 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Immunotherapy (IO) is rapidly reshaping the treatment landscape for solid tumors, with survival improvements demonstrated in multiple disease types. In the context of metastatic triple-negative breast cancer (mTNBC), the use of IO is based on the results from two phase III clinical trials: Impassion130 and KEYNOTE-355. In both trials, the addition of IO to first-line chemotherapy has shown an improvement in clinical outcomes restricted to patients with PD-L1 expressing tumors.
Impassion130 demonstrated a statistically significant improvement in progression-free survival (PFS) with the addition of atezolizumab to nab-paclitaxel in the intention-to-treat (ITT) population, with a greater improvement in the PD-L1+ population, namely PD-L1 stained tumor-infiltrating immune cells (IC) covering ≥1% of the tumor area, as determined by the VENTANA PD-L1 (SP142) as a companion diagnostic test (CDx) [Citation1]. Although the Food and Drug Administration (FDA) granted accelerated approval for the combination of atezolizumab and nab-paclitaxel as frontline treatment of PD-L1+ mTNBC, this study failed to demonstrate a statistically significant improvement in the co-primary endpoint of overall survival (OS) in ITT population due to its hierarchical statistical design, despite a numeric difference in OS [Citation2]. This ultimately led to the withdrawal of the indication in the United States by the agent’s developer. Thus, atezolizumab will no longer be available for the treatment of PD-L1+ mTNBC in the United States, while it is still available in Europe at the time of writing.
In contrast, in KEYNOTE-355, the addition of pembrolizumab to chemotherapy improved both progression-free survival (PFS) and overall survival (OS) compared to chemotherapy alone in PD-L1+ (combined positive score, CPS ≥ 10) mTNBC patients. PD-L1 status was defined using the IHC 22C3 pharmDx (Dako North America, Inc.) and a specific scoring system (CPS) [Citation3,Citation4]. Based on these results, the FDA granted full approval to pembrolizumab for locally recurrent unresectable or mTNBC expressing PD-L1 as assessed with 22C3 Dako CDx with a CPS ≥ 10, and the same agent may soon gain approval in Europe as well.
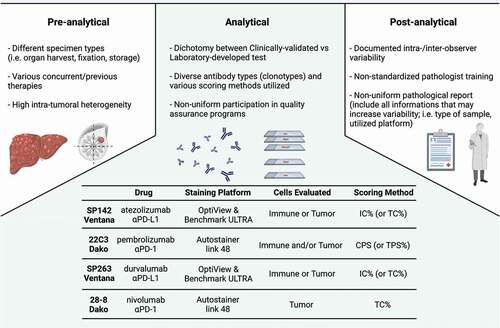
Two clinical trials, two drugs, and two CDx to answer the same question: how to identify mTNBC patients who might benefit the most from IO? It is worth noting that such discordance pervades the selection of patients for IO across various tumor histologies [Citation5]. Indeed, there are four anti-PD-(L)1 agents approved for PD-L1-restricted indications, together with four distinct CDx to assess the expression of PD-L1: nivolumab (28–8, Dako), pembrolizumab (22C3, Dako), atezolizumab (SP142, Ventana), and durvalumab (SP263, Ventana) [Citation5]. Moreover, the complexity of PD-L1 assessment itself presents many key sources of variability () [Citation5]. First of all, PD-L1 expression can be evaluated only on tumor cells (TC), only on IC, or on both (as for CPS). In addition, each CDx uses a different method for scoring calculation (see ) and there is no consensus for the cut-off value, even for the same CDx across different tumor histologies [Citation5]. Another important source of variability is the potential intratumoral heterogeneity in PD-L1 expression within each single lesion, or between primary and metastatic samples (as well as among different metastatic anatomic locations) [Citation6]. Last but not least, different assays use different immunohistochemistry (IHC) staining protocols, primary antibody clones (SP142, SP263, and 22C3), IHC platforms, and detection kits [Citation5].
Table 1. Scoring methods for PD-L1 assessment
Figure 1. Sources of variability in PD-L1 testing. Main sources of variability in PD-L1 testing are represented according to pre-analytical, analytical, and post-analytical phases. All these steps may represent sources for implementation of strategies aiming at homogenizing and standardizing PD-L1 assessments by immunohistochemistry (or other approaches). Acronyms: TC, tumor cells; IC, immune cells; TPS, tumor proportion score; CPS, combined positive score; aPD-L1, anti programmed cell death-ligand 1; aPD-1, anti programmed cell death-1.
