ABSTRACT
Introduction
We evaluated a potential move from one rapid-acting insulin analog to another, or their biosimilars, to aid better and faster decisions for diabetes management.
Methods
A systematic literature review was performed according to PRISMA reporting guidelines. The MEDLINE/EMBASE/COCHRANE databases were searched for randomized control trials (RCTs) comparing aspart/lispro in type-1 (T1D) and type-2 (T2D) diabetes. The methodological quality of the included studies was assessed using the Cochrane Collaboration’s risk of bias assessment criteria.
Results
Of the 753 records retrieved, the six selected efficacy/safety RCTs and the additional three hand-searched pharmacokinetics/pharmacodynamics RCTs showed some heterogeneity in the presentation of the continuous variables; however, collectively, the outcomes demonstrated that lispro and aspart had comparable efficacy and safety in adult patients with T1D and T2D. Both treatments yielded a similar decrease in glycated hemoglobin (HbA1c) and had similar dosing and weight changes, with similar treatment-emergent adverse events (TEAE) and serious adverse event (SAE) reporting, similar hypoglycemic episodes in both T1D and T2D populations, and no clinically significant differences for hyperglycemia, occlusions or other infusion site/set complications.
Conclusions
Aspart and lispro demonstrate comparative safety and efficacy in patients with T1D/T2D. Since both are deemed equally suitable for controlling prandial glycemic excursions and both have similar safety attributes, they may be used interchangeably in clinical practice.
PROSPERO registration number
CRD42023376793
1. Introduction
Diabetes is a leading cause of morbidity and mortality, and the number of adults (20–79 years) now living with diabetes (>500 million) will rise to nearly 650 million by 2030 and nearly 800 million by 2045 [Citation1,Citation2]. Insulin therapy is the standard of care in all patients with type-1 diabetes (T1D) and in those patients with type-2 diabetes (T2D) who are uncontrolled on other oral or injectable antihyperglycemic agents. The use of insulin in patients with T2D is estimated to increase from 516.1 million per year in 2018 to 633.7 million per year in 2030 [Citation3]. The global burden of T1D is also likely to double by 2040, and so would be the requirement of insulin [Citation4]. In 2021, the diabetes-associated health expenditure was estimated to be at least USD 966 billion dollars, which was a 316% increase in expenditure over the last 15 years, and this is a significant economic burden with about one in 10 adults being affected by diabetes worldwide [Citation1]. Insulin analogs, first- and second-generation, and their biosimilars have expanded the treatment options for patients with diabetes. Insulin biosimilars have a potential economic benefit by positively impacting healthcare budgetary redistribution through increased affordability and access [Citation5].
Rapid-acting insulin analogs (RAIAs; lispro [Humalog®, 1996]; aspart [Novolog®, 2002], and glulisine [Apidra®, 2004]) closely mimic the normal mealtime insulin excursions, with their pharmacodynamic (PD) profiles closer to that of endogenously released insulin [Citation6]. RAIAs are preferred in patients with symptomatic hyperglycemia or those with glycemia uncontrolled by basal insulin alone because of their faster onset and offset of action, lower hypoglycemia rates, and lower intra-patient variability [Citation7]. Insulin lispro, the first clinically available insulin analog, has the natural amino acid sequence of the B-chain at positions 28 and 29 reversed, resulting in proline at position 28 and lysine at position 29, similar to insulin-like growth factor-1. For insulin aspart, the proline at position 28 of the B-chain is replaced by aspartic acid [Citation8]. These substitutions deter the self-association of the RAIAs into dimers and hexamers, thereby increasing the rate of absorption into the blood after subcutaneous administration [Citation9]. A new class of insulins, ‘ultra-rapid-acting’ insulins, has been introduced recently. These are faster-acting insulin aspart (Fiasp®, 2017) [Citation10] and insulin lispro-aabc (URLi; Lyumjev®, 2020) [Citation11]. With these, the clinical practice has several bolus insulin options, with short-, rapid-, or ultra-rapid-acting time-action profiles, to help manage postprandial glucose levels and hyperglycemia incidences [Citation6].
The current systematic literature review (SLR) focuses on the RAIAs, Lispro and Aspart, to evaluate a potential switch from one to another or their biosimilars to better manage diabetes. There is evidence about the bioequivalence of lispro and aspart. There are some PD differences in the time of action, but head-to-head studies comparing the clinical outcomes in T1D and T2D are very limited. The objective of this SLR was to compare randomized controlled trials (RCTs) of insulin lispro versus insulin aspart regarding pharmacokinetics (PK)/PD, clinical efficacy, and safety outcomes in patients with T1D and T2D. The results from this SLR can aid prescribers, payors, and formularies in making better and faster decisions.
2. Methods
The SLR was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines [Citation12]. A protocol specifying the inclusion and exclusion criteria and the method for evaluating study quality, outcomes, and statistics was developed a priori. This protocol was registered with the Prospective Register of Systematic Reviews (PROSPERO; CRD42023376793), the international prospective register for systematic reviews.
