1. Introduction
Over the last decades, immunotherapy has achieved meaningful results in Oncology by radically changing the therapeutic approach of several solid tumors, including renal cell carcinoma (RCC) [Citation1]. Remarkable advances have been made with the introduction of immune checkpoint inhibitors (ICIs) either alone or in combination with other agents (such as tyrosine kinase inhibitors – TKIs) in the treatment landscape of RCC () [Citation2]. ICIs are monoclonal antibody designed to block the programmed death-1/ligand-1 (PD-1/PD-L1) axis and the cytotoxic T lymphocyte antigen-4 (CTLA-4) pathway, as well as other important molecules, which are exploited by malignant cells to evade the T-cell-mediated immune response () [Citation1]. In RCC, PD-1 inhibitors firstly came out to treat advanced staged patients who progressed on first-line anti-vascular endothelial growth factor (VEGF) TKIs [Citation2]. Subsequently, PD-1 inhibitors combined with a CTLA-4 inhibitor (nivolumab/ipilimumab) or with anti-VEGF TKIs (nivolumab/cabozantinib, or pembrolizumab plus axitinib or lenvatinib) were shown to improve the depth of response along with long-term survival benefits in untreated metastatic RCC (mRCC), soon becoming the novel standard-of-care as first-line options [Citation2]. Furthermore, the efficacy of pembrolizumab as an adjuvant therapy has recently been demonstrated for high-risk RCC patients, after surgery [Citation3].
Figure 1. Timeline of the main PD-1 inhibitors (alone or in combination with other drugs) tested in advanced renal cell carcinoma. Created with BioRender.com.
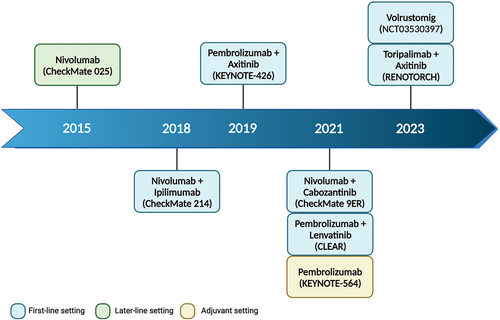
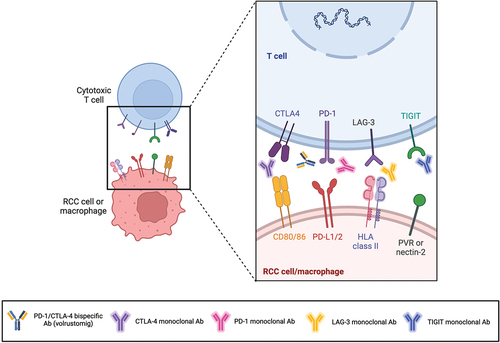
Figure 2. Monoclonal antibodies targeting PD-1 and CTLA-4 disrupt T-cell inhibitory responses, blocking PD-1 interaction with its ligands PD-L1/2 and CTLA-4 interaction with its ligand CD80/86. Notably, the bispecific antibody volrustomig targets both PD-1 and CTLA-4, in order to enhance the therapeutic benefit of this dual immune checkpoint blockade decreasing the risk of toxicity which is typically associated with anti-CTLA-4 agents. Furthermore, monoclonal antibodies targeting other immune checkpoints (such as TIGIT and LAG-3) aim to inhibit tumor-induced mechanisms that evade the host immune system, thereby reinvigorating the immune response against malignant cells. Created with BioRender.com. Abbreviations: RCC, renal cell carcinoma; PD-1, programmed death-1; PD-L1/2, programmed death ligand 1/2; CTLA-4, cytotoxic T lymphocyte antigen-4; LAG-3, lymphocyte activation gene-3; TIGIT, T-cell immunoglobulin and ITIM domain; HLA, human leukocyte antigen; PVR, poliovirus receptor; Ab, antibody.
Even though ICI-based strategies have strongly improved the outcomes in RCC, many patients still witness a primary resistance or acquire resistance after an initial response to these treatments. While the need to identify biomarkers for discerning responders from non-responders is apparent [Citation4], the exploration of novel immunotherapeutic agents has been simultaneously exploited to overwhelm this issue and to reach further progresses in this malignancy [Citation5]. Of note, the aim of this commentary is to focus on novel PD-1 inhibitors, which may represent an ‘alternative’ to the well-known and hugely used anti-PD-1 agents (such as pembrolizumab and nivolumab, that play a critical role in the therapeutic management of RCC), or novel agents, which may be used in combination with PD-1 inhibitors as treatment ‘alternatives’ in kidney cancer.
