ABSTRACT
Introduction
One of the most important aims in the management of systemic lupus erythematosus (SLE) is to avoid or delay the accumulation of organ damage. The first five years after diagnosis are crucial for prognosis.
Areas covered
This manuscript reviews available data on organ damage accrual in SLE and early therapeutic intervention as a possible strategy to prevent its long-term accrual.
Expert opinion
Organ damage can be minimized by controlling disease activity and risk of flares, reducing the dose of glucocorticoids, and ensuring a proper therapeutic intervention with an early introduction of the right therapies. The current standard treatment cannot provide clinical remission in all patients with SLE. Therefore, there is a clinical need for introducing new therapeutic strategies able to achieve the main therapeutic objectives. The addition of biologic and other therapeutic agents to the standard of care is effective for controlling disease activity and for preventing severe flares, enabling a reduced use of glucocorticoids, and presumably reducing organ damage progression. Considering its efficacy and safety, early inclusion of biologic agents in the first lines of the treatment algorithm, at least in certain patients, could be considered as an innovative treatment approach to decrease disease burden in SLE patients.
1. Introduction
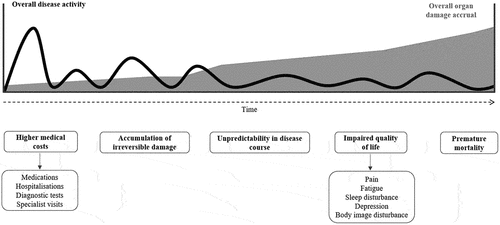
Systemic lupus erythematosus (SLE) is a chronic, multisystemic, autoimmune condition of unknown etiology and complex immune-mediated pathogenesis [Citation1,Citation2]. It affects predominantly women (9:1 ratio), ages between 15 and 45 [Citation3–5]. Older age at SLE onset is considered a risk factor for damage accrual [Citation6,Citation7]. Overall, the prevalence of SLE is 20 to 517.5 cases per 100,000 individuals in Europe [Citation4]. SLE can affect multiple organs (skin, joints, kidneys, heart, lungs, and brain), and has a wide variety of clinical manifestations and severity, ranging from mild cutaneous involvement or arthritis to end-stage renal disease, thromboembolic events, or central nervous system involvement [Citation5,Citation8]. The disease can follow three patterns of activity over time: chronically active, relapsing-remitting, and long quiescent [Citation9]. SLE has a great impact on the patient’s quality of life [Citation10,Citation11]. Chronic disease severity and flares are directly associated with higher medical costs (medications, hospitalizations, diagnostic tests, specialist visits), organ damage accrual and premature mortality (2–4:1 ratio, compared with the control population) () [Citation5,Citation12–15]. In SLE as in other diseases, organ damage is irreversible by definition. According to the Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index (SDI), organ damage is defined as such when it has been present for at least 6 months [Citation16]. Despite the improvement in the global management of disease, the current standard of care is still associated with the development of a high degree of organ damage, as measured with SDI [Citation17]. There is increasing evidence that early diagnosis and effective treatment improves outcomes and prognosis by avoiding damage [Citation18,Citation19]. The objective of the present manuscript is to review the available data on organ damage accrual and the benefits of early and effective therapeutic interventions in SLE to avoid this damage.
2. Therapeutic objectives for SLE
In recent years, the therapeutic strategy for SLE has evolved from the traditional symptom-based approach into a target-based one [Citation20]. Treat-to-target establishes a therapeutic goal for each patient, which is pursued within a planned time frame. Additionally, it emphasizes the active participation of patients at the time of making decisions. According to an international task force: ‘The treatment target of SLE should be remission of systemic symptoms and organ manifestations or, where remission cannot be reached, the lowest possible disease activity, measured by a validated lupus activity index and/or by organ-specific markers’ [Citation20]. It is necessary to state that there is no generally accepted definition for remission in SLE, and it remains a matter of controversy whether to include the symptoms and signs of the clinical disease activity and/or serological activity with or without active treatment [Citation21,Citation22]. The international group on definitions of remission in SLE (DORIS) have recently proposed a new single definition of remission, based on the following criteria: clinical SLE Disease Activity Index, SLEDAI, with 0 value; evaluator’s global assessment (PGA) <0.5 (on a 0–3 scale); 5 mg/day or less of prednisone usage; and stable treatment with antimalarials, immunosuppressive (IS) drugs and biologic agents [Citation23].
The DORIS group also suggested that the most appropriate outcomes for evaluating remission should be: ‘death, damage, flares and measures of health-related quality of life’ [Citation21]. There is also no general agreement on the definition of ‘lowest possible disease activity.’ In this line, one operational definition of lupus low disease activity state (LLDAS) has been proposed by the Asia-Pacific Lupus Collaboration, based on reduced risk for adverse long-term outcomes. When fulfilling the following domains: SLEDAI-2k ≤4 (excluding any major organ domains or hemolysis and gastrointestinal involvement); no new disease activity; PGA ≤1; prednisone dose (or equivalent) ≤7.5 mg per day; and absence of adverse events (AEs) of current IS treatment and approved biological agents, excluding investigational drugs [Citation24].