2.1. Search strategy
A systematic literature search of RCTs in the English language was conducted using an appropriate pre-specified search strategy in PubMed/Ovid MEDLINE, Embase, and Cochrane Library databases from their inception up to 18th August, 2023. The search string was constructed using controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings) and keywords. The search elements were kept very broad to pick up all comparators of lispro and aspart; the search strategy included an appropriate combination of keywords such as diabetes mellitus, diabetes, insulin, analogs or analogues, efficacy, benefits, safety, rapid-acting insulin analogs, insulin analogs, lispro, and aspart. In addition, the requirement of the article to be in English language and the time of publication were also used to impose additional constraints. The filters ‘Clinical Study’ and ‘Randomized Controlled Trial’ were used to limit the search output to clinical trials. Additionally, we hand-searched the reference lists and bibliographies from studies that fulfilled our eligibility criteria to identify additional relevant reports.
The eligibility criteria to shortlist the final articles followed the PICOS (participant, intervention, comparison, and study design) components ().
Table 1. Eligibility criteria based on the PICOS approach.
2.2. Screening and selection of articles
Two independent reviewers (GT and AP) identified relevant RCTs using the pre-defined search strategy, and all retrieved articles were imported and managed in Endnote X8. All retrieved articles were screened by independently reading the title and abstract, and duplicate studies were discarded. The reviewers read the full text and identified eligible studies that met the inclusion criteria. All articles identified as potentially meeting eligibility requirements were sourced and thoroughly reviewed. A third reviewer (SM) resolved any inconsistencies found during the study selection process.
2.3. Quality assessment
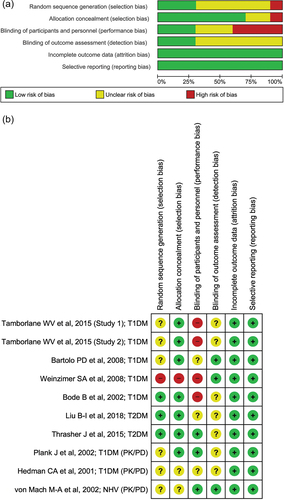
Two authors (GT and AP) independently assessed the methodological quality of the included studies using the Cochrane Collaboration’s risk of bias assessment criteria [Citation13]. Its specific contents included the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). Each domain was considered as high, unclear, or low risk and was represented by color codes, as red (high), yellow (unclear), and green (low), respectively. The quality of the evidence was assessed in terms of risk of bias, stability of effect size, openness, precision, and dissemination bias. Any conflicts were resolved after discussion and consensus with a third author (SM).
2.4. Outcome measures
Efficacy RCT studies were included if they reported at least one of the following outcomes: change from baseline in HbA1c, fasting plasma glucose (FPG), body weight, basal insulin dose, time to target range, and safety outcomes (incidence and severity of hypoglycemic events, nocturnal hypoglycemic events, total adverse events [AEs], treatment-emergent serious AEs [TESAEs], treatment-emergent AEs like hypersensitivity and injection-site reactions, and withdrawal because of AEs.
2.5. Data extraction
Two reviewers (GT and AP) independently extracted key data elements from the selected RCTs. A pre-designed standard Excel form was used to record and manage the extracted key data items. Reviewers first extracted key data elements from the selected RCTs, including general information (first author, country, year of publication), methodological characteristics (number of study centers, study population, sample size, randomization, duration of trial, interventions compared, number of intervention arms), and clinical characteristics (frequency of participants in the respective intervention groups, mean age, type of diabetes, and outcome measures reported). Disagreements between the reviewers were resolved through a third reviewer (SM).
3. Results
3.1. Study selection and characteristics of the included studies
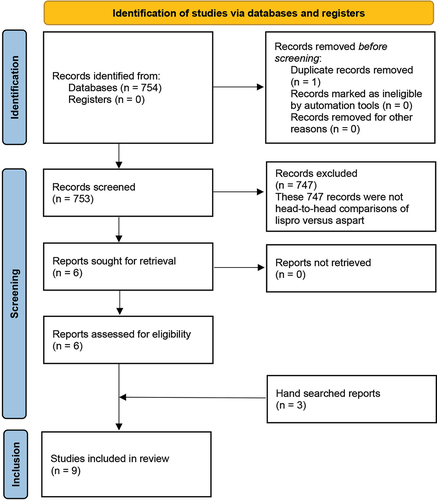
The electronic search retrieved 754 records, of which one duplicate was excluded. Of the remaining 753 unique records, 747 were excluded and six studies were considered eligible for data extraction. Three PK/PD RCTs were added upon hand searching. The search strategy for RCTs from databases carried out from their inception until 18 August 2023, is provided in . The PRISMA flowchart for identifying relevant studies and reasons for exclusion is depicted in . The characteristics of all the nine studies included in the SLR are presented in . These studies were published between 2001 and 2018, with sample sizes ranging from 14 to 298 patients. A total of 1016 patients with diabetes were evaluated in the included studies, with nearly equal distribution in the lispro and aspart groups. Four of the six included efficacy studies recruited T1DM patients (N = 726), while two recruited T2DM patients (N = 232). All studies, including the PK/PD studies, were RCTs comparing lispro and aspart in an open-label (n = 4), single-blind (n = 2), or double-blind setting (n = 3). All the efficacy studies included insulin infusion via continuous subcutaneous insulin infusion (CSII) pump and compared the efficacy and safety of lispro and aspart. The three PK/PD trials identified were head-to-head RCTs carried out in either the T1D population (n = 38) or healthy subjects (n = 20) (). Our search yielded no trials with subcutaneous injections, and we excluded premixed insulin and biphasic insulin studies to get a direct one-to-one comparison. As the trials identified were heterogenous regarding patient population, outcome measures, and consistency in the necessary continuous variables, we did not draw conclusions based on categorical summaries. Hence, we have not run a meta-analysis but presented the search results as an SLR. Even as an SLR, there were not enough homogenous outcome measures to be collated to present as Forest plots. Hence, all included studies’ characteristics, including outcome measures, results, and conclusions, are presented in a table format ().
