1. Introduction
The use of autologous tumor-infiltrating lymphocytes (TILs) as an advanced cancer therapy has been well documented since the late 1980s. Despite steady progress in the field, it remains to be adopted as a standard of care. Early pioneering work by Steve Rosenberg’s team at the National Cancer Institute (NCI), USA, first documented outcomes in 1988, with response rates as high as 60% in treatment-naïve patients with advanced melanoma [Citation1]. However, over the subsequent two decades limited progress was observed, likely in part a consequence of complexities of clinical implementation, but also the transformative introduction of immune checkpoint inhibition (ICI) for this disease subtype. Recently, however, there has been a resurgence in enthusiasm for TILs across multiple tumor subtypes and it now appears closer than ever to being established as a standard of care therapy.
2. Manufacturing/Administration
The starting material for TIL therapy is tumor tissue. This is most commonly obtained via surgical resection, but trial-based efforts are also exploring the potential of biopsy specimens [Citation2]. In its simplest form, TILs are isolated and expanded in a polyclonal manner; typically with use of anti-CD3 ± CD28 antibodies, feeder cells, and interleukin-2 (IL-2) (). TILs then undergo a rapid expansion phase (REP), before harvesting, quality control release, and appropriate storage [Citation3].
Figure 1. Tumor infiltrating lymphocyte (TIL) manufacture/administration. 1) Surgical resection of tumor tissue. 2) TIL isolation. 3) Rapid expansion protocol (REP): This process, originally developed by the Rosenberg team at the NCI, involves the use of IL-2, feeder cells and anti CD3/CD28 monoclonal antibodies to produce a therapeutic TIL product. 4) Quality control (QC) and storage. 5) Non myeloablative- lymphodepletion (NMA-LD). 6) TIL infusion. 7) Adjuvant IL-2: Dose and route of administration vary between clinical trials. Image created with Biorender.Com.
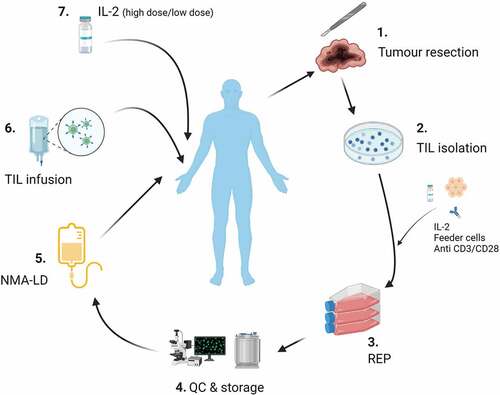
Adoptive transfer of TILs consists of three phases: non-myeloablative lymphodepletion (NMA-LD), infusion of TILs and adjuvant IL-2 ().
3. Early TIL trials
Interest in TILs as a therapeutic anti-cancer strategy was first established by Steve Rosenberg and team at the NCI [Citation1]. Initial investigation focused on the use of lymphokine activated killer cells (LAKs) [Citation4]. LAKs represent isolated peripheral blood leukocytes, which when stimulated ex vivo with IL-2, demonstrate the ability to lyse tumor cells, whilst sparing nonmalignant cells [Citation4]. Subsequent efforts focused on isolation of specific lymphocyte populations beyond the peripheral compartment [Citation5]. Following the successful isolation and expansion of lymphocytes from surgically resected tumor specimens, the first trial demonstrating the efficacy of adoptively transferred TILs in patients with metastatic melanoma was documented by Rosenberg et al in 1988 [Citation1]. Twenty patients were enrolled; 15 treatment-naïve and 5 having received prior IL-2 therapy. Patients received a single lymphodepleting dose of cyclophosphamide at 25 mg/kg, 36 hours prior to TIL infusion, with a cell dose range of 3 × 1010- 75 × 1010. IL-2 at a dose of 100,000 IU/kg was then administered every 8 hours until dose-limiting toxicity occurred. Objective responses were observed in 60% (9/15) of treatment-naïve and 40% of IL-2 pre-treated patients respectively. Toxicity, although significant, was attributed to IL-2 and there were no treatment-associated deaths [Citation1].
