Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.1.0 Introduction
Cervical cancer continues to be a significant cause of morbidity and mortality among women worldwide despite global efforts to increase access to care for both prevention and therapy. Cervical cancer is the fourth most common cancer in women globally and the death rate has decreased by less than 1% in the last two decades [Citation1]. Research into the biology of solid tumors and their microenvironment during recent years has led to investigation of novel therapies. The resulting clinical trials have yielded approval of several classes of targeted therapies for solid tumors including antiangiogenic agents, immune checkpoint inhibitors (ICI) and antibody-drug conjugates (ADC). These groundbreaking therapeutic agents have shifted the existing treatment paradigms for cervical cancer, particularly for those patients diagnosed with recurrent or metastatic cervical cancer (rmCC). This paper will focus on current and evolving treatment standards as well as the significance of a more novel therapy, tisotumab vedotin (TV), an ADC comprised of an anti-tissue factor antibody and a microtubule inhibitor.
2.0 Current treatment standards in recurrent and advanced metastatic cervical cancer
The pharmacologic standard of care (SOC) for rmCC was platinum-based chemotherapy until 2014, when the US Food and Drug Administration (FDA) approved the addition of bevacizumab, an antiangiogenic agent targeting vascular endothelial growth factor (VEGF), to SOC cytotoxic chemotherapy for patients with persistent or rmCC. This approval was based on results from the phase III randomized, controlled trial (RCT), Gynecologic Oncology Group (GOG)-240 (NCT 00803062) showing benefit in both progression free survival (PFS) and overall survival (OS) (8.2 vs 5.9 months; hazard ratio (HR) = 0.67; 95% confidence interval (CI) 0.54-0.82; p= 0.0002 and 16.8 vs 13.3 months; HR 0.77 95% CI 0.62-0.95; p=0.007, respectively) [Citation2]. Based on these findings and the growing understanding of the role of the immune system in solid tumors, other novel therapies were investigated. Pembrolizumab, an ICI with action against programmed cell death protein 1 (PD-1), was investigated in the phase II clinical trial, Keynote-158 (NCT 02628067). The study assessed the safety and efficacy of pembrolizumab monotherapy in the second line (2L) management of solid tumors. The clinically meaningful and durable survival benefits observed in this study allowed for accelerated FDA approval [Citation3]. The phase III, confirmatory RCT, Keynote-826 (NCT 03635567), investigated the addition of pembrolizumab to platinum-based chemotherapy with or without bevacizumab in first line (1L) management of rmCC. Survival benefit was seen in a subset of patients that received pembrolizumab and had a programmed death ligand-1 (PD-L1) combined positive score (CPS) of ≥1. Data showed a statistically significant difference in PFS (10.4 versus 8.2 months; HR= 0.62; 95% CI= 0.5-0.77; p=<0.001) and OS (53.0% versus 41.7%; HR= 0.64; 95% CI= 0.5-0.81; p=<0.001) [Citation4]. This regimen was approved by the FDA on October 13, 2021, for patients with persistent or rmCC with a PD-L1 CPS ≥1 and has since become the SOC [Citation5]. The role of pembrolizumab in earlier disease has also been investigated. Keynote-A18 (NCT 04221945), a phase III RCT of concurrent chemoradiotherapy (CRT) with or without pembrolizumab in patients with high-risk, locally advanced cervical cancer (LACC) showed an improvement in PFS with the addition of pembrolizumab compared to CRT alone (67.8% versus 57.3% at 24 months (HR= 0.70; 95% CI 0.55-0.89). This resulted in accelerated FDA approval on January 12, 2024, demonstrating pembrolizumab’s potential to shift the landscape of treatment for these patients as well [Citation6].