Hence, the next question easily arises: do different assays ultimately identify different patient populations? Across tumor histologies, SP142 stains fewer TC and IC compared to other clones [Citation7]. In mTNBC, a hint is given by a post hoc analysis from Impassion130, where IHC for PD-L1 status using SP263 and 22C3 was evaluated for analytical concordance with SP142 and patient-associated outcomes in a subset of tumor samples [Citation8]. Prevalence rates using PD-L1 IC ≥ 1% as a threshold for SP142, SP263, and 22C3 were, respectively, 46.4% (95% confidence interval [CI] = 42.5% to 50.4%), 74.9% (95% CI = 71.5% to 78.3%), and 73.1% (95% CI = 69.6% to 76.6%). The prevalence of PD-L1 22C3 CPS ≥ 1 was 80.9%. The analytical concordance, namely overall percentage agreement (OPA) for IC ≥ 1% (+), between SP142 and SP263 or 22C3 was 69.2% and 68.7%, respectively. Even if almost all SP142+ cases were classified as PD-L1+ by other assays (double positive), 29.6% of SP263+ and 29.0% of 22C3+ cases were SP142– (single positive) [Citation8]. Importantly, the IO clinical activity in SP263+ and 22C3+ patients could be mainly ascribed to double-positive cases rather than single-positive cases. Thus, authors used a mathematical model to identify an exploratory cutoff for SP263 and 22C3 that could replicate the patient population SP142 IC ≥ 1%: however, these harmonized cutoffs for SP263 (IC ≥ 4%) and 22C3 (CPS ≥ 10) led to an unsatisfying OPA (about 75%) [Citation8]. In fact, using harmonized cutoffs, the prevalence of SP142+/SP263- and SP142-/SP263+ were 12.2% and 12.4%, respectively, while the prevalence of SP142+/22C3- (i.e. CPS < 10) and SP142-/22C3+ (i.e. CPS ≥ 10) were 10.4% and 16.9%, respectively. This would imply that there is about 25% of the population that could be classified as PD-L1 positive with one assay and negative with another, conditioning the potential eligibility to the treatment. It must be noted that the differences in classification using harmonized cutoffs mirrored a difference in survival outcomes: for instance, patients identified as positive with 22C3 (CPS ≥ 10) did not show the same PFS benefit from the addition of atezolizumab to nab-paclitaxel of SP142 IC ≥ 1% patients [Citation8].
Expert opinion
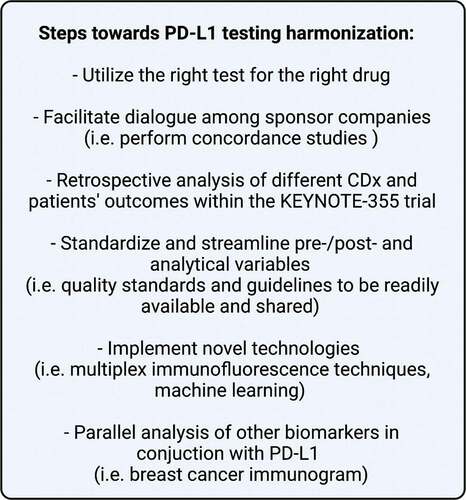
Considering these data, we can conclude that different PD-L1 assays are not equivalent to each other in the selection of patients affected by mTNBC who might benefit from atezolizumab: different assays actually identify patient populations that could not be entirely overalapped. Therefore, in order to identify patients who could actually benefit from the monoclonal antibody we are planning to use, the right assay should be paired with the right drug. However, there is an urgent need to harmonize the PD-L1 evaluation, maximizing the cost-effectiveness without losing accuracy because there is a real possibility that not all the laboratories across the globe could afford the use of four different CDx. () Thus, the sponsors involved in the development of IO strategies should dedicate extensive effort in the attempt of harmonizing the assessment of PD-L1 in oncology. One initial step, specular to that conducted in IMpassion130, should involve a retrospective comparison of outcomes based on the CDx in KEYNOTE-355, comparing CPS with the other two assays (SP263 and SP142) in terms of predictive potential. Moreover, assay protocols should be standardized as much as possible in order to prevent the risk of misclassifying patients. Additionally, new technologies for the assessment of PD-L1 status should be deeply investigated, including multiplex immunofluorescence techniques [Citation9] or machine learning approaches [Citation10]. Finally, it is important to remark that, although it is currently used with a binary fashion, PD-L1 is a continuous variable in nature. For certain cancers, it has been demonstrated that very high (>90%) PD-L1 expressions coincide with marked responsiveness to IO [Citation11]. In this regard, efforts should therefore be placed to improve the interpretation of this biomarker, possibly in conjunction with other emerging biomarkers of IO response, such as in the framework of a comprehensive breast cancer immunogram [Citation12,Citation13]. In the meantime, to maximize the access to IO for patients affected by breast cancer in countries where more than one IO agent is approved, testing with multiple assays should be evaluated in daily clinical practice, if feasible.