Subsequent to these pioneering studies, there have been a number of early trials with relatively consistent outcomes. Initially, these were completed at the NCI [Citation5,Citation6], but also undertaken, among others, at the Sheba Medical Centre [Citation7], MD Anderson [Citation8], and the Center of Cancer immune Therapy, Herlev Hospital, Copenhagen [Citation9,Citation10]. Throughout this early work, TIL therapy for metastatic melanoma has resulted in objective responses in 40–50% of patients, with complete response rates of 10–20% [Citation9].
4. TIL therapy in a contemporary melanoma population
With a relative paucity of data derived from a contemporary melanoma population, specifically those refractory to ICI’s, a significant advance came with data from C-144-01 study from Iovance Biotherapeutics [Citation3]. Sixty-six patients with heavily pre-treated advanced melanoma received the TIL product lifileucel as part of the phase II clinical trial (NCT02360579). All patients had received prior anti-PD-1/PD-L1 treatment, with 80% having received anti-CTLA-4 therapy and 23% having received BRAF ± MEK inhibitors. Lifileucel was produced via a streamlined 22-day process, and lymphodepletion was achieved with cyclophosphamide (60 mg/kg) once daily for 2 days followed by fludarabine (25 mg/m2) once daily for 5 days. IL-2 at a dose of 600, 000 IU/kg was then infused every 8–12 h for up to 6 doses. The overall response rate (ORR) was 36% (24/66) with 33% (22/66) partial response (PR) and 2 complete responses. Within the primary refractory cohort, an ORR of 41% (17/42) was achieved, with 15 PRs (36%). All patients experienced at least one treatment-related adverse event (trAE), the most common being thrombocytopenia (82%). These were however all attributed to the known toxicity profile of NMA-LD and IL-2.
Interestingly, exploratory phenotypic analysis did not display any association between T cell lineage, memory subset, youth, activation, exhaustion or trafficking with response [Citation3].
A further milestone was met in September 2022, with Haanen et al presenting data from the first randomized phase III TIL study in solid tumors [Citation11]. Here, 186 patients with unresectable melanoma and progressive disease after a maximum of one line of systemic therapy (excluding ipilimumab), were randomized 1:1 to either TILs or ipilimumab. Lymphodepletion and IL-2 strategy was the same as that used in C-144-01. ORR was 49% (41/84) for TILs and 21% (18/84) for ipilimumab, with a notable complete response rate of 20% (17/84) vs 7% (6/84) respectively. PFS was significantly improved at 7.2 months for TILs compared to 3.1 months for ipilimumab.
5. TILs in combination with ICI
Activated T cells are subject to immune regulation, as such there is clear rationale to combine TIL therapy with ICIs; not only to impact the activity of adoptively transferred TILs, but also potential waves of endogenous T cell immunity that may follow [Citation12].
Multiple studies (NCT04165967, NCT03475134, NCT03374839, NCT01701674, NCT03638375, NCT02652455) are seeking to determine the impact of combination approaches, including IOV-COM-202 (NCT03645928); a phase II multicentre study of TILs in combination with pembrolizumab in patients with ICI naïve melanoma, head and neck cancer (HNSCC) and non-small cell lung cancer (NSCLC)[Citation13].
Patients enrolled receive a single dose of pembrolizumab 400 mg (after tumor procurement but prior to lymphodepletion), as well as adjuvant IL-2 per the C-144-01 study; pembrolizumab is continued post TIL infusion 6-weekly for up to 24 months.
Initial data was presented at The Society for Immunotherapy for Cancer’s (SITC) annual meeting 2021, and although only 10 patients were assessed within the melanoma arm, an objective response rate of 60% (6/10) was observed. All efficacy evaluable patients experienced a reduction in tumor burden, with three patients achieving a complete response. Safety was deemed in keeping with the known profile of NMA-LD IL-2 and pembrolizumab. Grade 3/4 trAE were seen in all patients, however of note these predominantly occurred during in-patient admission in the two weeks following TIL’s infusion [Citation14].
6. Neoantigen-specific TILs
Translational studies conducted in patients undergoing polyclonal TIL therapy demonstrate neoantigen-reactive TIL are key orchestrators of anti-tumor immunity and underlie efficacy [Citation15,Citation16]. It follows that enriching TIL products for neoantigen reactivity might be an attractive method of optimizing the activity of TIL therapy.
