ABSTRACT
Introduction: The number of deaths and prevalent cases of cirrhosis are increasing worldwide, but there are no licensed antifibrotic or pro-regenerative medicines and liver transplantation is a limited resource. Cirrhosis is characterized by extreme liver fibrosis, organ dysfunction, and complications related to portal hypertension. Advances in our understanding of liver fibrosis progression and regression following successful etiological therapy betray vulnerabilities in common and disease-specific mechanisms that could be targeted pharmacologically.
Area covered: This review summarizes the cellular and molecular pathogenesis of cirrhosis as a preface to discussion of the current drug development landscape. The dominant indication for global pharma R&D pipelines is cirrhosis related to nonalcoholic steatohepatitis (NASH). We searched Clinicaltrials.gov, GlobalData, Pharmaprojects and PubMed for pertinent information on emerging synthetic drugs for cirrhosis, with a focus on compounds listed in phase 2 and phase 3 trials.
Expert opinion: Although cirrhosis can regress following successful etiological treatment, there are no specific antifibrotic or pro-regenerative drugs approved for this condition. Obstacles to drug development in cirrhosis include intrinsic biological factors, a heterogeneous patient population, and lack of acceptable surrogate endpoints. Nevertheless, several synthetic drugs are being evaluated in clinical trials and the NASH field is rapidly embracing a drug combination approach.
1. Background
Cirrhosis is characterized by extreme liver scarring (fibrosis), loss of organ function and serious complications related to portal hypertension (high blood pressure in the hepatic portal vein and its branches). It represents a generic end-stage for a variety of chronic liver diseases (CLD) including nonalcoholic fatty liver disease (NAFLD), alcohol-related liver disease and chronic viral hepatitis. NAFLD is now the commonest etiology worldwide, affecting 1 in 4 adults [Citation1], and the progressive form that leads to patient harm (nonalcoholic steatohepatitis (NASH)) is predicted to increase by 63% between 2015 and 2030 [Citation2], representing a global cohort of at least 100 million individuals. Cirrhosis is typically classified as either compensated or decompensated. In compensated cirrhosis, the liver can maintain its important functions and patients are generally asymptomatic. In decompensated cirrhosis the liver no longer functions adequately, and patients develop life-threatening problems including bleeding varices (varicose veins in the esophagus), ascites (abnormal buildup of fluid in the abdomen) and hepatic encephalopathy (altered brain function).
Cirrhosis is a growing healthcare challenge worldwide. The Global Burden of Disease Study 2017 reported that there were 112 million prevalent cases of compensated cirrhosis, 10.6 million prevalent cases of decompensated cirrhosis, and more than 1.32 million deaths caused by cirrhosis (33.3% in females and 66.7% in males) [Citation3]. For NASH, the number of prevalent cases more than doubled for compensated cirrhosis and more than tripled for decompensated cirrhosis between 1990–2017 [Citation3]. Crucially, cirrhosis impairs health-related quality of life (HRQoL) [Citation4] and typically affects people of working age, meaning that there are also broad socio-economic impacts.
Although 90% of cirrhosis is due to preventable causes, three-quarters of people are diagnosed at a late stage when the impact of lifestyle changes (e.g., weight loss, alcohol abstinence) or etiological treatment (e.g., antiviral therapy) is attenuated. Liver transplantation is the most effective therapeutic option for end-stage liver disease but is a scarce resource. There are currently no Food and Drug Administration (FDA) or European Medicines Agency (EMA) approved antifibrotic or pro-regenerative drug therapies for cirrhosis. However, there is intense activity in drug development, especially for liver fibrosis and cirrhosis related to NASH. In this article, we review the current drug development landscape in cirrhosis, with a specific focus on emerging synthetic drugs that are being evaluated in phase 2 or phase 3 trials.