Figure 2. Steps toward PD-L1 testing harmonization. Key proposals to ease and speed up PD-L1 testing harmonization for the use of immunotherapy (anti-PD-L1 or anti-PD-1) in metastatic TNBC patients. Acronyms: TNBC, triple-negative breast cancer; PD-L1, programmed cell death-ligand 1; PD-1, programmed cell death-1; CDx, companion diagnostic test.
Declaration of interests
P Tarantino served as advisor/consultant for AstraZeneca. J Cortes has declared consulting role for Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, MSD, GlaxoSmithKline (GSK), Leuko, Bioasis, and Clovis Oncology; honoraria from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, MSD, and Daiichi Sankyo; research funding to the institution from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GmbH–Servier Affaires, Bayer Healthcare, Eisai, F Hoffman-La Roche, Guardant Health, MSD, Pfizer, Piqur Therapeutics, Puma C, and Queen Mary University of London and intellectual property for MedSIR. HS Rugo reports funding for sponsored studies paid to the University of California San Francisco from Pfizer, Novartis, Lilly, Roche–Genentech, Macrogenics, OBI, Merck, Eisai, Immunomedics, Daiichi Sankyo, Seattle Genetics, and Odonate; travel support for educational meetings from Daiichi Sankyo, Mylan, Pfizer, Merck, AstraZeneca, Novartis, and Macrogenics; and consulting fees from Samsung and Puma, outside the submitted work. G Curigliano received honoraria for speaker, consultancy, or advisory rule from AstraZeneca, Roche, Pfizer, Novartis, Seattle Genetics, Lilly, Ellipses Pharma, Foundation Medicine, Daiichi Sankyo, and Samsung. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One of the peer reviewers declares speakers honoraria from MSD and Roche, advisor/consultant for MSD and Roche, and travel grants from Roche. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121 .
- Emens LA, Adams S, Barrios CH, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: iMpassion130 final overall survival analysis. Ann Oncol. 2021;32:983–993.
- Cortes J KEYNOTE-355: final results from a randomized, double-blind phase 3 study of first-line pembrolizumab + chemotherapy vs placebo + chemotherapy for metastatic TNBC. ESMO Congress 2021 LBA16. 2021 .
- Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828.
- Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–362.
- Rozenblit M, Huang R, Danziger N, et al. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J Immunother Cancer. 2020;8:e001558.
- Scott M, Scorer P, Barker C, et al. Comparison of patient populations identified by different PD-L1 assays in in triple-negative breast cancer (TNBC). Ann Oncol. 2019;30:iii4.
- Rugo HS, Loi S, Adams S, et al. PD-L1 immunohistochemistry assay comparison in atezolizumab plus nab -paclitaxel–treated advanced triple-negative breast cancer. JNCI J Natl Cancer Inst. 2021;113(12):1733–1743 .
- Sanchez K, Kim I, Chun B, et al. Multiplex immunofluorescence to measure dynamic changes in tumor-infiltrating lymphocytes and PD-L1 in early-stage breast cancer. Breast Cancer Res. 2021;23:2.
- Liu J, Zheng Q, Mu X, et al. Automated tumor proportion score analysis for PD-L1 (22C3) expression in lung squamous cell carcinoma. Sci Rep. 2021;11:15907.
- Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol. 2019;30:1653–1659.
- Blank CU, Haanen JB, Ribas A, et al. The “cancer immunogram”. Science. 2016;352:658–660.
- Tarantino P, Curigliano G. Defining the immunogram of breast cancer: a focus on clinical trials. Expert Opin Biol Ther. 2019;19:383–385.
