Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.1. Introduction
One of the most distinctive features of psoriatic arthritis (PsA) lies in is its extraordinary clinical variability, which translates into different manifestations, both cutaneous-ungual and musculoskeletal, which tend to overlap each other over time, giving this entity its own and exclusive phenotype within the rheumatological conditions. The Group for Research and Assessment of Psoriasis and PsA (GRAPPA) currently recognizes 6 domains of the disease and proposes a treatment algorithm for each of them. Among these domains there is one referring to nail disease, which gives an idea of the importance that this component of psoriatic disease may have in terms of its more comprehensive management [Citation1].
2. The nail in psoriatic disease
The nail unit is composed of 4 epithelial structures—the nail matrix, the nail bed, the hyponychium, and the proximal and lateral nail folds, which function to produce, attach, and protect the nail plate [Citation2]. In the field of psoriasis, nail disease is considered a difficult to treat location, also associated with a more extensive and severe skin disease, as well as a greater impact on quality of life. Furthermore, nail involvement has been considered a classic risk marker for arthritis among patients with psoriasis [Citation2].
2.1 Implications for PsA diagnosis
The nail is connected to the underlying distal phalanx bone through a complex mini-enthesis network that is fused with the extensor tendon crossing the distal interphalangeal (DIP) joint [Citation2]. In fact, finger extensor tendon enthesopathy at the DIP joint is a common finding in patients with psoriatic onychopathy [Citation2]. These intricate anatomical interactions between the DIP joint and the nail make up what is currently known as the synovio-entheseal complex (SEC) of the nail unit. For this reason, nail involvement has been considered a classic risk factor for arthritis development among psoriasis patients, being currently considered as a long-term risk factor for the joint condition [Citation2]. On average, patients with nail disease have an almost 3-fold higher risk of developing PsA than patients with psoriasis who do not have signs of nail dystrophy [Citation2].
2.2 Implications for PsA prognosis
Apart from the known connection to the risk of PsA, the effect that nail involvement has on the characteristics, burden and prognosis of PsA has been much less studied. In the Corrona Psoriatic Arthritis/Spondyloarthritis Registry (currently CorEvitas), patients with nail psoriasis had higher disease activity, higher tender and swollen joint counts, and increased likelihood of having enthesitis and dactylitis. Also, patients with nail psoriasis had worse pain, fatigue, and work and activity impairment than those without nail disease [Citation3]. In another study, the presence of nail lesions (HR 2.08), among other factors, increased the risk of PsA-related musculoskeletal surgery [Citation4]. Moreover, there is also a close connection between nail disease and structural damage. One study showed that any form of psoriatic nail dystrophy was associated with erosion at the DIP joints of the corresponding digit (OR 1.9) and this association was primarily driven by the presence of nail onycholysis (OR 1.72). Results from the same study showed that subungual hyperkeratosis was more strongly associated with joint space narrowing, erosions, and osteoproliferation at the corresponding DIP joint [Citation5]. Recently, nail involvement in PsA has also been proposed as an independent risk factor for carotid plaque development, thus increasing cardiovascular risk in this PsA subpopulation [Citation6]. Finally, in a novel prospective study carried out in patients with recent-onset PsA, a predictive model based on machine learning was able to demonstrate a clear association between the number of fingers with onychopathy and the risk of PsA flares over two years of follow-up [Citation7].
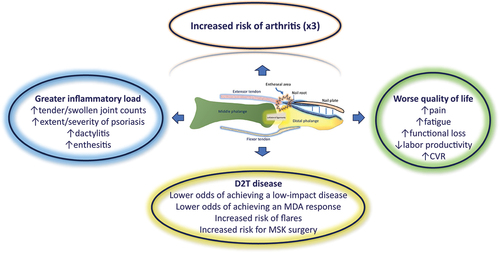
All these considerations, taken together, seem to indicate that the nail condition in psoriatic disease is not only relevant for the detection of PsA, but is also closely linked to a worse overall outcome (). Furthermore, the presence of nail lesions, especially the most severe and deforming ones, can be perceived as something embarrassing and stigmatizing by patients, thus leading to a greater risk of anxiety and depression [Citation2].
Figure 1. Disease burden associated with nail involvement in psoriatic disease. The synovio-entheseal complex (SEC) linked to the nail unit is a complex network of ligamentous, tendinous and capsular structures, woven around the nail and the distal interphalangeal joint. In addition to practically tripling the risk of suffering from arthritis in the long term, nail damage has a huge impact on the patient’s overall health. In this way, patients with involvement of the nail SEC present a disease with a greater inflammatory burden, poorer quality of life, greater need for musculoskeletal surgery, greater structural damage, and greater difficulty in terms of the therapeutic approach.