2. Medical need
The transition from compensated cirrhosis to decompensated cirrhosis occurs at a rate of about 5% to 7% per year [Citation5]. Once decompensation has occurred, cirrhosis becomes a systemic disease with multi-organ involvement associated with a dysregulated inflammatory state [Citation6]. Decompensation represents a key prognostic inflection point in the natural history of CLD, as the median survival drops from more than 12 years for compensated cirrhosis to about 2 years for decompensated cirrhosis [Citation5]. Accordingly, treatment strategies in cirrhosis may vary depending on the disease stage as well as the underlying etiology (). Broadly, the goals of treatment for compensated cirrhosis are to slow, halt or reverse progression of fibrosis and prevent decompensation events, whereas for decompensated cirrhosis the focus is on preventing further decompensation and death (e.g., by improving liver function) and treating complications related to portal hypertension. Importantly, any treatment strategy in cirrhotic patients should not increase the risk of hepatocellular carcinoma (HCC) and, consistent with FDA guidance, should ultimately improve how a patient ‘feels, functions or survives’. In the absence of specific antifibrotic or pro-regenerative drug therapies, liver transplant is the only available option for end-stage disease. Liver transplantation consistently improves outcomes in cirrhosis, including HRQoL measures, but this may not necessarily apply to pharmacological agents. Other clinical endpoints are also likely to be meaningful in patients with decompensated cirrhosis, such as the rates of hospitalization, unscheduled clinic and emergency room visits, tests performed, and lost work days [Citation7].
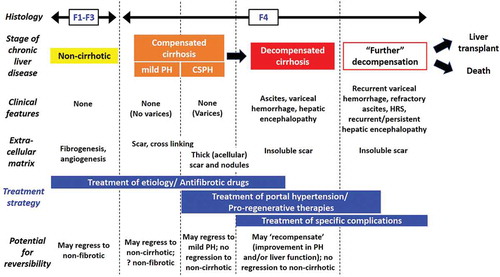
Figure 1. Clinical-pathological classification of cirrhosis and implications for treatment. Cirrhosis consists of several stages, with a range of potential outcomes based on the severity of histological and clinical correlates. Some cases of cirrhosis are more likely to improve than others, especially those of recent onset, characterized by relatively thin fibrous septa; here, etiological or specific antifibrotic therapies may induce regression of cirrhosis. Conversely, mature hepatic scar with thick, acellular, heavily cross-linked fibrous septa, such as those seen in established cirrhosis, may be irreversible; here, treatment of cirrhosis complications or regenerative approaches are likely to be more relevant. F1-4, fibrosis stage 1–4; (CS)PH, (clinically significant) portal hypertension, HRS, hepatorenal syndrome. Figure modified with permission from Wiley© from Garcia-Tsao et al, Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis, Hepatology (2010)
Another urgent requirement for drug development in advanced CLD (aCLD), particularly NASH, is validation of noninvasive liver tests that can accurately stratify disease severity, track changes in disease activity/stage and, crucially, are acceptable surrogates of future clinically meaningful outcomes (e.g., decompensation, death). This is now a global effort driven by large European (LITMUS) and US consortia (NIMBLE). A crowded field of emerging candidates includes serum markers/panels and imaging markers all with varying strengths and limitations [Citation8]. Although invasive assessment of portal hypertension by hepatic venous pressure gradient (HVPG) measurement is the best predictor of complications and mortality in patients with aCLD [Citation9], no noninvasive test is sufficiently validated to supplant HVPG.
3. Existing treatments
Existing treatments in cirrhosis comprise established etiological therapies (to remove the underlying drivers of disease), treatments for specific complications of hepatic decompensation and liver transplantation.
3.1. Curing or controlling the primary disease
Eradication of the etiological factor(s) causing liver injury is the foundational treatment strategy for all patients with aCLD and is, currently, the only effective antifibrotic approach [Citation10]. Successful etiological treatment (e.g., response to antiviral drugs in chronic HBV or HCV, weight loss, alcohol abstinence) has been shown to ameliorate portal hypertension, prevent decompensation and improve outcome in patients with compensated cirrhosis [Citation11–15]. However, results in patients with decompensated cirrhosis are generally less consistent, even after etiological cure [Citation16–18].
