1. Introduction
Atherothrombosis of the coronary, cerebral, or peripheral arteries may lead to acute coronary syndromes (ACS), stroke/transient ischemic attack (TIA) and acute limb ischemia (ALI) respectively, contributing to premature mortality and chronic morbidity. The central pathological process in these conditions is platelet activation, usually precipitated by rupture/erosion of an atherosclerotic plaque, with concurrent contact activation of coagulation and release of prothrombotic factors [Citation1].
Whilst platelet activation during thrombosis involves multiple pathways, the platelet P2Y12 receptor (‘P2Y12’), activated by adenosine diphosphate (ADP) that is released from platelets in dense granules, plays a central role in amplifying the response to a range of agonists [Citation1]. P2Y12 therefore represents a powerful therapeutic target in arterial thrombosis. Several P2Y12 antagonists have now been developed. Orally-administered agents include the thienopyridines clopidogrel and prasugrel that irreversibly inhibit P2Y12, and the cyclo-pentyl triazolopyrimidine ticagrelor, which reversibly binds to P2Y12 [Citation2]. A parenterally administered P2Y12 antagonist, cangrelor, is also available. Cangrelor is reversibly binding with rapid onset and offset, but requires intravenous access and a maintenance infusion after an initial bolus reducing its practicability, as well as significant cost implications [Citation3].
The effectiveness of P2Y12 antagonists in preventing cardiovascular events in patients with atherothrombosis is well established. Large randomized clinical trials (RCTs) have shown benefits of P2Y12 antagonists added to aspirin in ACS [Citation2] or substituted for it in cerebrovascular disease and peripheral arterial disease (PAD) [Citation4]. Furthermore, in ACS, particularly when managed by percutaneous coronary intervention (PCI), the more potent oral P2Y12 antagonist ticagrelor, in all ACS, and prasugrel, in the setting of ACS managed by PCI, offer clinical benefits over clopidogrel [Citation2]. Similarly, intravenous cangrelor reduces ischemic complications of PCI when compared to oral clopidogrel without increasing severe bleeding [Citation3].
2. Development and pharmacology of selatogrel
Selatogrel (4‐((R)‐2-|[6-((S)‐3-methoxypyrrolidin-1-yl)-2-phenylpyrimidine-4-carbonyl]amino|-3-phosphonopropionyl)piperazine-1-carboxylic acid butyl ester), known earlier in development as ACT-246475, is a novel P2Y12 antagonist for subcutaneous (SC) administration. A selective, potent and reversibly binding 2-phenyl-pyrimidine-4-carboxamide analog, it shares a pyrimidine core with ticagrelor and other aspects of its structure with a family of molecules previously investigated as reversible P2Y12 antagonists (BX 048, BX 667; Berlex Biosciences) () [Citation5].
Figure 1. (a) Molecular structure of the novel reversibly binding P2Y12 antagonist selatogrel, shown for comparison alongside the oral P2Y12 antagonist ticagrelor. (b) Illustrative figure demonstrating the temporal effect profile of a single subcutaneous dose of selatogrel on platelet reactivity, compared with an oral loading dose of ticagrelor with (solid line) and without (dotted line) concurrent morphine exposure. ADP, adenosine diphosphate; SC, subcutaneous; PO, per orum.
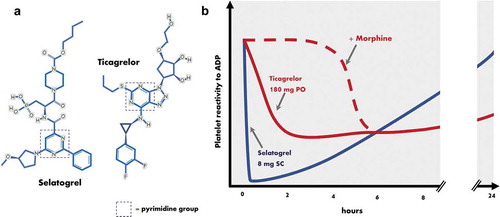
In vitro studies characterized selatogrel’s basic pharmacology: using Chinese hamster ovary cells expressing P2Y12, the concentration required for 50% inhibition was 4.8 nmol/L, and 31 nmol/L using light transmittance aggregometry of human platelet-rich plasma with 3 μmol/L ADP as an agonist [Citation5]. It was determined that the drug was not metabolized by cytochrome P450 enzymes but was a substrate for the hepatic uptake transporters organic-anion-transporting polypeptide 1B1 and 1B3 [Citation6]. In animal studies, the drug led to significantly less bleeding whilst maintaining a significant antithrombotic effect compared with clopidogrel or ticagrelor in an experimental rat model of arterial injury. The investigators concluded this suggested a wider therapeutic window than these two existing agents, although no human studies have been powered to assess this [Citation7, Citation8].