2. Novel PD-1 inhibitors in RCC: beyond pembrolizumab and nivolumab …
In 2023, data from the phase III trial RENOTORCH unveiled the clinical activity and tolerability of the anti-PD-1 toripalimab in combination with axitinib for intermediate-/poor-risk mRCC patients [Citation6], thus becoming the first ICI-based combination to be approved in China as a first-line strategy in this disease. The combination of the humanized anti-PD-1 IgG4k toripalimab plus axitinib showed a significant improvement in median progression-free survival (PFS) compared to sunitinib (18.0 vs 9.8 months, p = 0.0028), with a notably higher objective response rate (ORR − 56.7% vs 30.8%). Overall survival (OS) data, though still immature, tended to favor the immunocombination (not reached vs 26.8 months) [Citation6].
Two Asian phase II trials are currently assessing the role of toripalimab in non-metastatic RCC patients, both as adjuvant monotherapy in non-clear cell histologies (NCT05768464) and as neoadjuvant treatment in combination with axitinib (NCT05738694). The design of these two studies provides, respectively: 30 estimated nccRCC patients enrolled for the first trial, and 246 estimated patients with resected ccRCC at a high-risk of recurrence after surgery (T2,G3/4 or T3/4 or N1) enrolled for the second trial.
Other innovative PD-1 inhibitors are currently under investigation in mRCC, among which zimberelimab seems to be characterized by an interesting preclinical activity [Citation7]. An ongoing phase I umbrella trial is evaluating the safety of zimberelimab combined with the anti-CD39 IgG1 AB509 in treatment-naïve metastatic patients with many solid tumors (estimated number of enrolled patients = 81), including mRCC (NCT05891171). Study completion is scheduled for August 2025.
3. PD-1/CTLA-4 bispecific antibodies: the volrustomig experience
Lately, the so-called bispecific antibodies are getting even more interested in the therapeutic management of several malignancies. Focusing on RCC, volrustomig is a monovalent PD-1/CTLA-4 bispecific antibody, which distinctly leads to PD-1 blockade with a preferential CTLA-4 inhibition on activated PD-1+ T-cells. In a first-in-human phase Ib study enrolling treatment-naïve mRCC patients, volrustomig showed promising efficacy (ORR 58%) at the dose of 1500 mg administered every 3 weeks (Q3W), but with a safety profile limited by many adverse events (AEs), mainly represented by severe liver toxicities [Citation8]. Lowering the dose to 500 mg (V500) and 750 mg (V750) Q3W, volrustomig continued to exhibit an encouraging activity (ORR 48% with V750, 46% with V500) reducing severe AEs’ frequency and treatment discontinuation rate [Citation9]. Responses appeared to be durable, and V750 notably improved both ORRs (especially in intermediate-/poor-risk patients) and primary resistance (<10%) [Citation9]. Both V500 and V750 led to enhanced T-cell activation in the RCC, demonstrating higher T-cell proliferation and activation levels than clinical practice doses of anti-CTLA-4 [Citation9]. A combination of volrustomig with lenvatinib or axitinib is currently being assessed in the first-line setting within a phase Ib study (NCT04522323), involving an estimated number of 179 treatment-naïve mRCC patients to enroll.
4. Combining novel ICIs with PD-1 inhibitors for novel therapeutic options in RCC
To advance the field and achieve even more sustained benefits for RCC patients, exploring novel immunotherapeutic strategies beyond the PD-(L)1 and/or CTLA-4 blockage is needed. As a matter of fact, ever-growing evidence is highlighting that targeting a second immune receptor may improve the efficacy of PD-(L)1 inhibition [Citation10].
In recent years, the lymphocyte activation gene-3 (LAG-3) represents one of the immune checkpoints mostly studied as a target of novel ICIs. LAG-3 is expressed on several cells such as activated T-, B-, and NK-cells, and inhibits T-cell proliferation, thus creating an immune-depleted microenvironment (TME) [Citation11]. LAG-3 is particularly expressed in the RCC TME [Citation11]. Favezelimab (MK-4280) is a humanized IgG4 monoclonal antibody against LAG-3, which is currently under investigation combined with pembrolizumab in many malignancies including mRCC in the phase Ib/II substudies MK-3475-03A (NCT04626479) and MK-3475-03B (NCT04626518). As a matter of fact, these two substudies are parts of a larger research umbrella study (the phase Ib/II MK-3475-U03 umbrella trial), which aims to evaluate the safety and efficacy of experimental combinations of investigational agents (among which favezelimab/pembrolizumab) in RCC. The substudy 03A (MK-3475-03A) involves participants with advanced untreated ccRCC (estimated n = 400), including a safety lead-in phase and an efficacy phase. On the other hand, the substudy 03B (MK-3475-03B) focuses on the second-line setting, enrolling pretreated mRCC patients (estimated n = 370). Considering the promising data obtained in melanoma with anti-PD-1/anti-LAG-3 combinations, results from these trials are strongly awaited in mRCC to further expand the therapeutic armamentarium.