Since damage predicts subsequent damage and death, prevention of damage has been proposed as a major therapeutic goal for SLE patients [Citation20]. Consequently, the recommendations made by the current European League Against Rheumatism (EULAR) for SLE management include the treatment goals of preventing organ damage accrual, minimizing drug-related AEs and reducing the dose of glucocorticoids (GCs) to as low as possible [Citation25]. Over the last decades, the standard treatment of SLE has been based on nonsteroidal anti-inflammatory drugs, GCs, antimalarials (especially hydroxychloroquine, HCQ), and IS agents [Citation26]. Advances in the diagnosis and treatment have contributed to a substantial improvement in prognosis. The 5- and 10-year overall survival has significantly increased between the years 1950 and 2000 (from 74.8% to 94.8%; and from 63.2% to 91.4%, respectively) [Citation27]. However, despite improvements in survival, outcomes of the contemporary standard treatment of SLE remain unacceptable, bearing in mind that 25–40% of patients show: inadequate response or refractory disease; organ damage accrual (up to 70% of patients in 10 years); a high percentage of drug-related AEs (mainly from GCs and IS); and increased mortality rates [Citation12–15,Citation28]. Pego-Reigosa et al. evaluated the disease activity and response to conventional treatment in 3,658 Spanish patients with SLE found in the Spanish Society of Rheumatology Lupus Register (RELESSER) [Citation29]. Authors reported that 24.5% of patients showed refractory SLE at some stage. Furthermore, the long-term use of GCs and IS agents may lead to the development of AEs, and in turn an increase in morbidity and mortality [Citation30]. Targeted biologic agents, mainly belimumab and rituximab, emerged to address unmet clinical needs. Belimumab, a humanized monoclonal antibody that inhibits the B-cell activating factor, is the only biological agent approved by both the US Federal Drug Administration (FDA) and the European Medicines Agency (EMA) in 2011 and indicated for adult patients with active, autoantibody-positive SLE who are receiving standard therapy [Citation31,Citation32]. Rituximab, a chimeric murine/human monoclonal antibody that binds the CD20 protein, is used off-label for severe and refractory SLE, although there are no randomized controlled trials supporting its use [Citation33]. Indeed, rituximab is recommended as a treatment for lupus nephritis by both the American College of Rheumatology and EULAR guidelines. Despite 2019 EULAR recommendations for the treatment of SLE [Citation25], the use of therapeutic agents in routine clinical practice is not always in line with their level of evidence. Regarding HCQ and belimumab, both with recommendation grade A, the first one is underused [Citation34], and belimumab is mainly restricted to the persistence of refractory manifestations [Citation35]. It is necessary to highlight that belimumab has met its endpoints in several both non-renal and, recently, renal lupus trials. The two-year, randomized, multicenter, double-blind, placebo-controlled BLISS-LN trial, evaluating the efficacy and safety of belimumab in lupus nephritis patients, showing that a significantly higher percentage of patients in the belimumab group achieved a primary good efficacy renal response at week 104 than placebo [Citation36]. However, this trial showed that belimumab may have no benefit being used with cyclophosphamide, in-class V LN or in patients with nephrotic-range proteinuria at baseline [Citation37]. These results could be explained due to: 1) the sample size of the cyclophosphamide/azathioprine subgroups, 2) the time required for the proteinuria to decrease enough to meet the specifications of the primary efficacy renal response endpoint and 3) different mechanisms of disease in class V LN patients [Citation6].
3. Organ damage accrual
In SLE, disease activity and organ damage are concepts that must be well differentiated for establishing appropriate management and therapeutic strategies [Citation38]. Disease activity refers to reversible manifestations which are usually related to active inflammation, whereas organ damage is defined as irreversible injuries in organs [Citation39]. Organ damage is determined by factors such as severity and persistency of disease activity, patient characteristics, and comorbid factors, as well as the toxic effect of therapy [Citation40–42]; and it is measured mainly with SDI [Citation43,Citation44].
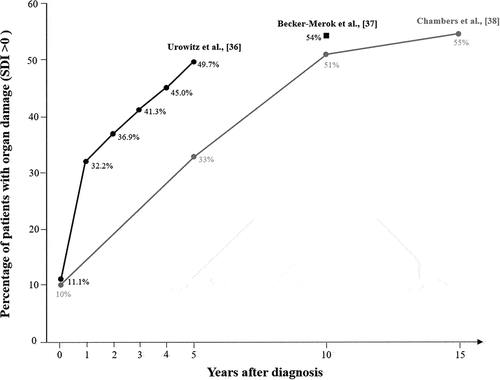
Damage frequently occurs in the musculoskeletal, cardiovascular, renal, neuropsychiatric, and pulmonary systems [Citation45]. Older age at diagnosis, male sex, Hispanic and African ethnic origin, dyslipidemia, number of organs/systems involved, cardiorespiratory involvement, and major flares have been associated with damage accrual [Citation46–48]. Several prospective and retrospective cohorts have shown that renal and cardiovascular damage, have the strongest impact on SLE mortality [Citation49,Citation50]. Chronic disease activity, especially in the initial years of the disease, predicts damage [Citation51,Citation52]; conversely, remission for at least 2 consecutive years protects against damage [Citation53]. Organ damage occurs at the very early stage of the disease, the 40% of patients can develop it in the first year [Citation28] and depending on the study, 28 to 56% of patients can develop this organ damage within 5 years of diagnosis [Citation28,Citation54]. In addition, according to the data from the Spanish Rheumatology Society register (RELESSER), the rate of damage accrual is highest in the first two years post-diagnosis [Citation55]. The accumulation of damage within the first 5 years of disease has been associated with an increased risk of cardiovascular complications and mortality [Citation56,Citation57]. Furthermore, damage triggers further damage. Patients with damage at baseline (SDI > 0) are more likely to experience additional damage over time than those with no baseline damage (SDI = 0) [Citation47]. Several studies have also demonstrated that, despite an apparent control of disease activity, organ damage increases slowly over time, eventually affecting approximately 50% of the patients [Citation34,Citation35,Citation57]. Some examples are represented in with results from the studies of Urowitz et al. [Citation40], Becker-Merok et al. [Citation41] and Chambers et al. [Citation42]. Similar results were also observed in a Swedish cohort where after a mean SLE duration of 17 years, 59% of patients have accrued damage [Citation58].
Figure 2. Evolution of the percentage of patients with organ damage over the course of the disease in several cohorts. SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index.