Together, these studies have provided important proof of principle that fibrosis regression in cirrhosis is feasible and is associated with improved patient outcomes. In a substantial proportion of patients with cirrhosis, however, treatment of the underlying cause is either ineffective or not possible; these individuals are potential candidates for antifibrotic therapies. Etiological treatment studies have also shown that remodeling of fibrosis in aCLD is a slow process. Following bariatric surgery in NASH, reduction of fibrosis began during the first year but continued through 5 years [Citation19]. Likewise, reversal of fibrosis/cirrhosis in patients with chronic hepatitis B treated with entecavir was generally only evident in long-term (3–7 years) follow-up biopsies [Citation20], indicating that it could take several years to demonstrate efficacy of an antifibrotic drug in clinical trials that rely on a biopsy endpoint.
3.2. Treatments for specific complications of decompensated cirrhosis
There are a multitude of established treatments for specific complications of decompensated cirrhosis (including ascites, variceal hemorrhage and hepatic encephalopathy) that are beyond the scope of this article. As decompensated cirrhosis is now considered a systemic disease, with multi-organ pathology associated with dysregulated inflammation, a number of mechanistic approaches have been explored to prevent disease progression in patients with decompensated cirrhosis, including i) targeting microbiome abnormalities and bacterial translocation (e.g., rifaximin); ii) improving abnormal circulatory function (e.g., long-term albumin); iii) treating the inflammatory milieu (e.g., statins); and iv) targeting portal hypertension (e.g., nonselective beta-blockers) [Citation21]. In particular, there has been considerable interest in the therapeutic potential of statins in patients with aCLD [Citation22]. Statins decrease the activity of small GTPases (Rho, Ras) and their downstream signaling pathways in the liver and have been shown to reduce portal pressure, improve endothelial dysfunction, attenuate fibrogenesis, protect against acute-on-chronic liver failure (ACLF) and HCC [Citation23,Citation24]. An active phase 4 trial (STATLiver; NCT04072601) is examining the effect of atorvastatin on survival and hospitalizations. A further phase 2 multicenter European trial (LIVERHOPE_EFFICACY; NCT03780673) is investigating the combination of simvastatin plus rifaximin in patients with decompensated cirrhosis to prevent ACLF development.
3.3. Liver transplantation
Around 8,000 liver transplants were performed in the US alone in 2019, with estimated associated healthcare costs of 878,400 USD per transplant [Citation25]. Outcomes are generally excellent, with overall 1-year and 5-year patient survival for adult elective deceased-donor first liver transplants of around 94 and 83%, respectively [Citation26]. However, because of a shortfall of deceased-donor organs to meet growing demand, around 25% of people on the waiting list die before receiving a transplant. NASH is the most rapidly increasing indication for liver transplantation in the US (and is now the leading indication in women) [Citation27].
4. Market review
There is no reliable drug intelligence data to estimate the overall liver cirrhosis market size (i.e. including all cirrhosis etiologies). This would require comprehensive epidemiological data for all CLD and would need to account for/exclude drugs that are used to target earlier disease stages prior to cirrhosis. In terms of NASH related cirrhosis, GlobalData are currently anticipating that 27% of the 7 major NASH markets ($7.3B; US, 5EU, and Japan) will be accounted for by cirrhosis (fibrosis stage F4) patients by 2029. The forecasted drugs targeting F4 patients in 2029 include: Ocaliva® (obeticholic acid), CC-90,001, aldafermin, belapectin, Ozempic® (semaglutide) and BMS-986,036.
5. Current research goals
Two major research goals in cirrhosis are the development of effective therapies to improve clinically meaningful patient outcomes and the identification and validation of noninvasive biomarkers.
Drug discovery and development approaches for liver fibrosis and cirrhosis are becoming ever more sophisticated, leveraging human ‘big data’ resources [Citation28] and incorporating high-throughput methods to investigate novel drugs/combinations (e.g., liver-on-a-chip devices, hepatic organoids/spheres) [Citation29]. Increasingly, preclinical efficacy assays with closer proximity to the patient (e.g., precision-cutting human liver slices [Citation30]) are being sought to obviate some of the shortcomings of animal models and increase confidence for clinical translation.