2.3 Implications for PsA therapy
That being said, how relevant is the nail unit when it comes to guiding treatment in PsA? In line with the aforementioned, nail disease can hinder the achievement of treatment objectives. Thus, patients with DIP joint involvement (most with onychopathy) are less likely to achieve a low disease impact status according to the PsA impact of disease (PsAID) questionnaire [Citation8]. Furthermore, nail involvement with DIP arthritis is one of the main barriers to achieving a minimal disease activity (MDA) response in subjects with PsA under systemic treatment [Citation9]. On the other hand, although the GRAPPA guidelines issue a strong recommendation for the use of any biological DMARD or PDE4 inhibitor in the treatment of nail psoriasis, there is no solid evidence on which drug could be superior to the others in the control of this disease domain [Citation1]. However, this evidence may be emerging now. In a recent network meta-analysis (NMA), the absolute probability of achieving complete resolution of nail psoriasis at weeks 24–28 was highest for ixekizumab at 46.4%, followed by brodalumab (37.1%), bimekizumab (30.3%), adalimumab (28.3%), guselkumab (27.7%), ustekinumab (20.8%), and infliximab (1.2%). The Bayesian NMA also showed that ixekizumab had the highest absolute probability to be ranked best (79%), followed by brodalumab (17%) and bimekizumab (4%). However, differences between biologic treatments were smaller and not statistically different in the long-term than in the short-term NMA, indicating that duration of treatment, rather than the neutralized cytokine pathway, is essential for treatment success [Citation10]. Other very recent studies, reached similar conclusions [Citation11]. The aforementioned works refer to potential differences between biological therapies for the treatment of the nail domain in patients with psoriasis, but not necessarily with PsA. Is there any similar information from studies focused on PsA? Actually, yes. A very recent post hoc analysis of the SPIRIT-H2H (NCT03151551) trial used a combined outcome measure to assess the proportion of finger units with simultaneous resolution of DIP joint involvement and resolution of adjacent nail psoriasis at different time points from week 12 to 52. Results showed that a greater resolution of DIP joint tenderness, swelling and adjacent nail psoriasis was achieved at all time points over 52 weeks through targeting IL-17A with ixekizumab than TNF-α with adalimumab [Citation12]. The difference between treatment arms was apparent by week 12 (38.8% vs 28.4%, p < 0.0001) and sustained out to week 52 (64.9% vs 57.5%, p < 0.0055). Thus, this finding reinforces the importance of the IL-17A pathway in nail and periungual immunobiology and also support the idea of a greater impact of IL-17A inhibition compared with TNF-α inhibition in the DIP-nail musculoskeletal appendage [Citation12,Citation13].
3. Expert opinion
The nail is a specialized appendicular epithelial structure with great relevance for understanding psoriatic disease pathogenesis. The idea of a close relationship between nail disease and DIP joint in PsA has been supported, above all, by both ultrasound and magnetic resonance imaging studies. This has led to the SEC theory to explain the extraordinary nail-DIP joint connection. For its part, nail disease triples the risk of arthritis among patients with psoriasis, which opens up the exciting possibility of preventing PsA by specifically addressing this psoriasis domain. This becomes especially relevant if we take into account that evidence is beginning to emerge that not all high-impact therapies address this component of the disease with the same effectiveness. However, although therapies that target IL17A seem to have a competitive advantage in addressing nail disease, it has not been possible to demonstrate that therapies directed against this pathway are superior to others in reducing the incidence of PsA. In fact, therapies against the IL23 pathway could have theoretical advantages in this latter regard [Citation14].
However, the nail-DIP appendage pathogenic relationship based on the SEC concept is not free of controversy. Some microanatomical and immunohistochemical studies show that DIP joint arthritis is not simply an extensor tendon enthesitis and that the nail unit remains a microanatomical structure independent of the extensor enthesis, while an inflammatory infiltrate is usually not found in the fascia connecting the nail with the extensor tendon [Citation15]. Because of this, it is possible to move away from a purely anatomical explanation of the strong association between nail psoriasis and PsA, and rather propose immunological factors based on the hypothesis that CD8+ T cells play a more crucial role in the pathogenesis of nail psoriasis through a pathogenic pathway similar to that of PsA and different from that of the skin (more dependent on CD4+ T cells). This, in turn, should lead us to treat the disease more comprehensively and not just guide treatment by specific domains, such as the nail.
Although PsA is characterized by its extraordinary phenotypic variability with multiple domains affected, its treatment should be aimed at addressing the disease as a whole, based on a common inflammatory pathogenesis for all disease manifestations (perhaps with subtle differences). Nail disease is of capital importance for the diagnosis and prognosis of PsA, although we still lack solid evidence to suggest that a differential management should be done for this specific component of the disease.
