stimulation loop on keratinocytes possibly involved in skin inflammation driven by Th17 and Th2. Brodalumab blocks IL17A, IL-17 F, and IL-17 C, suggesting a lesser frequency of eczematous reaction. Rather, brodalumab may be beneficial in patients treated with other anti-IL-17As and this paradoxical adverse event [Citation30].
Our findings also highlight the versality of the use of brodalumab, in that it can provide efficacy across a wide spectrum of patients burdened with a range of comorbid diseases and established risk factors for poor psoriasis outcome.
In this regard, brodalumab was also observed to be effective even in obese patients. Following both univariate and multivariate statistical analysis, no statistically significant association was observed between PASI response and body weight or BMI. This is particularly relevant since the majority of patients (67.8%) were overweight or obese (44.5% and 23.3%, respectively). In the real-word setting, there is evidence of various biological drugs yielding only a partial response or loss of efficacy or even primary ineffectiveness in patients with excess adipose tissue [Citation31–33], which represents a fundamental reservoir of inflammatory cytokines and adipokines with repercussions on skin inflammation [Citation34]. Since obesity can compromise the effectiveness of systemic treatments, it is important to identify obesity and early obesity-related diseases [Citation35].
It is important to highlight only two patients dropped out of the study due to loss of efficacy and efficacy was maintained through 48 weeks without any significant decrease. Longer follow-up studies are needed to verify if efficacy can be maintained up to 2 to 3 years.
In terms of safety, one patient (Patient #5) experienced a cerebral infarction and an endarterectomy of the right carotid during treatment with brodalumab. This was not judged to be associated with brodalumab treatment. Overall, results observed in this small cohort of 90 patients in a real-life setting corroborate the excellent safety profile observed from AMAGINE trials [Citation15,Citation36], a real-life case series [Citation26] as well as a systematic review [Citation37], that brodalumab for moderate-to-severe psoriasis is similar to that of other IL-17 antagonists [Citation38]. Furthermore, the proportion of patients with serious AEs, cardiovascular disease and discontinuations due to AEs in patients treated with IL‐17 antagonists compared to placebo was not significantly different [Citation37].
The significant improvement achieved in cases of erythrodermic psoriasis with a rapid onset of action was beneficial in this challenging phenotype of the disease presented in patients with a long history of other biologics or other previous failed treatments.
The management of erythrodermic psoriasis is difficult, often requiring a complete evaluation of patients with strong awareness of the psychological burden associated with this severe form of psoriasis [Citation39]. The efficacy and safety are relevant parameters for the chosen drug as a new first-level therapeutic option for erythrodermic psoriasis [Citation40,Citation41].
The effects of withdrawal and retreatment of brodalumab were among the objectives of the AMAGINE-1 trial [Citation36]. After Week 12 of the induction phase (brodalumab 140 or 210 mg/day), patients who achieved sPGA success and PASI 100 and PASI 90 were randomized to placebo or retreated with brodalumab and underwent withdrawal. In the placebo group, treatment withdrawal led to a relapse in 56 days, whereas in the brodalumab group (140 or 210 mg/day), patients maintained the PASI 100 and PASI 90 at Week 52 [Citation36].
After the withdrawal of brodalumab treatment, the severity of the disease is expected to increase. A retrospective cohort study of 77 patients collected from three RCTs (AMAGINE-1, 2, and 3) assessing the effects of the suspension of brodalumab has highlighted a 30% of psoriasis relapse with a median time-to-relapse of 46 days [Citation42]. Moreover, the study has reported 30% of the patients with a PASI higher than that before starting the treatment with brodalumab, 5% displayed a severe form of psoriasis (erythrodermic/pustular flares), and 10% developed symptoms suggestive of psoriatic arthritis despite having no history of PsA [Citation42].
The benefits of the brodalumab treatment and retreatment can be sustained in patients who suspend the treatment. In the long-term (Week 120) subgroup analysis of the AMAGINE-1 trial, the median time to PASI 90 for patients who received brodalumab 210 mg/day was 6 weeks and to PASI 100 was 12 weeks, but the median time to recapture sPGA 0/1 for these patients was four weeks after induction [Citation43].