Validated noninvasive biomarkers are urgently sought for both therapeutic trials and clinical practice, to identify ‘high risk’ populations (i.e., patients with advanced fibrosis), to provide prognostic information, and for monitoring treatment response. There is consensus that the field must move beyond liver biopsy to determine drug effects and although there have been great strides in this area, no new technologies have yet been deemed acceptable by regulators to replace histological assessment of fibrosis. Tests that show promise as surrogate efficacy endpoints include imaging measures (e.g., Magnetic Resonance Imaging-Proton Density Fat Fraction (MRI-PDFF), MR elastography, iron-corrected T1 relaxation (cT1)) and serum markers (e.g., AST/ALT, Enhanced Liver Fibrosis (ELF) test, PRO-C3). Critically, recent trial data demonstrate that biomarkers track the histological regression of fibrosis and therefore may be suitable for monitoring drug response [Citation31–34]. If liver biopsy is performed, evaluation using artificial intelligence (AI)-based digital pathology is increasingly recommended to extract more information that is objective and quantitative [Citation35]. In patients with cirrhosis, tests that can reliably measure changes in liver function (e.g., HepQuant SHUNT test [Citation36]) or portal hypertension [Citation37] are also a high priority. Notably, HVPG has recently been used as a primary outcome measure in trials in cirrhosis due to NASH (e.g., simtuzumab, belapectin), but the FDA have not yet approved reduction in HVPG as an accepted endpoint for registration trials in cirrhosis.
6. Scientific rationale
Data from rodent models and a variety of successfully treated human liver diseases has demonstrated unequivocally that liver fibrosis is reversible and even established cirrhosis can regress substantially. Moreover, our understanding of the key cellular and molecular players that mediate fibrogenesis, sinusoidal ‘capillarization’ and microcirculatory dysfunction, in addition to regression of fibrosis and liver regeneration in different liver diseases has revealed specific targets for newly developed or repositioned antifibrotic drug candidates [Citation38].
Although there are important disease-specific nuances (see sub-section on ‘Pathogenesis of NASH’), common mechanisms have been identified that pertain to all CLD [Citation39]. A central event in liver fibrosis is the activation of hepatic stellate cells (HSC), by various inflammatory stimuli, to myofibroblast-like cells that are proliferative, contractile, immunomodulatory and synthesize excessive amounts of scar extracellular matrix (ECM). Consequently, the activation, function and fate of HSC are prominent targets for antifibrotic therapies [Citation40]. In animal models, proof of principle for a variety of mechanistic treatments has been demonstrated, such as deactivation of HSC [Citation41], reduced proliferation of HSC [Citation42], decreased ECM deposition [Citation43] or removal of activated HSC via forced apoptosis [Citation44].
In response to liver injury, liver sinusoidal endothelial cells (LSECs) also rapidly de-differentiate, acquiring a so-called ‘capillarized’ phenotype that is characterized by loss of fenestrae, development of a basement membrane, reduced nitric oxide bioavailability and production of proinflammatory, profibrogenic and vasoconstrictor factors that dysregulate neighboring cells (especially HSC) and alter the sinusoidal microcirculation [Citation45,Citation46]. The LSEC and associated sinusoidal communications are therefore also a prime target for antifibrotic and portal hypertension therapy.
Hepatic macrophages have also been identified as key regulators of both fibrogenesis and fibrosis regression. Whereas conditional depletion of ‘scar-associated macrophages’ during liver injury in mice was antifibrotic, their removal during the resolution phase of liver injury impaired tissue repair [Citation47]. Circulating Li6Chi monocytes have been identified as the source of profibrogenic hepatic macrophages in murine liver fibrosis [Citation48]. However, following injury removal, these cells undergo a phenotypic switch to a restorative macrophage phenotype that release matrix metalloproteinases (MMPs) to promote fibrotic ECM degradation, as well as factors that dampen the inflammatory response and drive liver regeneration [Citation49]. Although comparative data in humans is limited [Citation50], hepatic macrophages have emerged as antifibrotic drug targets, for example through inhibiting the infiltration of inflammatory monocytes (e.g., CCR2/CCR5 antagonism [Citation51]) or disrupting the activity of macrophage-derived factors (e.g., galectin-3 (gal-3)) [Citation52]. Thus far, therapeutic strategies that promote macrophage polarization to a restorative phenotype in situ, have only been examined in rodent models [Citation53].