3.1. Role of glucocorticoids on organ damage
The GCs have been the cornerstone in the treatment of SLE for more than half a century [Citation59,Citation60]. The use of high-dose steroids has demonstrated more rapid, durable, and stronger effects than lower oral prednisone doses [Citation60,Citation61]. According to a systematic review on the use of GCs, a low-dose oral prednisone (<7.5 mg every day) is frequently prescribed for mild manifestations, an intermedium dosage (7.5–30 mg) for moderate manifestations, and 30–60 mg or intravenous pulse doses (>100 mg) for severe or life-threatening manifestations [Citation60,Citation61]. Remarkably, a significant proportion of damage accrual in SLE is attributable to the long-term use of GCs [Citation45,Citation62–64]. Cumulative exposure to GCs was significantly associated with an increased risk of cataracts and osteoporosis [Citation64,Citation65]. Sheane et al. [Citation63] compared disease outcomes between 86 GC-naive and 173 GC-exposed patients with SLE, which revealed that overall mortality in patients receiving GCs was significantly higher (13.3%) compared to those who did not (4.7%). Higher cumulative doses of GCs leading to an increase in the mortality risk was also confirmed by Tarr et al. [Citation64]. However, Thamer et al. [Citation66] revealed that low doses of prednisone do not result in a substantially increased risk of irreversible organ damage [Citation67]. Similarly, recent data from a multicenter Italian inception cohort (‘Early Lupus Project’) which only included patients within 12 months of SLE classification (1997 ACR criteria), suggested little effect of GC on damage in the early stages of the disease [Citation47]. Authors explain this observation as being due to the shorter disease duration and the lower daily dose of prednisone received by their patients.
4. Strategies to prevent organ damage accrual
Overall, data strongly indicate that persistent disease activity during the first 5 years after diagnosis and GC-related toxicity cause progressive organ damage [Citation40,Citation41,Citation42]. Therefore, prompt and sustained disease activity control, with early therapeutic intervention, and limiting GC have become important strategies in the management of SLE, preventing organ damage accrual. Taking into account that the rate of damage accrual is greater in the first few years and the burden of the damage-derived disease seems to be high in that critical period, perhaps we could be talking about a ‘window of opportunity.’
Another strategy to prevent early organ damage induced by treatment is to use biomarkers to monitor damage and evaluate specific interventions for each patient. However, distinguishing symptoms caused by active disease from those that arise following permanent organ damage has been challenging for clinicians [Citation68]. Since SLE is a heterogeneous disease, new biomarkers and future precision medicine approaches could provide new tools to facilitate the risk-stratification and enable personalized medicine and a more efficient treat-to-target approach [Citation20,Citation69].
4.1. Early control of the disease activity
In the treat-to-target strategy for SLE, remission or the lowest possible disease activity is the main treatment target to avoid organ damage and thus mortality [Citation20]. An early treatment not only avoids organ damage associated with mortality but also has an impact on the quality of patient’s life and the disease costs [Citation70]. The prevention of flares is also strongly recommended, since they, most notably the severe ones, contribute to organ damage accrual and lead to worse outcomes [Citation20,Citation25,Citation71,Citation72,Citation73].
From the available therapeutic arsenal, only belimumab has consistently demonstrated effectiveness in reducing the risk of new severe flares in RCT [Citation74,Citation75]. Efforts to identify risk factors for severe flare are required. Recently, Nikolopoulos et al. performed a multivariable logistic regression to identify baseline factors associated with the transition in severity, from initially non-severe disease progressing to severe disease, in a monocentric cohort of patients with early disease. During the disease course, transition to more severe forms was seen in 44.2%, especially men and patients with positive anti-double-stranded DNA or neuropsychiatric involvement at onset [Citation76].
Earlier achievement of remission provides better and maintained long-term responses and outcomes [Citation19,Citation50,Citation77]. Piga et al. [Citation77] showed that failure to achieve low disease activity (measured by LLDAS) within 6 months of the diagnosis is associated with early organ damage accrual. Similarly, Nossent et al. [Citation51] correlated not achieving early disease remission with higher relapse rates and subsequent disease activity (measured with SLEDAI). In higher disease activity patients, the administration of GCs in combination with antimalarials and/or IS/biologic agents, in an early treatment approach of SLE, has demonstrated a subsequent decrease in disease activity over time [Citation78]. Recently, Gatto et al. [Citation79] in a retrospective real-world study (BeRLISS, ‘Belimumab in Real Life Setting Study’) demonstrated that the early use of belimumab in patients with active SLE and low baseline damage predicts favorable outcomes regarding low disease activity, faster tapering of GCs, and less accrual damage. Therefore, the use of belimumab before the appearance of damage may increase the probability of response.
4.2. Tapering of glucocorticoid therapy
The efficacy of GCs for the rapid control of symptoms is well established; however, their long-term use has diverse detrimental effects including organ damage [Citation80]. Indeed, as previously mentioned, GCs are one of the most important causes of permanent organ damage [Citation62,Citation81]. For this reason, and according to 2019 EULAR recommendations, the use of GCs should be, when possible, withdrawn [Citation25]. Alternatives may include the use of intravenous pulses of GCs, as opposed to oral prednisone, with or without the early initiation of other effective agents such as IS or biologic drugs, to lower GC doses or even avoid them completely, in the treatment of SLE. Ruiz-Arruza et al. [Citation82] in an observational study with 74 SLE patients compared the general and specific damage accrual between a traditional treatment approach (using high GC doses) and another regimen characterized by later and lower doses of prednisone together with more pulses of methylprednisolone, earlier use of IS agents, and extensive administration of HCQ. The group with reduced use of prednisone showed a significantly lower damage accrual in terms of GC‐related, cardiovascular disease, and overall damages.