2.1 Cemiplimab as an acceptable option for 2L management
FDA approval of pembrolizumab in the 1L and 2L management of rmCC met a significant clinical need for patients with PD-L1 positive tumors. Under concurrent investigation was the PD-1 inhibitor, cemiplimab, in the EMPOWER trial (NCT 03257267) which showed survival benefit for patients receiving cemiplimab monotherapy in 2L compared to chemotherapy (OS 12.0 versus 8.5 months; HR, 0.69; 95% CI, 0.56-0.84; two-sided P<0.001) [Citation7]. Based on these results, cemiplimab has been included as an acceptable option for 2L management according to National Comprehensive Cancer Network (NCCN) guidelines [Citation8].
2.2 Accelerated FDA approval of tisotumab vedotin
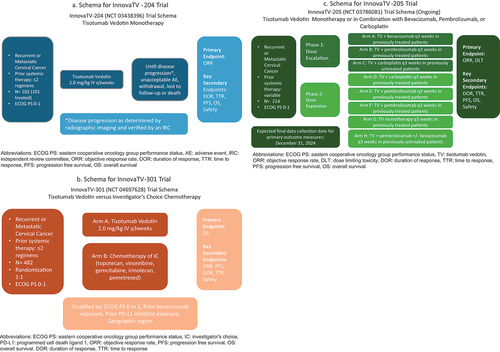
InnovaTV-204 (NCT 03438396) (), the phase II trial investigating the ADC, tisotumab vedotin (TV), in pre-treated rmCC patients, showed a clinically meaningful antitumor response (objective response rate (ORR) 24%; 95% CI= 15.9%-33.3%, p=0.0002) [Citation9]. These findings led to the accelerated FDA approval of TV for pretreated patients with rmCC on September 20, 2021 [Citation10]. The ongoing confirmatory trial, InnovaTV-301 (NCT 04697628), will be discussed in a subsequent section of this paper. While FDA approval of pembrolizumab in 2L is restricted to patients with PD-L1 positive tumors, the accelerated FDA approval of TV has no such biomarker restrictions and therefore offers a 2L option for those patients with PD-L1 negative tumors or those with progression despite prior ICI in the 1L.
3.0 Treatment related adverse events of TV
TV is a microtubule inhibitor, similar to the cytotoxic chemotherapeutic agent, paclitaxel. Despite sharing the same intracellular target, studies investigating paclitaxel and those investigating TV have highlighted differing toxicities. As a microtubule stabilizer, paclitaxel is strongly associated with peripheral neuropathy, affecting up to 90% of treated patients to varying degrees across studies [Citation11]. Conversely, a lower overall incidence has been reported in TV clinical trials affecting 42% of participants [Citation9]. Additionally, the profound dose-limiting neutropenia affecting patients treated with paclitaxel (≥grade 3, 52%) has not been observed in studies investigating TV (≥grade 3, 3%). Furthermore, while alopecia is frequently observed in clinical trials investigating paclitaxel (87%), its occurrence is notably less common in studies assessing TV (38%) [Citation9], [Citation11].
3.1 Unique toxicology profile of TV
A unique toxicity of bleeding events has been reported with TV and is inherent to one of its cellular targets, tissue factor (TF). TF, also known as thromboplastin, is a protein found on the surface of cells, most notably those within blood vessels but also can be overexpressed in certain cancers. This overexpression allows for TV to directly bind these cancer cells. However, the interaction between TV and TF can also affect normal blood vessels. Blood exposure of TF is the initial step in the extrinsic pathway of the clotting cascade in response to vascular endothelial injury and, as such, is vital in hemostasis efforts. By targeting TF, TV may interfere with this process, potentially leading to a disruption in the body’s ability to form blood clots effectively. Bleeding events were therefore a prespecified adverse event in the innovaTV-204 investigation of TV and occurred in 39% of patients in the experimental arm; most were grade 1 and 2 treatment-related adverse events (TRAE). These bleeding events did not have a substantial impact on the clinical management of these patients and the most common events included epistaxis (30%), vaginal hemorrhage (7%) and hematuria (3%) [Citation9].