Following preclinical studies, several human phase I and II trials of selatogrel have now been performed (). In summary, a standard oral formulation of ACT-281959, a pro-drug of selatogrel, failed to achieve a satisfactory reduction in platelet reactivity (PR), even at high doses. An aqueous-organic solution of ACT-281959 achieved a better response but had low palatability limiting dose escalation [Citation9]. Subsequently, an SC-administered preparation of selatogrel was tested in healthy volunteers and its pharmacodynamics (PD) and pharmacokinetics (PK) determined [Citation6, Citation10]. A dose-dependent potent effect on PR with rapid time to peak effect (30 min to 1 h) and offset (significant by 8 h, almost total by 24 h) was demonstrated, with good tolerance and safety. No plasma major metabolites were identified and elimination was largely fecal with a small urinary component. The investigators concluded that inhibitors or inducers of drug-metabolizing enzymes are unlikely to affect selatogrel’s plasma profile, but could not rule out drug–drug interaction with agents affecting the hepatic uptake transporters organic-anion-transporting polypeptide (OATP) 1B1 and 1B3 as well the efflux transporter multidrug resistance-associated protein 2 (MRP2), of which selatogrel is a substrate. This includes drugs such as cyclosporine, eltrombopag, lapatinib, lopinavir, rifampicin and ritonavir, and patients receiving these were excluded from clinical studies.
Table 1. Human studies reported to date of the pharmacodynamics, pharmacokinetics, and safety of selatogrel.
Two phase 2 studies of SC selatogrel have now been reported. In the largest, a total of 345 patients were randomized in a 1:1:1 ratio to receive an SC injection of either selatogrel 8 mg, 16 mg, or placebo [Citation11]. Platelet function tests were performed before injection and at serial timepoints up to 24 h after. Both doses of selatogrel rapidly and powerfully inhibited ADP-induced platelet activation by 30 min and with significant inhibition for at ≥8 h, reversing by 24 h. Whether the injection site was the thigh or abdomen made no difference to the profile of effect. Significantly, in those patients already receiving maintenance therapy with an oral P2Y12 antagonist, including clopidogrel or ticagrelor, selatogrel provided an additive effect. No severe or serious adverse events occurred. There was, however, an increased incidence of mild or moderate dyspnea in those receiving selatogrel when compared to placebo, not seen in phase I studies. There was some evidence of this being a dose-dependent effect: 5% reported dyspnea after 8 mg and 9% after 16 mg, compared to no reports after placebo. Certain P2Y12 antagonists (ticagrelor and cangrelor) are associated with an increased rate of dyspnea, sharing with selatogrel the property of reversible binding, suggested as a factor in this nonpathogenic phenomenon [Citation3]. No other adverse effects appeared more common following drug rather than placebo, with the possible exception to dizziness, which occurred in 4% and 3% receiving 8 mg and 16 mg respectively, and 1% receiving placebo, and injection site bruising (3% vs. 2% vs. 0%). No significant bleeding occurred in any participant and there were no changes in hemodynamic or electrocardiographic parameters.
A study of 47 patients given selatogrel 8 or 16 mg SC in the early phase of ACS confirmed similar PD/PK findings [Citation12].
3. Expert opinion: potential clinical roles for selatogrel
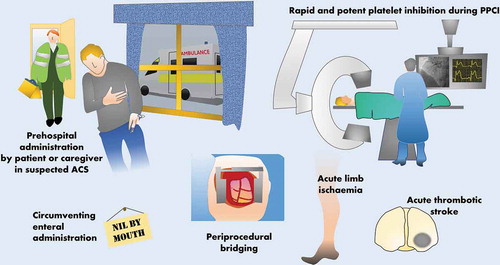
Selatogrel is currently neither commercially available nor licensed for clinical use. Whilst only phase 2 trials have been conducted at present, the attractive prospect of a rapid-onset parenterally administered potent and reversible P2Y12 antagonist raises the possibility of its use in a number of specific situations, especially, but not limited to, ACS (). Outcome-driven phase 3 data would, of course, be needed to make definitive recommendations but potential future roles can be speculated upon.