Table 2. Search strategy.
Table 3. Study characteristics and key results of the selected trials.
3.2. Risk of bias (quality assessment)
Most of the included studies had no bias concern except for high risk in performance due to the open-label setting. All nine studies included were considered to have a low risk of attrition bias. The study design included only RCTs, resulting in a low risk of selection bias for both T1D and T2D. However, few studies had unclear risks in domains like selection, detection, and reporting, as the publications did not describe the method for random sequence generation or blinding or the protocol was not available in the public domain to verify whether all data generated were reported. Based on the Cochrane Risk of Bias analysis, all studies were judged to be of moderate methodological quality. The risk of bias plots/summaries are presented in .
3.3. Results and outcomes
The selected RCTs comparing lispro and aspart were found to be heterogenous in terms of most intended outcomes stated in the methods section. Details of the results from the six efficacy trials and the three PK/PD trials are provided in . Three T1D trials were open label, Study 2 by Tamborlane et al.. (2015) and the study by Planck et al.. (2002) were double-blind, and the study by Hedman et al.. (2001) was single-blind [Citation14–18]. Two efficacy studies (n = 282) and two PK/PD studies (n = 38) had a cross-over design [Citation18–21]. A cross-over design in an efficacy trial is useful in evaluating the safe and effective use of drugs when patients switch from one medication to another [Citation22]. Data from cross-over studies can provide relevant information on switching treatments in a patient. In the two T2D trials comprising 232 patients, 122 patients were studied in a 16-week cross-over design [Citation20].
3.3.1. PK/PD
The PK/PD trial outcomes have been listed together; two trials were held in the T1D population and one trial was held in normal healthy volunteers. The trials were single- (n = 1) or double-blind (n = 2), randomized, cross-over studies, with one of them containing run-in patients with T1D in a PK/PD euglycemic clamp study setting [Citation21]. In the clamp study based on a complete head-to-head comparison, both insulin analogs were equally effective in controlling postprandial blood glucose excursions. In the other set of subjects with T1DM, free insulin profiles of aspart and lispro were reported to resemble each other, but lispro showed a more rapid uptake, reached the maximum peak concentration earlier, and showed a more rapid decline than aspart [Citation18].
However, in healthy volunteers, von Mach et al.. (2002) observed significant differences in the PK and PD profiles of insulin lispro and aspart [Citation23]. A significantly stronger reduction of blood glucose was observed in the aspart group, indicating a more rapid absorption of aspart compared to lispro. The lowest glucose concentrations were observed after 50 min in the aspart group (3.2 ± 0.1 mmol/L versus lispro 3.5 ± 0.1 mmol/L; p = 0.026) and after 60 min in the lispro group (3.4 ± 0.1 mmol/L).
3.3.2. T1DM
3.3.2.1. Efficacy
In a 16-week study comprising a pediatric population with T1DM, aspart provided glycemic efficacy similar to that of lispro (change in HbA1c: aspart, −0.15 ± 0.05%; lispro, −0.05 ± 0.07% [95% confidence interval {CI} of the treatment difference −0.27 to 0.07]; p = 0.241) [Citation16]. At week 16, 59.7% of the subjects in the aspart group (50.3% at baseline) and 43.8% of the subjects in the lispro group (40.4% at baseline) achieved ADA 2006 age-specific recommendations for HbA1c (p = 0.040, corrected for baseline percentage; ADA 2006 goals: <8.5% for subjects aged <6 years and < 8% for subjects aged 6–18 years).
The mean fasting plasma glucose values were comparable between treatments at baseline (aspart 170.8 ± 77.39 mg/dL; lispro 177.8 ± 67.61 mg/dL, p = 0.455) and at the end of study (aspart 166.5 ± 67.28 mg/dL; lispro 180.2 ± 82.58 mg/dL, p = 0.113). Body weight was also comparable between the treatment groups (aspart 1.82 ± 2.07 kg: lispro 1.6 ± 2.09 kg, p = 0.387). However, the mean weight-adjusted daily dose was significantly lower in the aspart group compared with the lispro group (0.86 ± 0.237 versus 0.94 ± 0.233 unit/kg, respectively, p = 0.018).