One such approach is being investigated in patients with advanced melanoma via the Thetis clinical trial from Achilles Therapeutics (NCT03997474). TILs are isolated from resected tumor and, in parallel, dendritic cells are isolated from peripheral blood. Following an initial phase of TIL expansion, a co-culture step is performed with dendritic cells loaded with peptides corresponding to putative clonal neoantigens. The final product is enriched for TILs reactive to clonal neoantigens (cNETs), potentially overcoming the well-recognized challenge of intra-tumoral heterogeneity in the neoantigen landscape. An interim observational analysis was presented at SITC 2021, demonstrating the ability to quantify and track cNeT pre and post transfer [Citation17].
7. Expert opinion
TIL therapy appears active in patients with cutaneous melanoma refractory to immune checkpoint inhibition [Citation3,Citation11]. Given TIL products derive from a site of disease, it is unclear what underlies such activity, but feasibly this could relate to a lack of penetration of ICI antibodies into the tumor microenvironment (TME), failure of expansion of endogenous TIL owing to the status of T cell differentiation, inefficient priming or a non-supportive cytokine milieu and/or competition within the TME. Within adoptive cell-based approaches, there is room for optimization of all key stages/interventions from NMA-LD to product manufacture to adjuvant therapy.
NMA-LD provides the opportunity to impact circulating (peripheral blood) factors that limit systemic immunity and also to positively ‘condition’ the tumor microenvironment via elimination of immunosuppressive subsets including regulatory T cells (Treg) and myeloid-derived suppressors cells (MDSCs). Almost universally, NMA-LD comprises cytotoxic chemotherapy, but one can envisage a future where more selective lymphodepletion and conditioning might be achieved via monoclonal antibody-based approaches and/or small molecule inhibition.
Ex vivo manipulation of TILs, even simple polyclonal expansion, provides the opportunity to ‘reinvigorate’ TILs in supportive culture medium with use of appropriate cytokines. Within TIL manufacture, ‘young’ TILs have been demonstrated to display greater efficacy in vivo and efforts are underway to evaluate the activity of TIL products that have undergone an enrichment or selection step; typically to increase the fraction of either neoantigen or more broadly tumor reactive cells [Citation18–20]. Molecular engineering approaches can also be incorporated into manufacturing practice including, for example, molecular editing of the programmed cell death-1 (PD-1) gene or of the T cell receptor and major histocompatibility complex towards generation of ‘off the shelf,’ allogeneic products. However, the activity of TIL therapy is thought to relate to enrichment for neoantigen-specific TILs; given neoantigens are tumour/patient-specific, for TIL therapy (in contrast to CAR-T/TCR therapy) allogeneic products are likely to be of limited utility [Citation21,Citation22]. Adjuvant approaches (post adoptive transfer) in TIL therapy have typically incorporated high or low dose IL-2, but in the context of clinical trials are increasingly incorporating more novel systemic therapies including immune checkpoint inhibition as previously described.
Within the spectrum of cellular therapy products, TILs are associated with a relatively favorable acute toxicity profile that does not typically include cytokine release syndrome (CRS) or immune effector cell associated neurotoxicity (ICANS) [Citation23]. However, high grade toxicity is observed related to both high dose lymphodepletion and intravenous IL-2; particularly when given at high doses via the intravenous route [Citation3,Citation11]. Red and white cell cytopenias, beyond desired lymphodepletion, are observed in a majority of patients following NMA-LD and managed with growth factor and blood product support [Citation23]. High dose IL-2 is associated with the potential for toxicity in multiple organ sites, including heart, lung, kidneys as well as systemic toxicity including capillary leak syndrome [Citation23]. Whilst prolonged inpatient admissions and peri-infusional toxicities require specialist input and may initially appear more challenging to manage than current standard of care interventions, TIL transfer is a ‘one-off’ intervention and almost all high-grade toxicity emerges and resolves during the inpatient admission itself; as such at the point of most severe toxicity, patients are likely to be under direct clinical supervision [Citation13,Citation14,Citation23]. The ‘one-off’ nature of this intervention is also attractive in terms of patient time, in contrast to a majority of current standard of care immunotherapy approaches, there is no requirement for regular repeat dosing with clear implications for both time and travel.