Figure 2. Potential clinical roles for selatogrel, a novel subcutaneous P2Y12 antagonist. ACS, acute coronary syndrome; PPCI, primary percutaneous coronary intervention.
3.1. Early P2Y12 inhibition in ACS
Understanding the natural history of an ACS event can help to identify when P2Y12 inhibition might be beneficial. Coronary thrombosis with resulting distal ischemia ± infarction is a dynamic process, with thrombus being formed and broken down, the net effect determining the extent of vessel occlusion, a process that can take hours or even days to reach its peak [Citation1]. Even in patients on preexisting oral antiplatelet therapy, factors such as increased platelet turnover, compliance, or reduced absorption may lead to reduced effect, and even small numbers of uninhibited platelets may initiate thrombosis. Whilst in high-risk groups there is evidence that long-term maintenance therapy with P2Y12 antagonists can help to prevent thrombotic events [Citation2], this comes at the expense of bleeding risk, limiting the attractiveness of this strategy in the wider population.
Hypothetically, delivering potent P2Y12 inhibition as early as possible during an ACS event may have clinical benefits, likely derived from a reduction in the impact of thrombosis on vessel occlusion and the most dangerous sequelae such as ST-elevation myocardial infarction (STEMI). However, early pre-hospital treatment with oral P2Y12 antagonists, for example, to those with STEMI, has proved disappointing when compared to in-hospital dosing. One factor potentially reducing the benefits of pre-treatment is that absorption of oral P2Y12 antagonists in the proximal small intestine is significantly delayed by the presence of opioid drugs, commonly administered in ACS [Citation13]. SC-administered selatogrel, with its rapid onset even during ACS, therefore represents an additional option to be investigated with the possibility of preventing the early thrombotic propagation and hence reducing hemodynamic impact within a coronary artery. The route of administration and the possibility of a pre-filled syringe are likely to be practical for pre-hospital administration by caregivers. Similarly, it is feasible that patients/carers could be trained to give selatogrel at the onset of a suspected ACS event, minimizing delay, perhaps akin to the use of pre-filled adrenaline pen by patients at high risk of anaphylaxis. Clearly, this strategy would require robust investigation in the target population before it could be recommended.
3.2. Use during primary percutaneous coronary intervention
Similarly, whether oral P2Y12 antagonists should be given before or after primary PCI (PPCI) for STEMI remains debated. Providing cover with a parenteral antiplatelet agent, such as a glycoprotein IIb/IIIa inhibitor, may reduce the risk of acute ischemic complications in those treated with opiates [Citation13]. Use of selatogrel to achieve rapid P2Y12 inhibition during PPCI, circumventing reduced enteral absorption related to morphine or other factors, might hypothetically reduce the need for additional parenteral antiplatelet agents, e.g. glycoprotein IIb/IIIa inhibitors, with the attendant risk of increased bleeding.
3.3. Peri-procedural bridging of P2Y12 inhibition
The risk of bleeding during major operative procedures in patients receiving oral P2Y12 antagonists is regarded as unacceptably high; hence, these are normally withheld in all but the most emergent cases. This includes patients who are admitted to hospital with ACS and require urgent coronary artery bypass grafting (CABG) shortly after diagnosis when their thrombotic risk remains high. The timing of discontinuation prior to procedures such as CABG may depend on the agent in question and is sometimes guided by platelet function testing [Citation14]. The effect of ticagrelor, for example, begins to reduce from 1 day after discontinuation but it may take at least 72 h for most patients to reach a level of PR deemed safe for surgery, longer if receiving clopidogrel or prasugrel [Citation14]. This may lead to a period of vulnerability to further thrombosis, which is prolonged further in cases of inevitable delay. Speculatively, given the rapid onset and offset in effect of SC selatogrel, it may be possible to provide adequate P2Y12 inhibition closer to the point of surgery than with oral drugs, similar to anticoagulation with SC low-molecular-weight heparin to cover a period of vitamin K antagonist discontinuation. Based on phase II data, it seems likely that P2Y12-mediated PR would fully recover in most patients by 24 h after the last dose [Citation15], thus allowing safe surgery sooner after discontinuation. Although the 8 and 16 mg doses tested in phase II showed relatively short periods of therapeutic effect (around 8 h) meaning multiple injections per day may be needed, data from phase I trials suggests that using a higher dose, e.g. 32 mg might prolong effect to around 12 h per dose whilst still returning to near baseline by 24 h after discontinuation, hence twice daily administration might be sufficient. Given kinetics may complicate multiple dosing regimens, prospective studies in this patient group would be needed to determine the appropriate doses and intervals, but the emergence of selatogrel is a promising development in this area.