In the two Tamborlane et al. studies (2015, ) with cross-over assessment in 265 patients, lispro did not achieve non-inferiority (self-monitored blood glucose [SMBG}; margin = 0.6 mmol/L [10.8 mg/dL]) to aspart on the primary outcome (Study 1: least-squares mean difference: 0.48 mmol/L [8.64 mg/dL]; 95% CI [0.20, 0.76]; Study 2: least-squares mean difference: 0.36 mmol/L [6.49 mg/dL]; 95% CI [0.06, 0.66]) [Citation14]. In Study 2 (12-week treatment period), aspart demonstrated a significantly lower HbA1c at the endpoint (p < 0.001) and a greater change from baseline (p < 0.001) compared with lispro [Citation14]. In both studies 1 and 2, there were no significant differences between the lispro and aspart treatments in daily basal, bolus, or total insulin dose. In an open-label, 16-week study comprising 146 subjects, the mean changes in baseline HbA1c values were not significantly different among the aspart and lispro groups (0.00 ± 0.51% and 0.18 ± 0.84%, respectively) [Citation17]. Di Bartolo et al.. (2008) reported that postprandial blood glucose was more stable with aspart than with lispro (absolute glucose 7.04 ± 3.16 versus 9.04 ± 4.2, P ˂ 0.0019); however, no differences in overall daily glucose stability were observed between the two treatments [Citation15]. The mean changes in baseline HbA1c values were not significantly different between the two groups [Citation15].
3.3.2.2. Safety
In the pediatric T1DM population, rates of overall (92.2% aspart; 81.3% lispro), minor (77.2% aspart; 66.0% aspart), and major (0.4% aspart; 0.3% lispro) hypoglycemic episodes and nocturnal hypoglycemia (5.7% aspart; 6.2% lispro), measured through SMBG, were similar in both lispro and aspart groups, with no statistically significant differences [Citation16]. Hyperglycemic episodes, mostly mild or moderate, were reported as AEs for 11% of subjects in the aspart group compared with 17% of subjects in the lispro group. Similar numbers and types of AEs were reported by 82% of subjects in the aspart and 83% in the lispro group, with the majority of events being mild in severity. Seven serious adverse events (SAEs) were reported for six subjects (five subjects [2.5%] in the aspart group [hypoglycemic seizure, diabetic keto-acidosis {DKA}, hypoglycemia with accidental overdose of insulin, hyperglycemia, and skin lacerations] and one subject [1.0%] in the lispro group [hypoglycemia]). The percentages of subjects who reported an infusion site reaction were similar between groups (aspart 17%, lispro 21%, p = 0.43).
The rates of self-reported hypoglycemic episodes per patient per month were similar (3.7 and 4.4 for the aspart and lispro groups, respectively), including nocturnal hypoglycemia, in an open-label RCT comprising 146 patients [Citation17]. During the 3-month maintenance period, a greater percentage of patients in the aspart group (41%) were free of nocturnal hypoglycemic episodes compared with the lispro (25%) group. Clogs/blockages in pumps or infusion sets were infrequent; most subjects (76% and 75% in the aspart and lispro groups, respectively) had one clog or blockage per 4 weeks during the trial.
In both Tamborlane studies [Citation14], subjects in the lispro group reported a significantly lower rate of hypoglycemic episodes per 30 days, which were self-reported as the glucose outcomes were measured through SMBG, than subjects in the aspart group for documented (Study 1: 9.39 versus 10.84, p = 0.003; Study 2: 7.57 versus 8.71, p = 0.012) and all reported hypoglycemia (Study 1: 15.26 versus 16.91, p = 0.006; Study 2: 16.74 versus 18.86, p < 0.001). There were no significant differences between the two treatments in asymptomatic or nocturnal hypoglycemic episode rates per 30 days. Subjects in both studies reported a significantly higher rate per 30 days of non-explained hyperglycemic episodes in the lispro compared with the aspart group (Study 1: 8.20 versus 6.79, p = 0.029; Study 2: 8.05 versus 6.54, p = 0.003). There were four episodes of DKA during the two studies in subjects receiving lispro. There were no significant differences between the two treatments for infusion set-clogging or other infusion site/set complications that led to premature reservoir or infusion set changes.
3.3.3. T2DM
3.3.3.1. Efficacy
A Phase-3b, multi-center, randomized, double-blind, active comparator, 2-period (16 weeks each), 2-sequence, 32-week cross-over trial demonstrated non-inferiority of lispro to aspart for the primary outcome of HbA1c (the upper limit of the 95% CI [−0.002, 0.210] for this treatment comparison was < 0.4%) [Citation20]. Daily insulin dose between lispro (80.41 units ± SE 4.78) and aspart (80.69 units ± SE 4.77) was not significantly different (least squares mean [LSM] difference: −0.28 units; 95% CI [−2.92, 2.35]; p = 0.831). Weight change from baseline was similar between lispro (0.31 kg ± SE 0.53) and aspart (0.89 kg ± SE 0.52), with an LSM difference of −0.58 kg (95% CI [−1.51, 0.34]) and no significant difference (p = 0.216) between treatments arms.