In established cirrhosis, the mature hepatic scar is less susceptible to remodeling due to a number of factors including lysyl oxidase (LOX) mediated ECM cross-linking and a paucity of scar-associated cells (myofibroblasts and macrophages) capable of secreting matrix metalloproteinases (MMPs). Critically, the ECM is not an inert structural framework; instead, ECM components and tissue stiffness actively modulate the phenotype and proliferation of the cells that are embedded or closely associated with it. Accordingly, ECM molecules, their receptors (e.g., av integrins) and ECM cross-linking enzymes have been investigated as therapeutic targets. Altering the balance between ECM degrading MMPs and their specific inhibitors (tissue inhibitor of metalloproteinases (TIMPs)), for example using MMP gene therapy [Citation54]), is also potentially antifibrotic but has only been shown in preclinical models.
Finally, it is clear that any successful therapy in cirrhosis must improve liver function. Hepatic regeneration is a feature of non-fibrotic healthy liver, but severe fibrosis represses regeneration. In mice, remodeling of ECM is required for a robust hepatic progenitor cell response [Citation55]. However, it is not known if an effective antifibrotic drug in patients with cirrhosis will be sufficient to unleash the liver’s inherent regenerative potential. Exploring the complex relationship between regeneration and fibrosis in the liver may identify new therapeutic approaches to augment liver function as a potential alternative to liver transplantation [Citation56].
6.1. Pathogenesis of NASH
The pathogenesis of NASH is represented as a model of substrate-overload liver injury, with genetic and environmental (e.g., microbiome-related) risk factors modifying disease susceptibility and progression [Citation57]. Indeed, the microbiome plays a major role in NAFLD progression through different mechanisms, including immune activation via toll-like receptors and potentially endogenous alcohol production by the gut bacteria [Citation58]. Modulation of the microbiome may play a role in our future therapeutic armamentarium, but more precision will be required in how to target it.
Free fatty acids are central to NASH development and originate from lipolysis of triglycerides in adipose tissue or from de novo lipogenesis (excess sugars converted to fatty acids) in the liver. When the catabolism of fatty acids through beta-oxidation or formation of triglyceride (TG) is overwhelmed, fatty acids can contribute to the generation of lipotoxic species that cause endoplasmic reticulum (ER) stress, oxidative stress, and inflammasome activation. These processes induce hepatocellular injury, inflammation, HSC activation and progressive accumulation of scar ECM. Elucidating these disease-specific pathways has provided a rational basis for drug development in pre-cirrhotic and cirrhotic NASH.
7. Competitive environment
7.1. Search strategy
We searched for recent and active phase 2 and phase 3 clinical trials of synthetic drugs for the treatment of liver cirrhosis using ClinicalTrials.gov GlobalData and Citeline’s Pharmaprojects. We focused on drugs directed against mechanistic targets rather than etiological therapies (such as antiviral drugs for chronic hepatitis B and C) or treatments for specific complications of cirrhosis. Background literature was explored using PubMed. Drug structures and chemical formulas were sourced from PubChem (an open chemistry database at the National Institutes of Health (NIH)).
7.2. Recent unsuccessful clinical trials in cirrhosis due to NASH
Before considering current clinical trial activity, it is important and informative to reflect on the disappointing results from recent major studies in cirrhosis due to NASH (summarized in ). These setbacks have highlighted several potential issues including the poor predictivity of preclinical models; inadequate duration of trials in cirrhosis that may require several years for substantial fibrosis remodeling to occur; drug mechanism of action which may be unfavorable in cirrhosis (or insufficient as monotherapy); lack of adequate biomarkers of target engagement; heterogeneous patient population; sampling variability of liver biopsy; and high placebo response rate.