Other meaningful immune checkpoints particularly expressed in RCC TME are represented by the T-cell immunoglobulin and ITIM domain (TIGIT) and the immunoglobulin-like transcript 4 receptor (ILT-4), both upregulated in immunosuppressive cancer-promoting pathways [Citation10].
TIGIT inhibitors are being studied in numerous cancers, alone or in combination with PD-(L)1 inhibitors. The anti-TIGIT vibostolimab is currently being tested in an arm of the above-mentioned phase Ib/II MK-3475-03A substudy (NCT04626479), after having demonstrated antitumor activity both as monotherapy and as immune-based combination with pembrolizumab in metastatic solid tumors (as lung cancer) [Citation12]. Moreover, an ongoing phase I study is assessing the efficacy of the combination of anti-TIGIT tiragolumab with atezolizumab and stereotactic body radiation therapy (SBRT) in various metastatic cancers, including RCC (NCT05259319) [Citation13]. As a matter of fact, SBRT was shown to increase expression levels of the inhibitory co-receptors TIGIT and PD-L1 in preclinical models of human solid tumors, thus defining the biological rationale of this combination [Citation13]. This phase I study has a four cohorts design (metastatic RCC, metastatic bladder cancer, metastatic non-small cell lung cancers and metastatic head and neck cancers), thus enrolling patients with a minimum of 3 measurable lesions, among which at least one lesion cannot be irradiated and at least 2 irradiable lesions (estimated n = 92).
On the other hand, anti-ILT-4 agents were shown to improve the immune response against tumor cells when administered with PD-(L)1 inhibitors. The anti-ILT-4 MK-4830 showed an encouraging antitumor activity with an acceptable safety profile alone and in combination with pembrolizumab in many malignancies [Citation14]. The combination of MK-4830 with pembrolizumab is currently under investigation in pretreated mRCC patients, who have been enrolled in the umbrella MK-3475-03B trial. CDX-585 is another novel ILT-4-inhibitor which is being evaluated in several advanced cancers, including pretreated mRCC, in a US phase I study (NCT05788484) (estimated n = 130).
Beyond the rise of novel ICIs, future results from ongoing trials focused on several other immunotherapeutic agents (including anti-cancer vaccines, ICI-based combinations plus bacterial products, chimeric antigen receptor (CAR) T-cells, and so on) will help overwhelm the above-mentioned issues [Citation5], further expanding the therapeutic options for this disease.
5. Expert opinion
In RCC, PD-(L)1 blockade has so far demonstrated considerable success as a therapeutic strategy, not only in treating metastatic disease (both in pretreated and in treatment-naïve patients) but also in the adjuvant setting. Nonetheless, providing durable clinical benefits for a huge number of RCC patients still represents an unmet need, shedding light on the necessity to broaden the range of therapeutic options at our disposal. In this regard, alternative PD-1 inhibitors (such as toripalimab, zimberelimab or bispecific antibodies) are currently under investigation, alone and in combinations with other agents (including TKIs). Moreover, as already seen with the nivolumab/ipilimumab combination, the addition of a second ICI targeting a different immune inhibitory pathway (i.e. LAG-3, TIGIT or ILT-4) seems to empower the host immunologic activity against malignant cells, further improving the clinical activity of PD-1 inhibitors.