A challenge will be extrapolation of this approach across multiple solid tumor types, particularly those recognized to be poorly immunogenic related to low putative neoantigen burden. Further, many patients will not have resectable sites of disease and/or be fit to undergo surgical resection. Such challenges underlie efforts to identify and isolate neoantigen-reactive T cells from peripheral blood [Citation24,Citation25]. For tumor subtypes where TIL therapy appears to hold promise (cervical carcinoma, cutaneous melanoma, HNSCC, and NSCLC), TIL harvesting in the context of refractory disease poses a significant challenge given a paucity of TIL infiltration is commonplace in progressing lesions; potentially related to multiple mechanisms of immune evasion. Harvesting and delivering TIL therapy earlier in a patient’s treatment pathway might therefore hold promise [Citation14]. Clonal evolution/neoantigen editing could limit the utility of a polyclonal TIL therapy manufactured early and delivered at a later time point, but targeting clonal neoantigens, as described above, might offer a potential solution [Citation26].
TIL therapy appears to offer the potential for durable disease control amongst a proportion of responding patients. Following the promising lifileucel data published by Sarnaik et al [Citation3], an updated pooled analysis of consecutive cohorts has been conducted [Citation27]. The full analysis set included 153 patients treated with lifileucel, including longer term follow-up of the 66 previously reported patients. Over a median study follow-up of 27.6 months, ORR was 31.4% (48/153), with 41.7% maintained for over 18 months; median duration of response was not reached and median OS was 13.9 months.
Rosenberg and colleagues have also reported longer-term outcomes amongst 93 patients with advanced melanoma treated with autologous polyclonal TIL therapy in three consecutive trials (Ref Rosenberg et al Clin Cancer Res (2011) 17 (13): 4550–4557.). Twenty of 93 patients (22%) achieved a complete response (CR), of these 19 have ongoing CRs beyond 3 years. The actuarial 3- and 5-year survival rates for the entire group were 36% and 29% respectively, but for the 20 complete responders were 100% and 93%. Further, within a small cohort of 10 patients investigated in the Netherlands, Haanen et al reported 2/10 patients who displayed complete responses persisting at seven years of follow-up [Citation28].
Beyond the recognized clinical and scientific challenges, such ‘living cell’ products are subject to greater regulatory oversight than standard investigational medicinal products (IMPs). In addition, there are technical, financial, and infrastructure-related challenges to the manufacture and delivery of TILs, which may limit implementation within healthcare systems to few privileged/specialist sites. Whilst such therapies do typically, at least in their current form, require inpatient admission they are most often ‘one-off’ interventions and, as such, one can envisage patients traveling some distance to specialist centers; as they might for bone marrow transplantation or other forms of immune effector cell therapy. Alongside the described challenges is great enthusiasm and an impression that although we only find ourselves in our current position as a consequence of major pre-clinical and clinical efforts, cell therapy, including TILs, remains in its infancy for solid tumors but possesses genuine potential to transform management and patient outcomes.
Declaration of interest
M Julve declares receipt of conference registration fee sponsorship from Amgen. AJS Furness declares an advisory role for Immunocore, Neogene and GSK, speaker’s bureau participation for BMS, Ipsen, Eisai and Merck, and travel, accommodation and expenses from ESMO. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N Engl J Med InternetAvailable from. 1988;319:1676–1680. DOI:10.1056/NEJM198812223192527
- Chesney JA, Schoenfeld AJ, Wise-Draper T, et al. Abstract CT130: trial in progress: a phase 2 multicenter study (IOV-LUN-202) of autologous tumor-infiltrating lymphocyte (TIL) cell therapy (LN-145) in patients with metastatic non-small cell lung cancer (mNSCLC). Cancer Res. 2022;82:CT130.
- Sarnaik AA, Hamid O, Khushalani NI, et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J Clin Oncol. 2021;39:2656–2666.
- Rayner AA, Grimm EA, Lotze MT, et al. Lymphokine-Activated Killer (LAK) cell phenomenon. IV. Lysis by LAK cell clones of fresh human tumor cells from autologous and multiple allogeneic tumors. JNCI. 1985;75:67–75. InternetAvailable from. DOI:10.1093/jnci/75.1.67
- Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240.
- Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. JNCI. 1994;86:1159–1166.
- Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655.
- Radvanyi LG, Bernatchez C, Zhang M, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18:6758–6770.
- Andersen R, Donia M, Ellebaek E, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res. 2016;22:3734–3745.
- Ellebaek E, Iversen TZ, Junker N, et al. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med. 2012;10:169.
- Haanen J, Rohaan M, Borch TH. LBA3 - Treatment with tumor-infiltrating lymphocytes (TIL) versus ipilimumab for advanced melanoma: results from a multicenter, randomized phase III trial. Ann Oncol. 2022;33:S808–869. DOI:10.1016/j.annonc.2022.08.036
- Draghi A, Chamberlain CA, Furness A, et al. Acquired resistance to cancer immunotherapy. Semin Immunopathol. 2019;41:31–40.
- O’malley D, Lee S, Psyrri A, et al. 492 phase 2 efficacy and safety of autologous tumor-infiltrating lymphocyte (TIL) cell therapy in combination with pembrolizumab in immune checkpoint inhibitor-naïve patients with advanced cancers. J Immunother Cancer. 2021;9:A523. Internet Available from http://jitc.bmj.com/content/9/Suppl_2/A523.abstract
- O’malley D Phase 2 efficacy and safety of autologous Tumor-Infiltrating Lymphocyte (TIL) cell therapy in combination with pembrolizumab in immune checkpoint inhibitor-naïve patients with advanced cancers. SITC 2021 [Internet]. (WA) DC; 2021. Available from: https://www.iovance.com/scientific-publications-presentations/. [cited 2022Dec6].
- Kristensen NP, Heeke C, Tvingsholm SA, et al. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes in melanoma. J Clin Investig. 2022;132. DOI:10.1172/JCI150535
- Lauss M, Donia M, Harbst K, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun. 2017;8:1738.
- Turajlic S, Jamal-Hanjani M, Furness A, et al. 543 sensitive quantification and tracking of the active components of a Clonal Neoantigen T cell (cNet) therapy: from manufacture to peripheral circulation. J Immunother Cancer. 2021;9:A572. InternetAvailable from https://jitc.bmj.com/content/9/Suppl_2/A572
- Yossef R, Tran E, Deniger DC, et al. Enhanced detection of neoantigen-reactive T cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight. 2018;3. DOI:10.1172/jci.insight.122467
- Warner A. Trial in progress: a phase 1/2 open-label study (IOV-GM1-201) of TALEN-mediated PD-1–inactivated autologous tumor-infiltrating lymphocytes (TIL; IOV-4001) in patients with advanced melanoma and NSCLC. SITC. Boston; 2022.
- Tran E, Robbins PF, Rosenberg SA. “Final common pathway” of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol. 2017;18:255–262. DOI:10.1038/ni.3682
- Menger L, Sledzinska A, Bergerhoff K, et al. TALEN-Mediated inactivation of PD-1 in tumor-reactive lymphocytes promotes intratumoral t-cell persistence and rejection of established tumors. Cancer Res. 2016;76:2087–2093.
- Pal S, Tran B, Haanen J, et al. 558 CTX130 allogeneic CRISPR-Cas9–engineered chimeric antigen receptor (CAR) T cells in patients with advanced clear cell renal cell carcinoma: results from the phase 1 COBALT-RCC study. Regular and young investigator award abstracts. BMJ Publishing Group Ltd; 2022; p. A584.
- Wolf B, Zimmermann S, Arber C, et al. Safety and Tolerability of Adoptive Cell Therapy in Cancer. Drug Saf. 2019;42:315–334.
- Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433–438.
- Gros A, Tran E, Parkhurst MR, et al. Recognition of human gastrointestinal cancer neoantigens by circulating PD-1+ lymphocytes. J Clin Investig. 2019;129:4992–5004.
- Rosenthal R, Cadieux EL, Salgado R, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019;567:479–485.
- Chesney J, Lewis KD, Kluger H, et al. Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study. J Immunother Cancer. 2022;10:e005755.
- van den Berg JH, Heemskerk B, van Rooij N, et al. Tumor infiltrating lymphocytes (TIL) therapy in metastatic melanoma: boosting of neoantigen-specific T cell reactivity and long-term follow-up. J Immunother Cancer. 2020;8:e000848.