In a single-blind RCT in newly diagnosed patients with T2D, the continuous glucose monitoring system (CGMS) data showed no statistically significant differences in 24-h mean blood glucose (6.49 ± 0.10 versus 6.49 ± 0.15 mmol/L), glucose concentration per hour, mean amplitude of glucose excursion (3.33 ± 0.27 versus 3.18 ± 0.19 mmol/L), standard deviation of mean blood glucose (1.30 ± 0.08 versus 1.28 ± 0.06 mmol/L), and number of glucose excursions (4.54 ± 0.27 versus 4.60 ± 0.21 times) between the aspart and lispro groups [Citation19].
3.3.3.2. Safety
In the newly diagnosed T2D population, the number of hypoglycemia events (<3.9 mmol/L; CGMS) in the aspart and lispro groups was 22 and 15, respectively, with no statistical differences found between the two groups (chi-square = 1.493, p = 0.222) [Citation19]. Thrasher et al. [Citation20] reported a similar overall incidence of hypoglycemia, measured through a mix of CGMS and SMBG, between the lispro (71.2%) and aspart (74.8%) groups. Nocturnal hypoglycemia was 44.1% for the lispro group and 49.6% for the aspart group, and severe hypoglycemia was 0% for the lispro group and 0.8% for the aspart group, with no statistically significant difference. In addition, the hypoglycemic episode rates per 30 days were similar for the lispro (2.24) and aspart (2.38) groups. No statistically significant differences were observed between lispro (51.7%) and aspart (54.6%) treatment for treatment-emergent adverse events (TEAEs) (p = 0.489). Most TEAEs were of mild or moderate severity. No occlusions or hyperglycemia related to pump malfunction/clogging events were reported in either group. SAE reporting was not statistically different (p = 0.909) between the lispro (12.7%) and aspart (11.8%) groups.
4. Discussion
Human regular insulin formulations show kinetics that are not ideal for prandial insulin replacement/augmentation when injected subcutaneously. This led to the development of insulin analogs with a more rapid onset and duration of action. Insulin aspart and lispro are the most widely used RAIAs. Their time-action profiles are highly similar as characterized by a euglycemic clamp study [Citation21]. Accordingly, no reproducible differences in terms of metabolic control should be seen in patients using either of the short-acting analogs in daily practice. This is confirmed by a comprehensive search of the literature, which did not reveal evidence from well-controlled studies that insulin lispro or aspart differ consistently in their ability to contribute toward meeting glycemic targets in insulin-treated patients with diabetes mellitus.
This SLR has shown heterogeneity in the presentation of the continuous variables; however, it is worth noting that, based on some consistent conclusions observed with the two analogs, the available evidence for similarities between the two is sufficient to deduce the potential benefits, or not, of using one versus the other. Collectively, the SLR outcomes demonstrate that lispro and aspart have comparable efficacy and safety in adult patients with T1D and T2D on CSII [Citation14–17,Citation19,Citation20]. Both aspart and lispro treatments yielded a similar decrease in HbA1c and had similar dosing and weight changes [Citation17,Citation19,Citation20], with similar TEAE and SAE reporting [Citation14–17,Citation19,Citation20], similar asymptomatic or nocturnal hypoglycemic episodes in both T1D and T2D populations [Citation14,Citation16,Citation17,Citation19,Citation20], and no significant differences for occlusions or other infusion site/set complications that may have led to premature reservoir or infusion set changes [Citation14,Citation17,Citation20]. Keeping aside the pediatric T1D population, where aspart provided similar glycemic efficacy to that of lispro, albeit at a significantly lower total daily dose [Citation16], and a significantly larger number of subjects in the aspart group (59.7%) achieved ADA age-specific recommendations for HbA1c than in the lispro group (43.8%) [Citation16], the rest of the efficacy/safety differential findings may be incidental and not essentially based on the distinctiveness of the products being used. The observation of significantly lower HbA1c at the endpoint with aspart and a significantly lower rate of hypoglycemic episodes with lispro in a Phase-3b study in T1D [Citation14] warrants more studies for any further consideration. Especially so, as the glucose monitoring and hypoglycemia incidences were done through SMBG and there is a chance that the self-reporting of hypoglycemia may not be very accurate.
There are not many studies comparing the PK/PD profiles between aspart and lispro, and the available studies are inconclusive. Of the three RCTs included in this SLR, the effects differed in the T1D population and normal healthy volunteers [Citation18,Citation21,Citation23]. While the PK and PD effects of aspart and lispro were observed to be equivalent in one set of T1D patients [Citation21], lispro showed a more rapid uptake, reached the maximum peak concentration earlier, and showed a more rapid decline than aspart in the other set of T1D patients [Citation18]. Whereas, in healthy volunteers, a more rapid absorption and significantly stronger reduction of blood glucose was observed in the aspart group with higher insulin concentrations, which could be advantageous in postprandial glucose control [Citation23].