3.2 Mitigation strategy for ocular toxicity
Another unique toxicity reported with TV use is ocular toxicity that was observed even during the phase I/II clinical trials. Given these findings, there have been management strategies implemented to mitigate these effects. The most frequent ocular TRAE reported were conjunctivitis (26%), dry eye (23%) and Keratitis (11%). Most events were mild to moderate, reported as grade 1 or 2 [Citation9], [Citation12]. The manufacturer released an eye care checklist involving resources and materials for providers and patients undergoing treatment with TV. This plan includes access to an eye care provider, premedication with eye drops, and cold packs for use during infusion. Dose modifications, when necessary, may also mitigate these TRAE [Citation12]. Patient education is important regarding the potential occurrence of these TRAE, signs and symptoms to look for while undergoing therapy, and management strategies. A detailed discussion with patients is a vital component of shared decision making and should occur prior to initiating therapy with TV.
4.0 Expert opinion
4.1 Role of tissue factor as a prognostic biomarker in cervical cancer
TF as discussed above is one of the cellular targets of TV and is also related to hemostasis given its expression on endothelial cells. In recent years, TF overexpression has also been noted on malignant tumor cells and is thought to play a role in solid tumor angiogenesis and metastasis. In some cervical tumors, upregulation of TF expression has been noted to be directly proportionate to increasing clinical stage and TF is therefore theorized to be a potential biomarker for cervical cancer [Citation13]. The FDA’s accelerated approval of TV, as a result of findings from the InnovaTV-204 trial, is noteworthy for its biomarker-unrestricted approach and differs from pembrolizumab’s approval, which was limited to patients positive for the PD-L1 biomarker. InnovaTV-204 showcased TV’s anti-tumor activity in patients with rmCC, irrespective of TF status. Although TF positivity was not a prerequisite for TV approval, the observed upregulation of TF in some cervical tumors suggests its potential as a biomarker for treatment response in those expressing the protein.
4.2 Future directions for TV therapy
The landscape for initial therapy of women with International Federation of Gynecology and Obstetrics (FIGO) stage III/IVA may be shifting to pembrolizumab with concurrent CRT based on the preliminary data from the Keynote-A18 study and resulting accelerated FDA approval. If this treatment regimen were to be adopted as SOC, then patients with prior ICI exposure that subsequently develop recurrent disease will be ineligible for the current standard 1L regimen proposed by Keynote-826 (platinum-based chemotherapy plus pembrolizumab with or without bevacizumab) [Citation4]. The ongoing phase III RCT, innovaTV-301 (NCT 04697628) () is the confirmatory trial for innovaTV-204 and is assessing the efficacy and tolerability of TV in pre-treated rmCC patients [Citation14]. If the antitumor activity and tolerability mirror the outcomes observed in InnovaTV-204, the prospect of full FDA approval for TV in 2L management of rmCC is encouraging. Additionally, the phase Ib/II trial, InnovaTV-205 (NCT 03786081) (), assessed 1L TV in combination with either bevacizumab, carboplatin or pembrolizumab and showed survival benefit with tolerable side effect profiles for these combination therapies [Citation15]. Therefore, TV could potentially emerge as a 1L option for rmCC patients as well. Subject to full approval, TV presents a prospective therapeutic avenue for patients experiencing recurrence after receiving initial ICI treatment, as per the proposed Keynote-A18 regimen [Citation6] ().