Table 1. Recent unsuccessful drug programs in cirrhosis due to NASH
7.2.1. Simtuzumab
Simtuzumab (GS-6624) is a subcutaneously (SC) administered humanized IgG4 monoclonal antibody, developed by Gilead Sciences, that specifically binds and inhibits lysyl oxidase like 2 (LOXL2), an enzyme that is thought to mediate collagen crosslinking in fibrosis. Initial enthusiasm for this approach was based on compelling evidence of both human tissue expression and preclinical data implicating LOXL2 in the pathogenesis of fibrosis in liver, lungs, and tumor xenograft models [Citation59]. However, the drug failed in phase 2b clinical trials as a monotherapy in patients with bridging fibrosis and compensated cirrhosis due to NASH [Citation60] and in compensated liver disease due to primary sclerosing cholangitis (PSC) [Citation61]. Although development of simtuzumab has been terminated, this may have been a target engagement issue and there could still be a role for small molecule inhibitors of LOXL2 (and/or other isoforms), possibly deployed earlier in fibrosis to slow progression rather than to reverse advanced disease.
7.2.2. Selonsertib
Selonsertib is an orally administered apoptosis signal-regulating kinase 1 (ASK1) inhibitor, also developed by Gilead Sciences. In the setting of oxidative stress, activation of ASK1, a serine/threonine signaling kinase, can lead to phosphorylation of p38 mitogen-activated protein kinase and c-Jun N-terminal kinase (JNK), leading in turn to activation of stress response pathways that worsen hepatic inflammation, apoptosis, and fibrosis. Moreover, hepatic steatosis, fibrosis, and TGFβ1 expression was significantly attenuated in ASK1-deficient mice fed a high-fat diet [Citation62]. However, selonsertib failed in two large and well-powered phase 3 trials in patients with advanced fibrosis (STELLAR 3) and compensated cirrhosis (STELLAR 4) due to NASH [Citation63]. Although selonsertib had dose-dependent effects indicating pharmacodynamic activity, and statistically non-significant improvements in noninvasive biomarkers were observed, it did not reach the primary efficacy endpoint of fibrosis improvement without worsening of NASH at week 48. The drug is still being explored in combination regimens (where efficacy might be amplified), but it is no longer being pursued as a monotherapy.
7.2.3. Emricasan
Emricasan is an orally administered pan caspase inhibitor developed by Conatus and Novartis. Inhibition of caspases may reduce the disease-driven loss of hepatocytes and production of apoptotic bodies and microparticles that promote progression of CLD. Moreover, emricasan was recently shown to improve liver sinusoidal microvascular dysfunction and portal hypertension in cirrhotic rats [Citation64]. However, despite showing pharmacodynamic effects on caspase inhibition, emricasan was ineffective in multiple phase 2 trials, including in patients with pre-cirrhotic NASH [Citation65] and compensated [Citation66] and decompensated [Citation67] NASH related cirrhosis. Interestingly, in a subgroup of patients with HVPG ≥16 mmHg in the ENCORE-PH study, there was a significant reduction of HVPG, suggesting efficacy in a more severe population [Citation66]. Nevertheless, following multiple setbacks, development of emricasan has been terminated.
7.2.4. Belapectin
Belapectin (GR-MD-02) is an intravenously (IV) administered gal-3 inhibitor under development by Galectin Therapeutics. Gal-3 is the most important galectin protein secreted in the disease state, mainly by macrophages, and it binds to the cell surface and ECM glycans to regulate a variety of physiological and pathological processes including cell apoptosis, adhesion, migration, angiogenesis, and inflammatory responses. Belapectin is a complex carbohydrate drug that improved pathology of NASH and reversed liver fibrosis/cirrhosis in animal models [Citation68]. However, in a phase 2 trial in patients with compensated NASH cirrhosis and HVPG ≥6 mmHg (NASH-CX), belapectin failed the primary endpoint of a reduction in HVPG in the total population [Citation69]. Nevertheless, in a post hoc analysis, patients without varices at baseline had a significantly reduced HVPG and lower incidence of varices development in the drug-treated group compared to placebo, although interestingly there was no dose-response effect. These results should be viewed cautiously and are now being validated in a further phase 2b/3 trial in NASH cirrhosis (NCT04365868).