4.3. Early effective therapeutic intervention
HCQ has been proven to reduce the incidence of non-severe flares, the risk of organ damage, and improve survival in patients with SLE [Citation83,Citation84]. Thus, early and extensive use of HCQ is postulated to prevent damage accrual. Interestingly, a retrospective study evaluating the effect of the administration of HCQ in 130 US military personnel concluded that early initiation of treatment with HCQ in patients with incomplete SLE was associated with a delayed onset of fully developed ‘complete’ SLE [Citation85]. Akhavan et al. [Citation86] in a nested case-control study determined whether HCQ was able to avoid early damage in 481 patients with SLE. The authors showed that HCQ was significantly associated with less organ damage at 3 years from diagnosis [Citation23]. On the other hand, poor adherence to HCQ appears to vary from 10% to 22% of SLE patients. Novel biological and targeted therapies, especially belimumab, in several randomized controlled trials, have been demonstrated to be effective in controlling disease activity and symptoms when added to ST [Citation26,Citation31,Citation50,Citation87,Citation88]. They also appear to be able to reduce the requirement of GCs [Citation89]. Furthermore, the incidence of organ damage accrual remains low in patients treated with long-term belimumab, regardless of baseline damage accrual [Citation90,Citation91]. Van Vollenhoven et al. [Citation91], evaluating long-term data from the BLISS extension studies, demonstrated that the organ damage progression after 8 years of follow-up was minimal. The SDI remained stable in most patients (87.7%). The subgroup of patients with baseline SDI ≥1 showed a slightly higher change in SDI (mean 1.6) than those with SDI = 0 (mean 0.0). Likewise, Urowitz et al. [Citation92] in a propensity score-matched comparative analysis (n = 99) revealed that organ damage progression in patients receiving belimumab is significantly lower (61%) than in those receiving ST. The annual probability of progression with belimumab was 3.5%, in contrast to 8.7% with ST alone. Parodis et al. [Citation93] also highlighted the importance of the early use of belimumab when there is no organ damage. Additionally, a clinical trial on the use of belimumab in early lupus is currently ongoing [Citation94].
An early treatment with biologics can be considered from two perspectives: closer to the time of diagnosis or positioned in the first therapeutic lines (with GCs and antimalarials, but before the administration of IS agents). Regarding the treatment with biologic agents closer to the diagnosis, the use of rituximab in newly diagnosed patients with SLE has shown to be clinically effective and able to reduce the use of GCs in observational studies [Citation95,Citation96] evaluated the long-term use of rituximab (up to 7 years) in 16 patients with SLE who started treatment at or close (3 months) to confirm the diagnosis. At 60 months, the mean cumulative prednisolone dose in patients who received rituximab was significantly lower (4.7 g) than those who did not (12.6 g). A systematic review from Cobo-Iñabez et al. also observed that rituximab has shown to be safe and effective in the treatment of non-renal SLE, especially in terms of disease activity, immunologic parameters and steroid-sparing effect [Citation97].
When extrapolating data from other autoimmune diseases, such as RA, a more aggressive treatment very early in the course of the disease has been shown to have a clinical benefit, with a longer duration of the effect and modification of the natural course of the disease [Citation98]. Biologic treatment for early RA (<2 years of evolution) has demonstrated better outcomes as compared with the usual strategy [Citation99]. On the other hand, although there are no studies specifically designed to address the early administration of biologic agents in SLE (before IS agents), there are patients who were included in pivotal trials BLISS-52 and BLISS-76 and who were not receiving IS agents before starting with belimumab. Indeed, Schwarting et al. [Citation100] in a post hoc, pooled analysis of these trials revealed that the most frequent treatment group (concomitant to belimumab) was GCs plus antimalarials (346 out of 834, 41.5%). Moreover, patients from this treatment group showed the greatest percentage of responders at week 52 (59% with belimumab 10 mg/kg versus 44% with placebo). Regarding cost-effectiveness, insufficient control of the disease activity results in an increase of flares, which is related to increased costs, especially for severe SLE patients [Citation10]. The introduction of belimumab has demonstrated improved clinical outcomes and reduced health resource use, thus leading to direct cost savings, and contributing to sustainability and decision-making [Citation101,Citation102]. According to 2019 EULAR recommendations for the management of SLE, belimumab could be considered with or ‘without’ IS agents in ‘patients with inadequate response to ST, defined as residual disease activity not allowing tapering of GCs and/or frequent relapses’ (add-on treatment with belimumab, grade recommendation 1a/A) [Citation25]. Additionally, although with a lower recommendation grade (2b/C), rituximab may be considered as an add-on treatment in cases of ‘organ-threatening disease refractory or with intolerance/contraindications to standard IS agents’ [Citation23].
Other effective therapeutic agents may also be deemed for an early approach in SLE. For example, anifrolumab, a human monoclonal antibody that blocks the activity of the type I interferon receptor, has been recently approved in the US (July 2021) for adult patients with moderate-to-severe SLE who are receiving standard therapy [Citation103]. In the MUSE phase II trial and two TULIP phase III trials, a higher percentage of patients treated with anifrolumab experienced a reduction in overall disease activity, especially in skin and joint involvement, and achieved a reduction of GCs usage, in comparison to placebo [Citation104,Citation105]. However, no data regarding damage prevention are yet available with this biological agent.
FDA recent approved voclosporin (calcineurin inhibitor) and belimumab for the treatment of lupus nephritis based on AURORA 1 and BLISS LN trials [Citation94]. Multitarget therapy has been shown an important advancement in the treatment of patients’ lupus nephritis. Triple therapy using voclosporin in combination with mycophenolate mofetil and low-dose steroids showed superior clinical renal response comparatively to mycophenolate mofetil and low-dose steroids alone [Citation106,Citation107]. Moreover, in the study performed by Liu et al., the authors showed that multitarget therapy consisting of tacrolimus, mycophenolate mofetil, and steroid provides superior efficacy compared with intravenous cyclophosphamide as induction therapy in these patients [Citation106]. Since the most damage occurs in the first year, this early combined therapy could reduce the accumulation of damage and chronic renal disease development in lupus nephritis patients [Citation36].