In RCTs, brodalumab, among other biologic treatments, has shown the best recapture rate in patients who prior achieved sPGA 0/1, with 95.2% of PASI 100 achieved in 24 weeks [Citation44].
Moreover, the rapid onset of the action of brodalumab showed in our retreatment case favored the motivation of the patient in persisting the treatment and eased the relationship between doctor and patient.
Rapid onset of action and high PASI response are the hallmarks of the efficacy profile of brodalumab compared with other biologic therapies [Citation45]. However, as psoriasis is a chronic disease, long-term efficacy as skin clearance and the safety profile of the treatments are essential [Citation46]. Brodalumab administered at a dose of 210 mg every two weeks was evaluated in an open extension study of the phase II trial at 5-years follow-up [Citation46]. The rapid onset of action of brodalumab in 181 patients with moderate-to-severe psoriasis according to sPGA, PASI 75 to <90 was associated with an improved DLQI 0/1 at week 12 and sustained up to 240 weeks [Citation46]. The long-term (120 weeks) efficacy of brodalumab was evaluated in the phase III AMAGINE-2 trial [Citation47]. Brodalumab (210 mg every two weeks) was administered in 274 patients after ustekinumab or continuously in 168 patients; it showed a sustained response in terms of skin clearance at 2 years with a 100% improvement of PASI from baseline with an acceptable safety profile. During the long-term extension time, brodalumab provided good results as skin clearance in many patients who received ustekinumab for 52 weeks as a prior treatment [Citation47].
To date, there are few real-life studies evaluating the efficacy of brodalumab in patients with moderate-to-severe psoriasis. The BRILLIANT Working Group was responsible for evaluating the efficacy and safety of brodalumab through a retrospective 24-week analysis of data derived from 17 Italian dermatological centers [Citation48]. Data from 78 patients with moderate-to-severe plaque-type psoriasis included demographics, comorbidities, disease features, and prior treatments. The mean age of the patients was 47.9 years, 71.8% were male, and the average duration of the disease was 16.8 years. The mean PASI score rapidly decreased just after four-weeks-treatment and then at 12 and 24 weeks compared with baseline (all P < 0.0001). In addition, the efficacy and safety of brodalumab were observed in patients who failed with previous treatment using other anti-IL17 therapies. At all the evaluating points, the efficacy measured by the PASI 90 score was associated with cardiometabolic comorbidities and prior therapies. During treatment with brodalumab, compared with baseline, the patients' quality-of-life showed a significant increase (P < 0.0001) as well as the patients’ satisfaction (P < 0.01). At Week 4, 55.6% of the patients achieved global PGA and 72.2% of patients achieved PGA-G scores of clear or minimal (0/1); at Week 12, 80.7% of patients achieved global PGA of 0/1 increasing to 92.6% at 24 weeks. Similarly, 88.9% of the patients achieved PGA-G scores of 0/1 at 12 weeks, which remained stable up to Week 24. Treatment with brodalumab was interrupted for adverse events in four, lack of efficacy in three, lost at follow-up in one, and surgical intervention in one patient [Citation48]. The results of the clinical response at Week 12 were consistent with data from AMAGINE-1/2/3 studies regarding the PASI 75, PASI 90, and PASI 100. However, these results were not consistent with those from a post-hoc analysis of the AMADINE-2 and AMAGINE-3 trials regarding the achievement of PASI 90 at Week 12 in that, in this real-life study, the majority of patients were bio-naïve (47.8% of patients). The overall good tolerability profile of brodalumab was confirmed [Citation48].
The case series by Facheris et al. (2020) [Citation49] regarding six patients treated with brodalumab (210 mg administered s.c. every 2 weeks) showed the efficacy, safety, and good tolerability of the drug. There was a significant reduction of PASI, BSA, PGA, DLQI, and long-term efficacy at week 52 [Citation49].
The limitations of the present study are related to the retrospective design, a relatively limited follow-up (1 year), essentially due to the recent introduction of biologic drugs. This study lacks a control group partially since most of the patients enrolled had a history of failures of other biologic or not treatments.