When presented with such limited data, it becomes pertinent that we critically assess the data at hand and comprehend the limitations of the selected studies before drawing any conclusions. The study by Thrasher et al. [Citation20] in patients with T2DM allowed for oral antidiabetic agents, albeit on a stable dose; however, such an accompanying regimen acts as a confounding factor. The Di Bartolo et al. study [Citation15] was open labeled, characterized by a small sample size, a short study duration (24 h on each treatment), and absence of a control for carry-over effects. Several other studies were also open label [Citation14,Citation16,Citation17], had small sample sizes [Citation18,Citation21,Citation23], had short follow-up periods [Citation15,Citation18,Citation21,Citation23], and seemed to have limited control over extraneous variability (e.g. in terms of diet, physical activity, baseline blood glucose control, and/or basal insulin therapy, etc.). Attributing any observed differences between treatment arms to the insulin used, given the multiple alternative explanations or confounders, would stand the risk of overinterpretation. The outcome of this SLR observes that both aspart and lispro are comparative in safety and efficacy in patients with T1D/T2D. In clinical practice, both can be deemed equally suited to control prandial glycemic excursions and have similar safety attributes in patients with diabetes mellitus.
In addition to the SLR of RCTs, in the real-world evidence setting, again, we found a paucity of Phase 4, investigator-initiated trials, observational studies, or real-world evidence studies that have a head-to-head comparison of aspart with lispro. Two longitudinal cohort studies performed in T1D and T2D populations from 2005 to 2013/2014 and published recently in 2023 assessed cardiovascular safety and mortality among lispro, aspart, and glulisine insulins users [Citation24,Citation25]. Results demonstrated no significant differences in the effectiveness and long-term cardiovascular safety and mortality between aspart and lispro. Overall, data from clinical practitioners for >7 years showed no major differences in the rates of hypoglycemia and hyperglycemia, weight, or long-term safety between aspart and lispro [Citation24,Citation25]. In another retrospective cohort of comparative effectiveness, Rasca et al. (2018) provided a comprehensive assessment of outcomes and costs between aspart and lispro using administrative claims data (commercial and Medicare health coverage; data collection period: 1 January 2007-31 December 2014) from a national database for approximately 16 million patients across the United States [Citation26]. This study showed no difference in clinical or economic measures between aspart and lispro, thereby aiding patients and providers to discuss and deduce from the therapy options available to them. In clinical practice, with similar efficacy and safety, lispro and aspart have a 1:1 dose conversion with each other [Citation27], although some prescribers recommend a 10% cut in dose while switching from lispro to aspart [Citation28]. With an earlier time to onset (aspart, 5–10 mins versus lispro, 15 mins), users report more flexibility with aspart regarding the timing of consuming their meals [Citation28]. The patient populations for aspart and lispro also differ as aspart is mainly prescribed to both adults and children at least 2 years old with T1D or T2D, whereas lispro is prescribed for adults and children >3 years with T1D and only adults with T2D [Citation29,Citation30].
On a parallel note, it is also important to state that the biosimilars of RAIAs are providing more affordable options with retail prices less than half of the originators of lispro and aspart [Citation31]. In the U.S.A., instead of meting out a retail price of $670 for a pack of originator insulin lispro Kwikpen (3 ml; 100 IU/ml; five pens), 60% savings were achieved by paying $261 for a pack of generic insulin lispro Kwikpen (3 ml; 100 IU/ml; five pens) [Citation31]. Similarly, the retail price for a carton of five originator insulin aspart 70/30 FlexPen (3 ml; 100 IU/ml) is $707.88 versus $345.53 for five FlexPens (3 ml; 100 IU/ml) of the generic version, showing a saving of nearly 50%. In both cases, the data are from claims from 1 October 2022 to 30 September 2023 [Citation31]. Similarly, in a single UK county, a cost-effective biosimilar of aspart has the potential to save the NHS ~£250K per year if all patients are switched over [Citation32]. We have observed that due to limited insurance coverage and limited formulary availability, this benefit is not being extended to many. Hence, it becomes imperative that switching between the two RAIAs or their biosimilars should enable and ensure faster and better outreach.
5. Conclusions
This systematic literature review demonstrates that both aspart and lispro are comparative in safety and efficacy parameters in patients with T1D and T2D. In clinical practice of diabetes mellitus, both may be deemed equally suitable in controlling prandial glycemic excursions and having similar safety attributes and hence may be used interchangeably.