5. Conclusions
One of the most important aims in the management of SLE is to avoid or delay the accumulation of organ damage. The first five years after the diagnosis of the disease are crucial for prognosis. Early therapeutic intervention with effective agents is a promising alternative for controlling the disease symptoms and preventing severe flares, reducing the use of GCs, and overall, minimizing organ damage. The early use of effective agents such as HCQ and belimumab in clinical practice should be in line with their level of evidence (grade A) established in the 2019 update of EULAR recommendations. Studies specifically designed to evaluate the early use of biological agents and their comparison with IS agents are required to corroborate this new therapeutic early approach.
6. Expert opinion
Preventing organ damage represents one of the most important goals in the management of SLE, as irreversible damage leads to more damage, health-related quality of life reduction and an increased risk of death [Citation20,Citation26]. Strategies aimed at meeting such objectives include the early control of the disease activity, using highly effective therapeutic agents and minimize GC therapy. Chronic disease activity, especially in the initial years, is strongly associated with organ damage [Citation52,Citation53]. By contrast, earlier achievement of clinical remission or lower disease activity protects against damage [Citation77]. Over the last decades, the standard treatment in SLE included nonsteroidal anti-inflammatory drugs, GCs, antimalarials (mainly HCQ), and several IS agents [Citation26]. However, unfortunately, the current standard treatment cannot provide clinical remission or low disease activity in all patients with SLE. Regarding GCs, despite being widely used and very effective for the rapid control of symptoms, their long-term use is responsible for a remarkable proportion of organ damage accrual and conditions, such as coronary artery disease, cataracts, osteoporotic fractures, stroke, and avascular necrosis [Citation61,Citation80,Citation81]. Therefore, there is an unmet clinical need for introducing new therapeutic strategies able to achieve the main therapeutic objectives. Based on the experience of autoimmune diseases like RA, aggressive treatments administered early in the course of the disease or positioned in the first therapeutic lines have shown to provide longer clinical benefits and modification of the natural course of the disease [Citation94,Citation95,Citation96,Citation97].
Biologic agents, mainly belimumab and rituximab, have demonstrated efficacy for controlling disease activity and preventing severe flares, enabling a reduced use of GCs. Moreover, diverse studies have revealed that organ damage accrual continues to be low for long-term treatment with belimumab, regardless of damage accrual at baseline [Citation88,Citation90]. Specifically in lupus nephritis, the early use of combination therapy with belimumab or calcineurin inhibitors (also referred to as ‘multitarget therapy’) namely tacrolimus and more recently voclosporin lead to improve the short-term outcomes as compared to standard therapy alone. Taking into account that most of the renal damage takes place in the first year of the renal flare, this kind of strategy seems promising in terms of prevention of chronic renal disease. Despite the failure of randomized controlled trials, observational studies have suggested that rituximab in newly diagnosed patients with SLE achieves significant clinical responses and minimizes the use of GCs. Similarly, and although none of the studies has been specifically designed to evaluate the early administration of biologic agents in SLE (before IS agents), data on the early use of belimumab in patients with active SLE and low baseline damage point out to favorable clinical outcomes in term of low disease activity, faster tapering of GCs, and less accrual damage [Citation79]. Some patients could benefit most from this early combination therapy such as 1) moderate to severe SLE, 2) patients who are not possible to reduce GC doses (lower than 7,5 mg/day) to control the activity, 3) patients at high risk of severe flares, 4) with poor long-term prognostic factors, 5) at high risk of infection. Some patients such as lower-moderate SLE would not require the combination therapy since most of them could respond to HCQ. The major limitation of this approach is the lack of good damage predictors that can help physicians to identify patients at risk [Citation6]. Moreover, it is necessary to take into account the timing of the expected response of a therapeutic approach, which is an organ-dependent (range varies from 3 to several months). Following EULAR recommendations, patients with LN who did not respond as early as 3 months [Citation25].
Taking into account the available literature, the early use of biological agents and combination therapy in the first lines of the treatment algorithm could be considered an innovative treatment approach to decrease the disease burden in SLE patients.
Article highlights
The first five years after diagnosis are crucial for prognosis in systemic lupus erythematosus (SLE).
Preventing organ damage is one of the most important goals in SLE management.
The current standard treatment cannot provide clinical remission or low disease activity in all patients with SLE.
The early use of innovative treatments such as biological agents in combination with standard of care in the first lines of the treatment could decrease the disease burden in SLE patients.
There is a need to define which SLE profiles could benefit from an early treatment strategy based on the basal characteristics of the disease.
Emerging biomarkers that help to perform a long-term prognostic in SLE patients may pave the way for choosing the best treatment strategy.
This box summarizes key points contained in the article.
Declaration of interests
C San Román Gutiérrez is full-time GlaxoSmithKline employee. FJ Hidalgo Bermejo was a full-time GlaxoSmithKline employee at the time of writing. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors wish to thank Pablo Vivanco on behalf of Meisys (Mediacion Cientifica, S.L.) for his support in the development of this manuscript.
Additional information
Funding
References
- Weiss JE. Pediatric systemic lupus erythematosus: more than a positive antinuclear antibody. Pediatr Rev. 2012;33(2):62–73.
- Rivas-Larrauri F, Yamazaki-Nakashimada MA. Lupus eritematoso sistémico: ¿es una sola enfermedad? Reumatol Clin. 2016;12(5):274–281.
- McMurray RW, May W. Sex hormones and systemic lupus erythematosus: review and meta-analysis. Arthritis Rheum. 2003;48(8):2100–2110.
- Cortés R, Pego-Reigosa JM, Seoane-Mato D, et al. Prevalence of systemic lupus erythematosus in Spain: higher than previously reported in other countries? Rheumatology (Oxford). 2020;59(9):2556–2562.
- Nat Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Rev Rheumatol. 2016;12:605–620.
- Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74(9):1706–1713.