3.4. Use in patients with prolonged poor enteral absorption
As well as circumventing poor absorption of oral P2Y12 antagonists in morphine-treated ACS patients, selatogrel may potentially find another theoretical niche in patients with longer periods of reduced enteral absorption. Whilst many patients who are ‘nil by mouth,’ e.g. because of swallowing difficulties or reduced consciousness can receive oral P2Y12 antagonists via a nasogastric tube or as an orodispersible preparation of ticagrelor, some individuals present challenges when enteral absorption itself is disrupted, most notably those with prolonged periods of critical illness, but also those with chronic conditions such as short bowel syndrome. For specialist use, selatogrel may offer a feasible alternative in those rare cases where a reliable regimen of maintenance therapy with an enterally active P2Y12 antagonist cannot be instigated.
3.5. Use in other thrombotic conditions
As well as ACS, dual antiplatelet therapy with aspirin and a P2Y12 antagonist may be of benefit in acute thrombotic stroke or high-risk TIA. For example, recent studies have suggested a reduction in ischemic complications from an acute stroke in those receiving aspirin and clopidogrel vs. aspirin alone, particularly early after the event [Citation16]. Similarly, although a recent study of ticagrelor monotherapy vs. aspirin failed to show a statistically significant difference in ischemic events after acute stroke or high-risk TIA, a posthoc analysis suggested those exposed to both drugs in the peri–infarct period may have improved outcomes compared to a single agent alone, a hypothesis now being tested prospectively in the THALES study (NCT03354429). If P2Y12 inhibition is beneficial in acute thrombotic stroke, it is plausible that delivering this as soon as possible after the onset of thrombosis may be optimal. The rapid onset of selatogrel, combined with delays in administering oral medication to patients with acute stroke due to concerns regarding safe swallowing, means the novel agent may be a good candidate for further investigation in this setting. Contrary to ACS, it would be difficult, however, to envisage how a patient or pre-hospital caregiver could administer selatogrel in this situation given the need first to exclude intracranial hemorrhage.
Similarly, in cases of ALI due to thrombosis, which may be treated by urgent angioplasty of the peripheral arteries, an oral P2Y12 antagonist, e.g. clopidogrel is frequently given. Limitations of oral P2Y12 antagonists detailed above are potentially relevant in this situation too; hence, selatogrel may have a role in improving the speed and reliability of antiplatelet therapy in acute manifestations of PAD.
3.6. Limitations of current evidence
Currently, there are no human data to support preclinical work suggesting that selatogrel may have a safer profile compared to other P2Y12 antagonists and larger clinical studies are required to assess the safety of selatogrel, including its effect on bleeding rates. Indeed, it is expected that the effects of selatogrel on hemostasis in humans will be consistent with its profile as a P2Y12 antagonist, as initially suggested by a numerical increase in injection-site bruising.
Cangrelor is known to block the binding of thienopyridine active metabolites to P2Y12 [Citation17] and further pharmacodynamic studies of selatogrel are required to investigate any potential negative interaction with clopidogrel and prasugrel in order to guide the switching from selatogrel to oral P2Y12 antagonists.
4. Conclusions
Selatogrel is a novel P2Y12 antagonist offering advantages of SC administration, rapid onset/offset and additive effect to maintenance oral therapy. An existing parenterally administered P2Y12 antagonist, cangrelor, is available. It similarly provides rapid, potent, and additive antiplatelet effect, and may improve clinical outcomes compared to oral agents in those undergoing PCI [Citation3], but requires intravenous access so is less suited to pre-hospital or repeated administration. Achievement of rapid and reliable P2Y12 inhibition in ACS, ALI, or acute thrombotic stroke is a logical goal and oral P2Y12 antagonists have limitations, as discussed. It should be emphasized that no phase 3 trials have yet been performed, but there is a clear rationale for investigating the use of selatogrel to improve clinical outcomes in patients with atherothrombotic conditions.