Table 1: Progression of Pharmacologic Management for Recurrent and Metastatic Cervical Cancer
List of abbreviations
| 1L | = | first line |
| 2L | = | second line |
| 3L | = | third line |
| ADC | = | Antibody Drug Conjugate |
| AE | = | adverse event |
| CI | = | confidence interval |
| CPS | = | combined positive score |
| CRT | = | chemoradiotherapy |
| DLT | = | dose limiting toxicity |
| DOR | = | duration of response |
| ECOG PS | = | Eastern cooperative oncology group performance status |
| FDA | = | Food and Drug Administration |
| FIGO | = | international federation of gynecology and obstetrics |
| GOG | = | gynecologic oncology group |
| HR | = | Hazard ratio |
| IRC | = | independent review committee |
| ORR | = | Objective response rate |
| OS | = | Overall survival |
| PD-1 | = | Programmed cell death protein 1 |
| PD-L1 | = | Programmed cell death ligand 1 |
| PFS | = | Progression Free Survival |
| RCT | = | Randomized controlled trial |
| rmCC | = | Recurrent/metastatic cervical cancer |
| SOC | = | Standard of care |
| TF | = | Tissue factor |
| TRAE | = | treatment-related adverse event |
| TTR | = | time to response |
| TV | = | Tisotumab Vedotin |
| VEGF | = | Vascular endothelial growth factor |
Declaration of interest
K Tewari has acted as a consultant for Merck, Pfizer, Genmab and Seagen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
REFERENCES
- Group, U.S.C.S.W. U.S. Cancer Statistics Data Visualizations Tool, based on 2022 submission data. (2023); Date accessed: 02/01/2024. Available from: https://www.cdc.gov/cancer/dataviz
- Tewari K, Sill M, Long H, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 390(10103), 1654–63, (2017).
- Marabelle A, Le D, Ascierto P, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 38(1), 1–10, (2020).
- Monk B, Colombo N, Tewari K, et al., First-Line Pembrolizumab + Chemotherapy Versus Placebo + Chemotherapy for Persistent, Recurrent, or Metastatic Cervical Cancer: Final Overall Survival Results of KEYNOTE-826. Journal of Clinical Oncology 41(36), 5505–11, (2023).
- Broderick, J. Pembrolizumab granted FDA approval for PD-L1+ Cervical Cancer. Date accessed 02/03/2024. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125514s034lbl.pdf
- Lorusso D, Xiang Y, Colombo N, et al. 254TiP ENGOT-cx11/KEYNOTE-A18: A phase III, randomized, double-blind study of pembrolizumab with chemoradiotherapy in patients with high-risk locally advanced cervical cancer. Annals of Oncology 31, S1341–42, (2020).
- Tewari K, Monk B, Vergote I, et al. EMPOWER-Cervical 1/GOG-3016/ENGOT-Cx9. N Engl J Med 386, 544–55, (2022).
- Abu-Rustum N, Yashar C, Arend R, et al. NCCN Guidelines(R) Insights: Cervical Cancer, Version 1.2024. Journal of National Comprehensive Cancer Network 21(12), 1224–33, (2023).
- Coleman L, Lorusso D, Gennigens C, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. The Lancet Oncology. 2021;22(5):609–619. doi: 10.1016/S1470-2045(21)00056-5
- Administration, F.D.A. FDA grants accelerated approval to tisotumab vedotin-tftv for recurrent or metastatic cervical cancer. FDA Approved Drugs (2021). accessed 02 04 2024. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tisotumab-vedotin-tftv-recurrent-or-metastatic-cervical-cancer.
- Pfizer. Paclitaxel Injection, USP Adverse Reactions. Pfizer medical information (2023). Date Accessed: 02/11/2024. Available from: https://www.pfizermedicalinformation.com/paclitaxel/adverse-reactions.
- Kim S, Ursell P, Coleman R, Monk B, Vergote I. Mitigation and management strategies for ocular events associated with tisotumab vedotin. Gynecologic Oncology. 2022;165(2):385–392. doi:10.1016/j.ygyno.2022.02.010
- Zhao X, Cheng C, Gou J, et al. Expression of tissue factor in human cervical carcinoma tissue. Exp Ther Med. 2018;16(5):4075–4081. doi: 10.3892/etm.2018.6723
- Bogani G, Coleman R, Vergote I, Raspagliesi F, Lorusso D, Monk B. Tisotumab vedotin in recurrent or metastatic cervical cancer. Current Problems in Cancer. 2023;47(3):100952. doi:10.1016/j.currproblcancer.2023.100952
- Vergote I, Nieuwenhuysen E, O’Cearbhaill R, et al. Tisotumab Vedotin in Combination With Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-cx8 Study. Journal of Clinical Oncology 41(36), 5536–49, (2023).