- Arkema EV, Jönsen A, Rönnblom L, et al. Case definitions in Swedish register data to identify systemic lupus erythematosus. BMJ Open. 2016;6(1):e007769.
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121.
- Tselios K, Gladman DD, Touma Z, et al. Disease course patterns in systemic lupus erythematosus. Lupus. 2019;28(1):114–122.
- Gallop K, Nixon A, Swinburn P, et al. Development of a conceptual model of health-related quality of life for systemic lupus erythematosus from the patient’s perspective. Lupus. 2012;21(9):934–943.
- Mazzoni D, Cicognani E, Prati G. Health-related quality of life in systemic lupus erythematosus: a longitudinal study on the impact of problematic support and self-efficacy. Lupus. 2017;26(2):125–131.
- Cervera R, Rúa-Figueroa I, Gil-Aguado A, et al. Coste económico directo del control y el tratamiento del lupus eritematoso sistémico activo y sus brotes en España: estudio LUCIE. Rev Clín Esp. 2013;213(3):127–137.
- Doria A, Amoura Z, Cervera R, et al. Annual direct medical cost of active systemic lupus erythematosus in five European countries. Ann Rheum Dis. 2014;73(1):154–160.
- Doria A, Gatto M, Zen M, et al. Optimizing outcome in SLE: treating-to-target and definition of treatment goals. Autoimmun Rev. 2014;13(7):770–777.
- Jorge AM, Lu N, Zhang Y, et al. Unchanging premature mortality trends in systemic lupus erythematosus: a general population-based study (1999–2014). Rheumatology (Oxford). 2018;57(2):337–344.
- Ghazali WSW, Daud SMM, Mohammad N, et al. SLICC damage index score in systemic lupus erythematosus patients and its associated factors. Medicine (Baltimore). 2018;97(42):12787.
- Sutton EJ, Davidson JE, Bruce IN. The systemic lupus international collaborating clinics (SLICC) damage index: a systematic literature review. Semin Arthritis Rheum. 2013;43(3):352–361.
- Doria A, Zen M, Canova M, et al. SLE diagnosis and treatment: when early is early. Autoimmun Rev. 2010;10(1):55–60.
- Gatto M, Zen M, Iaccarino L, et al. New therapeutic strategies in systemic lupus erythematosus management. Nat Rev Rheumatol. 2019;15(1):30–48.
- van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014;73(6):958–967.
- van Vollenhoven R, Voskuyl A, Bertsias G, et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis. 2017;76(3):554–561.
- Wilhelm TR, Magder LS, Petri M. Remission in systemic lupus erythematosus: durable remission is rare. Ann Rheum Dis. 2017;76(3):547–553.
- van Vollenhoven R, Bertsias G, Doria A, et al. OP0296 The 2021 DORIS definition of remission in sle – final recommendations from an international task force. Ann Rheum Dis. 2021;80(Suppl 1):181–182.
- Morand EF, Mosca M. Treat to target, remission and low disease activity in SLE. Best Pract Res Clin Rheumatol. 2017;31(3):342–350.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745.
- Mok CC. Biological and targeted therapies of systemic lupus erythematosus: evidence and the state of the art. Expert Rev Clin Immunol. 2017;13(7):677–692.
- Mak A, Cheung MW-L, Chiew HJ, et al. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum. 2012;41(6):830–839.
- Taraborelli M, Cavazzana I, Martinazzi N, et al. Organ damage accrual and distribution in systemic lupus erythematosus patients followed-up for more than 10 years. Lupus. 2017;26(11):1197–1204.
- Pego-Reigosa JM, Rúa-Figueroa Í, López-Longo FJ, et al. Analysis of disease activity and response to treatment in a large Spanish cohort of patients with systemic lupus erythematosus. Lupus. 2015;24(7):720–729.
- Doria A, Iaccarino L, Ghirardello A, et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med. 2006;119(8):700–706.
- Dubey AK, Handu SS, Dubey S, et al. Belimumab: first targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. 2011;2(4):317–319.
- Poh YJ, Baptista B, D’ CDP. Subcutaneous and intravenous belimumab in the treatment of systemic lupus erythematosus: a review of data on subcutaneous and intravenous administration. Expert Rev Clin Immunol. 2017;13(10):925–938.
- Schioppo T, Ingegnoli F. Current perspective on rituximab in rheumatic diseases. Drug Des Devel Ther. 2017;11:2891–2904.
- Schmajuk G, Yazdany J, Trupin L, et al. Hydroxychloroquine treatment in a community-based cohort of patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010;62(3):386–392.
- Gatto M, Iaccarino L, Zen M, et al. When to use belimumab in SLE. Expert Rev Clin Immunol. 2017;13(8):737–740.
- Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117–1128.
- Rovin BH, Furie R, Teng YKO, et al. A secondary analysis of the Belimumab International Study in Lupus Nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int. 2022 Feb;101(2):403–413.
- Feld J, Isenberg D. Why and how should we measure disease activity and damage in lupus? Presse Med. 2014;43(6):151–156.
- Handa R. Chapter 144. Management of systemic lupus erythematosus. In: Medicine update. Vol. 17; 2007. doi:10.5005/jp/books/12086_144.
- Urowitz MB, Gladman DD, Ibañez D, et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken). 2012;64(1):132–137.
- Becker-Merok A, Nossent HC. Damage accumulation in systemic lupus erythematosus and its relation to disease activity and mortality. J Rheumatol. 2006;33:1570–1577.
- Chambers SA, Allen E, Rahman A, et al. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford). 2009;48(6):673–675.
- Gladman D, Ginzler E, Goldsmith C, et al. Systemic lupus international collaborative clinics: development of a damage index in systemic lupus erythematosus. J Rheumatol. 1992;19(11):1820–1821.
- Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–369.
- Gladman DD, Urowitz MB, Rahman P, et al. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. 2003;30(9):1955–1959.