5. Conclusion
This cross-sectional retrospective analysis and case series of the Lazio Region with brodalumab confirmed the efficacy and safety profile of the drug observed in phase II and III RCTs and other real-world experiences. Moreover, the excellent efficacy profile for brodalumab was maintained even for psoriasis with challenging clinical presentations and combined with its favorable safety profile will allow to address problems of treatment persistence and adherence to therapy.
Declaration of interest
C De Simone has received honoraria as speaker and consultant for Abbvie, Almirall, Biogen, Eli Lilly, LEO Pharma, Novartis, Janssen, Sanofi, Pfizer, and UCB Pharma outside the submitted work. L Bianchi has served as speaker and as consultant for Abbvie, Novartis, Janssen-Cilag, Pfizer, UCB, and Leo-Pharma outside the submitted work. K Peris reports personal fees for advisory board meeting from Almirall, AbbVie, Biogen, Janssen, Eli Lilly, Celgene, Galderma, Leo Pharma, Novartis, Pierre Fabre, Sanofi, Sandoz, and Sun Pharma outside the conduct of the work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Reviewer disclosures
One of the peer reviewers on this manuscript has received research, speaking and/or consulting support from Arcutis, Dermavant, Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Helsinn, Arena, Forte, Informa, UpToDate and National Psoriasis Foundation. They also consult for others through Guidepoint Global, Gerson Lehrman and other consulting organizations. They are the founder and majority owner of www.DrScore.com. They are the founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. Three additional peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. .
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Farley E, Menter A. Psoriasis: comorbidities and associations. G Ital Dermatol Venereol. 2011;146(1):9–15.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–284.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675–1684. .
- Griffiths CEM, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. .
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. .
- Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. .
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431. .
- Galluzzo M, D’Adamio S, Silvaggio D, et al. Ustekinumab treatment for moderate-to-severe plaque psoriasis: eight-year real-life experience. Expert Opin Biol Ther. 2020;20(1):95–104. .
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6(6):383–392. .
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570.
- Gisondi P, Girolomoni G. Brodalumab in the treatment of chronic plaque psoriasis. Expert Opin Biol Ther. 2020;20(10):1175–1186.
- Egeberg A, Andersen YMF, A-s H-O, et al. Systematic review on rapidity of onset of action for interleukin-17 and interleukin-23 inhibitors for psoriasis. J Eur Acad Dermatol Venereol. 2020;34(1):39–46. .
- Lebwohl M, Strober B, Menter A, et al. Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med. 2015;373(14):1318–1328. .
- Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci [Internet]. 2019 [ cited 2021 Mar 31];20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6471628/.
- Papp KA, Gordon KB, Langley RG, et al. Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3 Br J Dermatol. 2018;179(2):320–328.
- Elewski B, Rich P, Lain E, et al. Efficacy of brodalumab in the treatment of scalp and nail psoriasis: results from three phase 3 trials. J Dermatolog Treat. 2020;1–5. DOI:https://doi.org/10.1080/09546634.2020.1749546.
- Gisondi P, Altomare G, Ayala F, et al. Italian guidelines on the systemic treatments of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(5):774–790. .
- Kyntheum | european Medicines Agency [Internet]. [ cited 2021 Apr 20]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kyntheum
- Papp KA, Fonjallaz P, Casset-Semanaz F, et al. Analytical approaches to reporting long-term clinical trial data. Curr Med Res Opin. 2008;24(7):2001–2008. .
- Czarnecka-Operacz M, Polańska A, Klimańska M, et al. Itching sensation in psoriatic patients and its relation to body mass index and IL-17 and IL-31 concentrations. Postepy Dermatol Alergol. 2015;32:426–430.
- Costa L, Perricone C, Chimenti MS, et al. Switching Between Biological Treatments in Psoriatic Arthritis: a Review of the Evidence. Drugs R D. 2017;17(4):509–522. .
- Kromer C, Wilsmann-Theis D, Gerdes S, et al. Changing within the same class: efficacy of brodalumab in plaque psoriasis after treatment with an IL-17A blocker - a retrospective multicenter study. J Dermatolog Treat. 2020. p. 1–5.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81(3):857–859. .