Declaration of interest
RF Storey reports institutional research grants/support from AstraZeneca, GlyCardial Diagnostics and Thromboserin; consultancy fees from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, GlyCardial Diagnostics, Haemonetics, Portola, and Thromboserin; and honoraria from AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, and Medscape. In addition, RF Storey was the Chief Investigator for the phase IIa study of selatogrel (unpaid). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Jackson SP. Arterial thrombosis–insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436.
- Parker WA, Storey RF. Long-term antiplatelet therapy following myocardial infarction: implications of PEGASUS-TIMI 54. Heart. 2016;102:783–789.
- Parker WA, Bhatt DL, Prats J, et al. Characteristics of dyspnoea and associated clinical outcomes in the CHAMPION PHOENIX study. Thromb Haemost. 2017;117:1093–1100.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329–1339.
- Caroff E, Meyer E, Treiber A, et al. Optimization of 2-phenyl-pyrimidine-4-carboxamides towards potent, orally bioavailable and selective P2Y(12) antagonists for inhibition of platelet aggregation. Bioorg Med Chem Lett. 2014;24:4323–4331.
- Ufer M, Huynh C, van Lier JJ, et al. Absorption, distribution, metabolism and excretion of the P2Y12 receptor antagonist selatogrel after subcutaneous administration in healthy subjects. Xenobiotica. 2020;50:427–434.
- Caroff E, Hubler F, Meyer E, et al. 4-((R)-2-{[6-((S)-3-methoxypyrrolidin-1-yl)-2-phenylpyrimidine-4-carbonyl]amino}- 3-phosphonopropionyl)piperazine-1-carboxylic acid butyl ester (ACT-246475) and its prodrug (ACT-281959), a novel P2Y12 receptor antagonist with a wider therapeutic window in the rat than clopidogrel. J Med Chem. 2015;58:9133–9153.
- Rey M, Kramberg M, Hess P, et al. The reversible P2Y12 antagonist ACT-246475 causes significantly less blood loss than ticagrelor at equivalent antithrombotic efficacy in rat. Pharmacol Res Perspect. 2017;5:e00338.
- Baldoni D, Bruderer S, Krause A, et al. A new reversible and potent P2Y12 receptor antagonist (ACT-246475): tolerability, pharmacokinetics, and pharmacodynamics in a first-in-man trial. Clin Drug Investig. 2014;34:807–818.
- Juif PE, Boehler M, Dobrow M, et al. Clinical pharmacology of the reversible and potent P2Y12 receptor antagonist ACT-246475 after single subcutaneous administration in healthy male subjects. J Clin Pharmacol. 2019;59:123–130.
- Storey RF, Gurbel PA, Ten Berg J, et al. Pharmacodynamics, pharmacokinetics, and safety of single-dose subcutaneous administration of selatogrel, a novel P2Y12 receptor antagonist, in patients with chronic coronary syndromes. Eur Heart J. 2019:ehz807. doi: 10.1093/eurheartj/ehz807.
- Sinnaeve PR, Fahrni G, Schelfaut D, et al. Inhibition of platelet aggregation after subcutaneous administration of a single-dose of selatogrel, a novel P2Y12 antagonist, in acute myocardial infarction: a randomised open-label phase 2 study. Eur Heart J. 2019;40(suppl 1):ehz746.0078.
- Zwart B, Yazdani M, Ow KW, et al. Use of glycoprotein IIb/IIIa antagonists to prevent stent thrombosis in morphine-treated patients with ST-elevation myocardial infarction. Platelets. 2020;31:174–178.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260.
- Storey RF, Gurbel PA, James S, et al. Selatogrel, a novel P2Y12 inhibitor for emergency use, achieves rapid, consistent and sustained platelet inhibition following single-dose subcutaneous administration in stable CAD patients. Eur Heart J. 2019;40(suppl 1):ehz748.0136.
- Kheiri B, Osman M, Abdalla A, et al. Clopidogrel and aspirin after ischemic stroke or transient ischemic attack: an updated systematic review and meta-analysis of randomized clinical trials. J Thromb Thrombolysis. 2019;47:233–247.
- Judge HM, Buckland RJ, Jakubowski JA, et al. Cangrelor inhibits the binding of the active metabolites of clopidogrel and prasugrel to P2Y12 receptors in vitro. Platelets. 2016;27:191–195.