- Maddison P, Farewell V, Isenberg D, et al. The rate and pattern of organ damage in late onset systemic lupus erythematosus. J Rheumatol. 2002;29(5):913–917.
- Mok CC, Ho LY, Cheung MY, et al. Effect of disease activity and damage on quality of life in patients with systemic lupus erythematosus: a 2-year prospective study. Scand J Rheumatol. 2009;38(2):121–127.
- Piga M, Floris A, Sebastiani GD, et al. Risk factors of damage in early diagnosed systemic lupus erythematosus: results of the Italian multicentre Early Lupus Project inception cohort. Rheumatology (Oxford). 2020;59(9):2272–2281.
- Danila MI, Pons-Estel GJ, Zhang J, et al. Renal damage is the most important predictor of mortality within the damage index: data from LUMINA LXIV, a multiethnic US cohort. Rheumatology (Oxford). 2009;48(5):542–545.
- Pego-Reigosa J, Lois-Iglesias A, Mouriño-Rodriguez C, et al. Chronological analysis of damage accrual in patients with systemic lupus erythematosus: results from the Spanish Registry of Patients with SLE of the Spanish Society of Rheumatology (RELESSER). Arthritis Rheumatol. 2016;68:1774.
- Nossent J, Kiss E, Rozman B, et al. Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus. 2010;19(8):949–956.
- Giannakou I, Chatzidionysiou K, Magder LS, et al. Predictors of persistent disease activity and long quiescence in systemic lupus erythematosus: results from the Hopkins Lupus Cohort. Lupus Sci Med. 2018;5(1):e000287.
- Zen M, Iaccarino L, Gatto M, et al. The effect of different durations of remission on damage accrual: results from a prospective monocentric cohort of Caucasian patients. Ann Rheum Dis. 2017;76(3):562–565.
- Rahman P, Gladman DD, Urowitz MB, et al. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus. 2001;10(2):93–96.
- McMahon M, Hahn BH, Skaggs BJ. Systemic lupus erythematosus and cardiovascular disease: prediction and potential for therapeutic intervention. Expert Rev Clin Immunol. 2011;7(2):227–241.
- McCarthy EM, Sutton E, Nesbit S, et al. Short-term efficacy and safety of rituximab therapy in refractory systemic lupus erythematosus: results from the British Isles Lupus Assessment Group Biologics Register. Rheumatology (Oxford). 2018;57(3):470–479.
- Stojan G, Petri M. The Risk Benefit Ratio of Glucocorticoids in SLE: have things changed over the Past 40 years? Curr Treatm Opt Rheumatol. 2017;3(3):164–172.
- Luijten RKMAC, Fritsch-Stork RD, Bijlsma JWJ, et al. The use of glucocorticoids in Systemic Lupus Erythematosus. After 60 years still more an art than science. Autoimmun Rev. 2013;12(5):617–628.
- Apostolopoulos D, Kandane-Rathnayake R, Raghunath S, et al. Independent association of glucocorticoids with damage accrual in SLE. Lupus Sci Med. 2016;3(1):e000157.
- Frodlund M, Reid S, Wetterö J, et al. The majority of Swedish systemic lupus erythematosus patients are still affected by irreversible organ impairment: factors related to damage accrual in two regional cohorts. Lupus. 2019;28(10):1261–1272.
- Conti F, Ceccarelli F, Perricone C, et al. The chronic damage in systemic lupus erythematosus is driven by flares, glucocorticoids and antiphospholipid antibodies: results from a monocentric cohort. Lupus. 2016;25(7):719–726.
- Davidson JE, Fu Q, Rao S, et al. Quantifying the burden of steroid-related damage in SLE in the Hopkins Lupus Cohort. Lupus Sci Med. 2018;5(1):e000237.
- Sheane BJ, Gladman DD, Su J, et al. Disease outcomes in glucocorticosteroid-naive patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2017;69(2):252–256.
- Tarr T, Papp G, Nagy N, et al. Chronic high-dose glucocorticoid therapy triggers the development of chronic organ damage and worsens disease outcome in systemic lupus erythematosus. Clin Rheumatol. 2017;36(2):327–333.
- Ruiz-Arruza I, Ugarte A, Cabezas-Rodriguez I, et al. Glucocorticoids and irreversible damage in patients with systemic lupus erythematosus. Rheumatology (Oxford). 2014;53(8):1470–1476.
- Thamer M, Hernán MA, Zhang Y, et al. Prednisone, lupus activity, and permanent organ damage. J Rheumatol. 2009;36(3):560–564. (REPETIDA A X4).
- Mok CC, Ho CTK, Wong RWS, et al. Damage accrual in southern Chinese patients with systemic lupus erythematosus. J Rheumatol. 2003;30(7):1513–1519.
- Bonakdar ZS, Mohtasham N, Karimifar M. Evaluation of damage index and its association with risk factors in patients with systemic lupus erythematosus. J Res Med Sci. 2011;16:427–432.
- Ugarte-Gil MF, Acevedo-Vásquez E, Alarcón GS, et al. The number of flares patients experience impacts on damage accrual in systemic lupus erythematosus: data from a multiethnic Latin American cohort. Ann Rheum Dis. 2015;74(6):1019–1023.
- Legge A, Doucette S, Hanly JG. Predictors of organ damage progression and effect on health-related quality of life in systemic lupus erythematosus. J Rheumatol. 2016;43(6):1050–1056.
- Schneider M. Pitfalls in lupus. Autoimmun Rev. 2016;15(11):1089–1093.
- Enocsson H, Wirestam L, Dahle C, et al. Soluble urokinase plasminogen activator receptor (suPAR) levels predict damage accrual in patients with recent-onset systemic lupus erythematosus. J Autoimmun. 2020;106:102340.
- van Vollenhoven RF, Petri MA, Cervera R, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. 2012;71(8):1343–1349.
- Zhang F, Bae S-C, Bass D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77(3):355–363.