Clinicians should begin to view nail involvement, not only as a characteristic of psoriasis linked to a higher risk of PsA, but as a factor clearly associated with a worse natural history of the disease. Considering that evidence is beginning to emerge that not all high-impact therapies address this disease domain with the same effectiveness, we should act with sufficient speed and forcefulness to achieve a complete resolution of the inflammatory process affecting the nail complex. If the latter is achieved, we could be marking a positive turning point for the natural history of the disease.
Declarations of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Note: CVR: cardiovascular risk; D2T: difficult to treat; MDA: minimal disease activity; MSK: musculoskeletal.
Additional information
Funding
References
- Coates LC, Soriano ER, Corp N, et al. Group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–479. doi: 10.1038/s41584-022-00798-0
- Kaeley GS, Eder L, Aydin SZ, Rich P, Bakewell CJ. Nail Psoriasis: Diagnosis, Assessment, Treatment Options, and Unmet Clinical Needs. J Rheumatol 2021; 48: 1208–20.
- Mease PJ, Liu M, Rebello S, McLean RR, Dube B, Glynn M, Hur P, Ogdie A. Association of Nail Psoriasis with Disease Activity Measures and Impact in Psoriatic Arthritis: Data from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol 2021; 48: 520–26.
- Kwok TSH, Sutton M, Cook RJ, et al. Musculoskeletal surgery in psoriatic arthritis: prevalence and risk factors. J Rheumatol. 2023;50(4):497–503. doi: 10.3899/jrheum.220908
- Antony AS, Allard A, Rambojun A, et al. Psoriatic Nail Dystrophy is Associated with erosive disease in the distal interphalangeal joints in Psoriatic arthritis: a retrospective cohort study. J Rheumatol. 2019;46(9):1097–1102. doi: 10.3899/jrheum.180796
- Colunga-Pedraza IJ, Galarza-Delgado DA, Azpiri-Lopez JR, et al. Nail involvement in psoriatic arthritis patients is an independent risk factor for carotid plaque. Ann Rheum Dis. 2021;80(12):1629–1631. doi: 10.1136/annrheumdis-2021-220782
- Queiro-Silva R, Seoane-Mato D, Laiz A, et al. POS0311 FLARES in PATIENTS with RECENT-ONSET PSORIATIC ARTHRITIS. PREDICTIVE MODEL BASED on MACHINE LEARNING. Ann Rheum Dis. 2022;81(Suppl 1):405.2–6. doi: 10.1136/annrheumdis-2022-eular.1778
- Queiro R, Cañete JD, Montoro M, et al. MAAPS study group. Disease features associated with a low disease impact in patients with psoriatic arthritis: results of a cross-sectional multicenter study. Arthritis Res Ther. 2020;22(1):82. doi: 10.1186/s13075-020-02168-1
- Bakirci S, Solmaz D, Al Osaimi N, Dalkilic E, Can M, Erden A, et al. What are the main barriers to achieve minimal disease activity in psoriatic arthritis in real life? Clin Exp Rheumatol 2019; 37: 808–12.
- Egeberg A, Kristensen LE, Puig L, Rich P, Smith SD, Garrelts A, et al. Network meta-analyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24-28 and 48-52 weeks. J Dermatolog Treat 2023; 34: 2263108.
- Husein-ElAhmed H, Husein-ElAhmed S. Bayesian network meta-analysis of head-to-head trials for complete resolution of nail psoriasis. Clin Exp Dermatol. 2023;48(8):895–902. doi: 10.1093/ced/llad136
- McGonagle D, Kavanaugh A, McInnes IB, Kristensen LE, Merola JF, Strober B, et al. Association of the clinical components in the distal interphalangeal joint synovio-entheseal complex and subsequent response to ixekizumab or adalimumab in psoriatic arthritis. Rheumatology (Oxford) 2024: keae060. doi: 10.1093/rheumatology/keae060.
- Pinto-Tasende JA, Queiro-Silva R Psoriatic nail complex: thick as thieves. Rheumatology (Oxford) 2024 May 6: keae249. doi: 10.1093/rheumatology/keae249.
- Loredo M, Braña I, Queiro R. Does pharmacological intervention prevent or delay the onset of psoriatic arthritis among psoriasis patients? Expert Opin Biol Ther. 2023;23(12):1159–1162. doi: 10.1080/14712598.2023.2273269
- Perrin C. Are there signs of enthesitis in nail psoriasis? An immunohistological study of nail psoriasis with and without psoriatic arthritis. Am J Dermatopathol. 2023;45(1):40–46. doi: 10.1097/DAD.0000000000002328