6. Expert opinion
Clinical trials comparing different insulin products are inherently difficult to interpret. The reason for this lies in the necessity to adapt insulin regimens and doses to individual patients’ needs, which are dynamic and inherently dependent on a wide range of variables. The magnitude of insulin action is confounded by patient factors including individual responsiveness to insulin (insulin sensitivity), design of insulin regimen (in particular, the basal insulin coverage or support when comparing short-acting bolus or prandial insulins), as well as the meal intake (in terms of size and composition). In addition, the injection site, injection technique, and ambient and skin temperature can impact insulin absorption. Although not intended, what is often actually being compared is the acumen of the patients to inject insulin in a reproducible manner and to adjust insulin doses to their needs, or, more broadly, the suitability of the insulin regimen (rather than product) for the patient. It is for this reason that the time-action profiles of insulin in terms of its ability to stimulate glucose uptake are usually used to compare different insulin products (i.e. glucose infusion rates as assessed by the euglycemic clamp studies). In cases like that of aspart and lispro, where such head-to-head comparisons have not been largely established, critical assessment of the data at hand is required keeping in mind the limitations of the available studies before drawing any conclusions. As stated earlier, attributing any differences to the two products, given the multiple alternative explanations or confounders, may lead to overinterpretation. To conclude the observations from this SLR, it is noted that even after >20 years of use in clinical practice, no robust evidence from well-controlled studies could be found that supports the conclusion that insulin lispro or insulin aspart are distinct in terms of achieved glycemic control. Clinically, both aspart and lispro are deemed equally suited to control prandial glycemic excursions and have similar safety attributes in patients with diabetes mellitus. Also to be noted is the fact that with the increasing use of automated insulin delivery systems, small differences in efficacy between insulins are unlikely to significantly impact outcomes as the learning algorithms would be able to compensate and adapt to slightly different insulin action profiles. Therefore, it is reasonable to take as read that aspart and lispro are very similar in terms of safety and efficacy and can be used interchangeably in clinical practice.
Keeping the future perspective in mind, biosimilars of RAIAs can benefit healthcare budgets impacted by spiraling costs of diabetes management through increased affordability and access. The need of the hour is also to develop standardized minimum methodological criteria for health technology assessments for biosimilars, the outputs of which can aid prescribers, payors, and formularies in making better and faster decisions.
Article highlights
With one in 10 adults living with diabetes (>500 million) worldwide, there is a significant health economic burden anticipated as the numbers will rise to nearly 650 million by 2030 and nearly 800 million by 2045.
Insulin analogs, first- and second-generation, including rapid-acting insulin analogs (RAIAs), have expanded the treatment options for patients with diabetes, but these come at a higher cost. Their biosimilars may potentially benefit by positively impacting healthcare budgetary redistribution through increased affordability and access.
Aspart and lispro, the two RAIAs, are comparable in safety and efficacy in patients with T1D/T2D, and, even after >20 years of use in clinical practice, no robust evidence from well-controlled studies is found that supports the conclusion that lispro or aspart is distinct in terms of achieved glycemic control.
Declaration of interest
R Kapur, G Tonpe, A P, P Raj, U Gudat, and SN Athalye are employees of Biocon Biologics Limited and are eligible for stocks and stock options. R Kapur was an employee of Novo Nordisk and has stocks of the same. S Mittra is engaged as a consultant with Biocon Biologics Limited. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the writing and review of the manuscript and have read and approved the final version of the manuscript.
Acknowledgments
The authors acknowledge Dr Alben Sigamani (Carmel Research Consultancy Pvt Ltd, Bengaluru, India) for his guidance on conducting the systematic literature search, preparing the study protocol, and providing editorial support toward the development of this article.
Additional information
Funding
References
- Diabetesatlas.org [Internet]. Brussels (Belgium): International Diabetes Federation. IDF Diabetes Atlas; 10th 2021 [cited 2023 Aug 21]. Available from: https://diabetesatlas.org/
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414
- Basu S, Yudkin JS, Kehlenbrink S, et al. Estimation of global insulin use for type 2 diabetes, 2018-30: a microsimulation analysis. Lancet Diabetes Endocrinol. 2019;7(1):25–33. doi: 10.1016/S2213-8587(18)30303-6
- Gregory GA, Robinson TIG, Linklater SE, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10(10):741–760. doi: 10.1016/S2213-8587(22)00218-2
- Yang L-J, Wu T-W, Tang C-H, et al. Efficacy and immunogenicity of insulin biosimilar compared to their reference products: a systematic review and meta-analysis. BMC Endocr Disord. 2022;22(1):35. doi: 10.1186/s12902-022-00944-5