- Stohl W, Schwarting A, Okada M, et al. Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus: a Fifty-Two–Week Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheumatol. 2017;69(5):1016–1027.
- Nikolopoulos DS, Kostopoulou M, Pieta A, et al. Transition to severe phenotype in systemic lupus erythematosus initially presenting with non-severe disease: implications for the management of early disease. Lupus Sci Med. 2020;7(1):e000394.
- Piga M, Floris A, Cappellazzo G, et al. Failure to achieve lupus low disease activity state (LLDAS) six months after diagnosis is associated with early damage accrual in Caucasian patients with systemic lupus erythematosus. Arthritis Res Ther. 2017;19(1):247.
- Hanly JG, Sayani A, and Doucette S, et al. Corticosteroids in early treatment pathways in SLE. Abstract Number: 688. 2014 ACR/ARHP Annual Meeting. Boston. Available from: https://acrabstracts.org/abstract/corticosteroids-in-early-treatment-pathways-in-sle
- Gatto M, Saccon F, Zen M, et al. Early disease and low baseline damage as predictors of response to belimumab in patients with systemic lupus erythematosus in a real-life setting. Arthritis Rheumatol. 2020;72(8):1314–1324.
- Fanouriakis A, Bertsias G. Changing paradigms in the treatment of systemic lupus erythematosus. Lupus Sci Med. 2019;6(1):e000310.
- Zonana-Nacach A, Barr SG, Magder LS, et al. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. 2000;43(8):1801–1808.
- Ruiz-Arruza I, Lozano J, Cabezas-Rodriguez I, et al. Restrictive use of oral glucocorticoids in systemic lupus erythematosus and prevention of damage without worsening long-term disease control: an observational study. Arthritis Care Res (Hoboken). 2018;70(4):582–591.
- Costedoat-Chalumeau N, Dunogué B, Morel N, et al. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med. 2014;43(6):167–180.
- Floris A, Piga M, Mangoni AA, et al. Protective effects of hydroxychloroquine against accelerated atherosclerosis in systemic lupus erythematosus. Mediators Inflamm. 2018;2018:3424136.
- James JA, Kim-Howard XR, Bruner BF, et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus. 2007;16(6):401–409.
- Akhavan PS, Su J, Lou W, et al. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J Rheumatol. 2013;40(6):831–841.
- Collins CE, Dall’Era M, Kan H, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. 2016;3(1):e000118.
- Terrier B, Amoura Z, Ravaud P, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 2010;62(8):2458–2466.
- Bruce IN, Urowitz M, van Vollenhoven R, et al. Long-term organ damage accrual and safety in patients with SLE treated with belimumab plus standard of care. Lupus. 2016;25(7):699–709.
- Urowitz MB, Ohsfeldt RL, Wielage RC, et al. Organ damage in patients treated with belimumab versus standard of care: a propensity score-matched comparative analysis. Ann Rheum Dis. 2019;78(3):372–379.
- van Vollenhoven RF, Navarra SV, Levy RA, et al. Long-term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a phase III study extension. Rheumatology (Oxford). 2020;59(2):281–291.
- Parodis I, Gomez A, Emamikia S, et al. Established organ damage reduces belimumab efficacy in systemic lupus erythematosus. Ann Rheum Dis. 2019;78(7):1006–1007.
- Clinical Trial [Internet]. Trial of belimumab in early lupus. Available from: https://clinicaltrials.gov/ct2/show/NCT03543839
- Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72(8):1280–1286.
- Asif S, Bargman J, Auguste B. A review of the Aurora and BLISS trials: will it revolutionize the treatment of lupus nephritis? Curr Opin Nephrol Hypertens. 2022;31(3):278–282.
- Gracia-Tello B, Ezeonyeji A, Isenberg D. The use of rituximab in newly diagnosed patients with systemic lupus erythematosus: long-term steroid saving capacity and clinical effectiveness. Lupus Sci Med. 2017;4(1):e000182.
- Cobo-Ibáñez T, Loza-Santamaría E, Pego-Reigosa JM, et al. Efficacy and safety of rituximab in the treatment of non-renal systemic lupus erythematosus: a systematic review. Semin Arthritis Rheum. 2014;44(2):175–185.
- Finckh A, Bansback N, Marra CA, et al. Treatment of very early rheumatoid arthritis with symptomatic therapy, disease-modifying antirheumatic drugs, or biologic agents: a cost-effectiveness analysis. Ann Intern Med. 2009;151(9):612–621.
- Castro-Rueda H, Kavanaugh A. Biologic therapy for early rheumatoid arthritis: the latest evidence. Curr Opin Rheumatol. 2008;20(3):314–319.
- Schwarting A, Dooley MA, Roth DA, et al. Impact of concomitant medication use on belimumab efficacy and safety in patients with systemic lupus erythematosus. Lupus. 2016;25(14):1587–1596.
- Cortés J, Andreu JL, Calvo J, et al. Evaluation of use of belimumab in clinical practice settings (Observe Study) in Spain: health resource utilization and labour absenteeism. Value Health. 2014;17(7):534.
- Cevey M, Calvo-Alén J, Crespo C, et al. Budget impact analysis of subcutaneous belimumab in patients with systemic lupus erythematosus in Spain. Clinicoecon Outcomes Res. 2019;11:757–765.
- Deeks ED. Anifrolumab: first approval. Drugs. 2021;81(15):1795–1802.
- Liossis SN, Staveri C. What’s new in the treatment of systemic lupus erythematosus. Front Med (Lausanne). 2021;8:655100.
- Keyes E, Grinnell M, Jacoby D, et al. Assessment and management of the heightened risk for atherosclerotic cardiovascular events in patients with lupus erythematosus or dermatomyositis. Int J Women’s Dermatol. 2021 Sep 9;7(5):560–575.
- Liu Z, Zhang H, Liu Z, et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med. 2015;162(1):18–26.
- Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (Aurora 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397(10289):2070–2080.