To answer the title question, the therapeutic management of RCC surely needs alternative PD-1 inhibitors (along with novel ICIs). Nevertheless, in the current era, a multitude of treatment modalities exists for RCC patients (especially as first-line therapies for mRCC). Consequently, novel immunotherapeutic agents should try to address the actual open questions in this field, by improving the depth of response in the first-line setting of mRCC, by overcoming primary and secondary resistance to available ICI-based therapies, or by facilitating extended treatment-free periods. Results from the above-mentioned clinical trials are awaited to better define the clinical activity along with the safety profile of the many novel ICI-based combinations and the many therapeutic alternatives to the currently used PD-1 inhibitors. Certainly, the real-world experience with these new molecules will be crucial to ensure their appropriate use, especially for novel drugs such as the bispecific antibodies (i.e. the bispecific anti-PD-1 and anti-CTLA-4 agent volrustomig). Furthermore, the intersection of these novel treatment options with radiogenomics, an evolving field in RCC research, promises significant future benefits for enhancing patient management [Citation15]. Lastly, further data is required to identify the most adequate treatment sequence for each RCC patient (both with a metastatic de novo diagnosis or with a disease progression after an initial response to post-nephrectomy adjuvant PD-1 inhibitor), in which novel ICIs and/or novel immune-based combinations may get their most appropriate space of action.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers in this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Yang L, Ning Q, Tang SS. Recent advances and next breakthrough in immunotherapy for cancer treatment. J Immunol Res. 2022;2022:8052212. doi: 10.1155/2022/8052212
- Yang J, Wang K, Yang Z. Treatment strategies for clear cell renal cell carcinoma: past, present and future. Front Oncol. 2023;13:1133832. doi: 10.3389/fonc.2023.1133832
- Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial [published correction appears in Lancet Oncol. 2023 Jan;24(1): e10]. Lancet Oncol. 2022;23(9):1133–1144. doi: 10.1016/S1470-2045(22)00487-9
- Rosellini M, Marchetti A, Mollica V, et al. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat Rev Urol. 2023;20(3):133–157. doi: 10.1038/s41585-022-00676-0
- Braun DA, Bakouny Z, Hirsch L, et al. Beyond conventional immune-checkpoint inhibition - novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol. 2021;18(4):199–214. doi: 10.1038/s41571-020-00455-z
- Yan XQ, Ye MJ, Zou Q, et al. Toripalimab plus axitinib versus sunitinib as first-line treatment for advanced renal cell carcinoma: RENOTORCH, a randomized, open-label, phase III study. Ann Oncol. [cited 2023 Oct 20];35(2):190–199. doi: 10.1016/j.annonc.2023.09.3108
- Lou B, Wei H, Yang F, et al. Preclinical characterization of GLS-010 (Zimberelimab), a novel fully human anti-PD-1 therapeutic monoclonal antibody for cancer. Front Oncol. 2021;11:736955. doi: 10.3389/fonc.2021.736955
- Albiges L, Rodriguez LM, Kim S, et al. Safety and clinical activity of MEDI5752, a PD-1/CTLA-4 bispecific checkpoint inhibitor, as monotherapy in patients (pts) with advanced renal cell carcinoma (RCC): preliminary results from an FTIH trial. J Clin Oncol. 2022;40(Suppl 16):107. doi: 10.1200/JCO.2022.40.16_suppl.107
- Voss MH, Garmezy B, Kim SH, et al. 1883MO - MEDI5752 (volrustomig), a novel PD-1/CTLA-4 bispecific antibody, in the first-line (1L) treatment of 65 patients (pts) with advanced clear cell renal cell carcinoma (aRCC). Ann Oncol. 2023;34(suppl_2):S1013–S1031. doi: 10.1016/j.annonc.2023.09.1113
- Patwekar M, Sehar N, Patwekar F, et al. Novel immune checkpoint targets: a promising therapy for cancer treatments. Int Immunopharmacol. 2024;126:111186. doi: 10.1016/j.intimp.2023.111186
- Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620
- Niu J, Maurice-Dror C, Lee DH, et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer☆. Ann Oncol. 2022;33(2):169–180. doi: 10.1016/j.annonc.2021.11.002
- Roussot N, Fumet JD, Limagne E, et al. A phase I study of the combination of atezolizumab, tiragolumab, and stereotactic body radiation therapy in patients with metastatic multiorgan cancer. BMC Cancer. 2023;23(1):1080. doi: 10.1186/s12885-023-11534-6
- Siu LL, Wang D, Hilton J, et al. First-in-class anti-immunoglobulin-like transcript 4 myeloid-specific antibody MK-4830 abrogates a PD-1 resistance mechanism in patients with advanced solid tumors [published correction appears in clin cancer res. 2022 Apr 14;28(8): 1734] [published correction appears in clin cancer res. 2022 Sep 15;28(18): 4158]. Clin Cancer Res. 2022;28(1):57–70. doi: 10.1158/1078-0432.CCR-21-2160
- Ferro M, Musi G, Marchioni M, et al. Radiogenomics in renal cancer management-current evidence and future prospects. Int J Mol Sci. 2023;24(5):4615. doi: 10.3390/ijms24054615