- Wong EY, Kroon L. Ultra-rapid-acting insulins: how fast is really needed? Clin. Clin Diabetes. 2021;39(4):415–423. doi: 10.2337/cd20-0119
- Nicolucci A, Ceriello A, Di Bartolo P, et al. Rapid-acting insulin analogues versus regular human insulin: a meta-analysis of effects on glycemic control in patients with diabetes. Diabetes Ther. 2020;11:573–584. doi: 10.1007/s13300-019-00732-w
- Guney Z. Insulin and its analogues–what are they for? (pros and cons). Trends Diabetes Metab. 2019;2(2). doi: 10.15761/TDM.1000113
- Homko C, Deluzio A, Jimenez C, et al. Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care. 2003;26(7):2027–2031. doi: 10.2337/diacare.26.7.2027
- Novo Nordisk. Fiasp [package insert]. Plainsboro (NJ): Novo Nordisk; 2019 [cited 2024 May 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208751s010s011lbl.pdf
- Eli Lilly and Company. Lyumjev. Package insert. (IN)polis: IN, Eli Lilly and Company; 2020 [cited 2024 May 21]. Available from: https://pi.lilly.com/us/lyumjev-uspi.pdf
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA–P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928
- Tamborlane WV, Renard E, Wadwa RP, et al. Glycemic control after 6 days of insulin pump reservoir use in type 1 diabetes: results of double-blind and open-label cross-over trials of insulin lispro and insulin aspart. J Diabetes. 2015;7(2):270–278. doi: 10.1111/1753-0407.12162
- Bartolo PD, Pellicano F, Scaramuzza A, et al. Better postprandial glucose stability during continuous subcutaneous infusion with insulin aspart compared with insulin lispro in patients with type 1 diabetes. Diabetes Technol Ther. 2008;10(6):495–498. doi: 10.1089/dia.2008.0013
- Weinzimer SA, Ternand C, Howard C, et al. A randomized trial comparing continuous subcutaneous insulin infusion of insulin aspart versus insulin lispro in children and adolescents with type 1 diabetes. Diabetes Care. 2008;31(2):210–215. doi: 10.2337/dc07-1378
- Bode B, Weinstein R, Bell D, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care. 2002;25(3):439–444. doi: 10.2337/diacare.25.3.439
- Hedman CA, Lindström T, Arnqvist HJ. Direct comparison of insulin lispro and aspart shows small differences in plasma insulin profiles after subcutaneous injection in type 1 diabetes. Diabetes Care. 2001;24(6):1120–1121. doi: 10.2337/diacare.24.6.1120
- Liu BL, Yin GP, Li FF, et al. Comparison of efficacy and safety of lispro and aspart evaluated by continuous glucose monitoring system in patients with newly diagnosed type 2 diabetes. Int J Endocrinol. 2018;2018:2087960. doi: 10.1155/2018/2087960
- Thrasher J, Bhargava A, Rees TM, et al. Insulin lispro with continuous subcutaneous insulin infusion is safe and effective in patients with type 2 diabetes: a randomized crossover trial of insulin lispro versus insulin aspart. Endocr Pract. 2015;21(3):247–257. doi: 10.4158/EP14242.OR
- Plank J, Wutte A, Brunner G, et al. A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care. 2002;25(11):2053–2057. doi: 10.2337/diacare.25.11.2053
- Wang T, Malone J, Fu H, et al. Crossover design and its application in late-phase diabetes studies. J Diabetes. 2016;8(5):610–618. doi: 10.1111/1753-0407.12412
- von Mach MA, Brinkmann C, Hansen T, et al. Differences in pharmacokinetics and pharmacodynamics of insulin lispro and aspart in healthy volunteers. Exp Clin Endocrinol Diabetes. 2002;110(8):416–419. doi: 10.1055/s-2002-36428
- Lak V, Svensson AM, Miftaraj M, et al. Clinical effects and safety of direct-acting insulin analogs in patients with type 1 diabetes: a nation-wide observational cohort study. Diabetes Ther. 2016;7(3):561–573. doi: 10.1007/s13300-016-0191-x
- Svensson AM, Miftaraj M, Franzén S, et al. Clinical effects, cardiovascular and renal outcomes associated with rapid-acting insulin analogs among individuals with type 2 diabetes: a nation-wide observational cohort study. Clin Diabetes Endocrinol. 2017;3(1):5. doi: 10.1186/s40842-017-0043-2
- Racsa PN, Meah Y, Ellis JJ, et al. Comparative effectiveness of rapid-acting insulins in adults with diabetes. J Manag Care Spec Pharm. 2017;23(3):291–298. doi: 10.18553/jmcp.2017.23.3.291
- Pharmacist.therapeuticresearch.com [Internet]. Delaware: therapeutic research center. How to switch insulin products. 2023 [cited 2023 Oct 9]. Available from: https://pharmacist.therapeuticresearch.com/Content/Segments/PRL/2016/Dec/How-to-Switch-Insulin-Products-10473
- Medpagetoday.com [Internet]. MedPage Today. Basen T. What to consider when changing rapid acting insulin products: key differences between the three major types. (NY) (NY); 2019 [updated 2019 Jun 20] [cited 2023 Oct 5]. Available from: https://www.medpagetoday.com/reading-room/endocrine-society/diabetes/80254
- Novo-pi.com [Internet]. Plainsboro: Novo Nordisk. Insulin Aspart PI; 2023 [updated 2023 Feb]; cited 2023 Oct 5]. Available from: https://www.novo-pi.com/insulinaspart.pdf
- Pi.lilly.com [Internet]. (IN)polis (IN): Eli Lilly and Company. Insulin Lispro USPI; 2023 [updated 2023 Sep] cited 2023 Oct 5]. Available from: https://pi.lilly.com/insulin-lispro-uspi.pdf
- Goodrx.com [Internet]. McQueen H, Li D. How much does insulin cost? Here is how 32 brands and generics compare. 2023 [webpage updated 2023 Oct 11; cited 2024 Jan 12]. Available from: https://www.goodrx.com/healthcare-access/research/how-much-does-insulin-cost-compare-brands
- Formulary.nhssomerset.nhs.uk [Internet]. Chapter 6.4.2 – Related guidance: A guide to insulin treated diabetes and driving (DVLA). [cited 2024 Jan 12]. Available from: https://formulary.nhssomerset.nhs.uk/?page_id=767