- Pinter A, Bonnekoh B, Hadshiew IM, et al. Brodalumab for the treatment of moderate-to-severe psoriasis: case series and literature review. Clin Cosmet Investig Dermatol. 2019;12:509–517.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44(4):355–362. .
- Munera-Campos M, Ballesca F, Richarz N, et al. Paradoxical eczematous reaction to ixekizumab. J Eur Acad Dermatol Venereol. 2019;33(1):e40–e42. .
- Napolitano M, Megna M, Fabbrocini G, et al. Eczematous eruption during anti-interleukin 17 treatment of psoriasis: an emerging condition. Br J Dermatol. 2019;181(3):604–606. .
- Caldarola G, Pirro F, Di Stefani A, et al. Clinical and histopathological characterization of eczematous eruptions occurring in course of anti IL-17 treatment: a case series and review of the literature. Expert Opin Biol Ther. 2020;20(6):665–672. .
- Naldi L, Addis A, Chimenti S, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the Psocare project. Dermatol (Basel). 2008;217(4):365–373.
- Mourad A, Straube S, Armijo‐Olivo S, et al. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181(3):450–458. .
- Kisielnicka A, Szczerkowska-Dobosz A, Nowicki RJ. The influence of body weight of patients with chronic plaque psoriasis on biological treatment response. Postepy Dermatol Alergol. 2020;37(2):168–173.
- Chiricozzi A, Raimondo A, Lembo S, et al. Crosstalk between skin inflammation and adipose tissue-derived products: pathogenic evidence linking psoriasis to increased adiposity. Expert Rev Clin Immunol. 2016;12(12):1299–1308. .
- Galluzzo M, Talamonti M, Perino F, et al. Bioelectrical impedance analysis to define an excess of body fat: evaluation in patients with psoriasis. J Dermatolog Treat. 2017;28(4):299–303. .
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. .
- Naik GS, Ming WK, Magodoro IM, et al. Th17 Inhibitors in Active Psoriatic Arthritis: a Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Dermatology. 2017;233(5):366–377. .
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a Review of Safety. Skin Therapy Lett. 2018;23(2):1–3.
- Galluzzo M, D’Adamio S, Campione E, et al. A clinical case of severe disease burden: an erythrodermic psoriatic patient treated with secukinumab. In: J Dermatolog Treat. 2018. p. 1–11.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176(3):741–751. .
- Xing X, Liang Y, Sarkar MK, et al. IL-17 Responses Are the Dominant Inflammatory Signal Linking Inverse, Erythrodermic, and Chronic Plaque Psoriasis. J Invest Dermatol. 2016;136(12):2498–2501. .
- Masson Regnault M, Konstantinou M-P, Khemis A, et al. Early relapse of psoriasis after stopping brodalumab: a retrospective cohort study in 77 patients. J Eur Acad Dermatol Venereol. 2017;31(9):1491–1496. .
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183(6):1037–1048. .
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-Year US Pharmacovigilance Report on Brodalumab. Dermatol Ther (Heidelb). 2021;11(1):173–180. .
- Armstrong AW, Puig L, Joshi A, et al. Comparison of Biologics and Oral Treatments for Plaque Psoriasis: a Meta-analysis. JAMA Dermatol. 2020;156(3):258–269. .
- Lebwohl MG, Blauvelt A, Menter A, et al. Efficacy, Safety, and Patient-Reported Outcomes in Patients with Moderate-to-Severe Plaque Psoriasis Treated with Brodalumab for 5 Years in a Long-Term, Open-Label, Phase II Study. Am J Clin Dermatol. 2019;20(6):863–871. .
- Puig L, Lebwohl M, Bachelez H, et al. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020;82(2):352–359. .
- Fargnoli MC, Esposito M, Dapavo P, et al. Brodalumab for the treatment of moderate-to-severe plaque-type psoriasis: a real-life, retrospective 24-week experience. J Eur Acad Dermatol Venereol. 2021;35(3):693–700. .
- Facheris P, Valenti M, Pavia G, et al. Brodalumab: a new way to inhibit IL-17 in psoriasis. Dermatol Ther. 2020;33(3):e13403.