7.3. Synthetic drugs currently being investigated in phase 2 or phase 3 trials for cirrhosis: monotherapy landscape
A number of drugs are currently in development for cirrhosis, predominantly due to NASH, and these are summarized in and .
Figure 2. Summary of synthetic drugs being evaluated in phase 2 or phase 3 trials in cirrhosis. Emerging drug candidates and mechanistic targets/pathways are depicted. The majority relate to pathogenesis of nonalcoholic steatohepatitis. CCR2/5, C-C chemokine receptor type 2/5; ER, endoplasmic reticulum; ROS, reactive oxidative species; UPR, unfolded protein response; FFA, free fatty acids; SERBP-1, sterol regulatory element-binding protein 1; SHP, small heterodimer partner; VLDL, very low-density lipoprotein; FGF19/21, fibroblast growth factor 19/21; FXR, farnesoid X receptor; HSP47, heat shock protein 47; p38, p38 mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; ACC, acetyl-CoA carboxylase; ASK-1, apoptosis signal-regulating kinase 1; GLP-1,glucagon-like peptide 1; WNT, Wingless-related integration site; b-catenin, beta-catenin; Akt, protein kinase B; EGFR, epidermal growth factor receptor; HSC, hepatic stellate cell; Z a1 AT, mutant alpha-1-antitrypsin Z; ECM, extracellular matrix
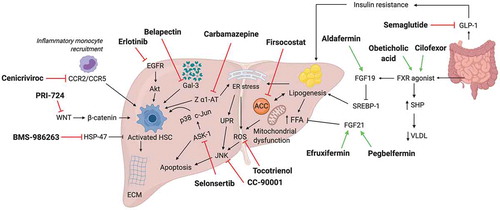
Table 2. Summary of emerging synthetic drugs for the treatment of cirrhosis under investigation in phase 2 or 3 trials
7.3.1. Obeticholic acid
Obeticholic acid (INT-747) is an orally administered synthetically-modified analog of chenodeoxycholic acid, under development by Intercept Pharmaceuticals as a first-in-class farnesoid X receptor (FXR) agonist for the treatment of primary biliary cholangitis (PBC) and NASH. FXR agonism has multifaceted effects on bile acid metabolism, FGF19 induction, gut microbiota, hepatic inflammation and fibrogenesis. It is an established modality for improving NASH histological endpoints, including fibrosis, and several steroidal and non-steroidal FXR ligands are in development [Citation70]. The results of the REGENERATE trial (NCT02548351) in patients with pre-cirrhotic NASH [Citation71] was hailed as a watershed moment in NASH drug development as this was the first positive phase 3 clinical trial in patients with NASH and stage 2–3 fibrosis. However, after 18 months of obeticholic acid treatment, fibrosis improvement (≥1 stage) was only observed in 23% of all drug-treated patients (compared to 12% on placebo) and there was no effect on NASH resolution. In June 2020, the FDA rejected a New Drug Application (NDA) for obeticholic acid because ‘the predicted benefit based on a surrogate histopathologic end point remains uncertain and does not sufficiently outweigh the potential risks’. Major adverse effects related to FXR agonists include pruritus and increased low‐density lipoprotein (LDL) cholesterol (LDL-C), both of which are dose-dependent, and decreased high-density lipoprotein cholesterol. Since cardiovascular disease is common (and is the leading cause of death) in patients with NAFLD, this rise in serum LDL-C is noteworthy. The relative impact on long-term outcomes of dyslipidemia associated with OCA therapy, compared to the observed histological benefit, remains undefined [Citation72]. However, if approved, it is likely that OCA will require regular monitoring of lipid profiles and treatment with statin therapy as indicated.
Intercept remains committed to the drug and a phase 3 trial (REVERSE; NCT03439254) is ongoing in patients with compensated NASH cirrhosis, with the primary endpoint of a one-stage reduction in fibrosis without worsening of NASH.
7.3.2. Cenicriviroc
Cenicriviroc (TAK-652; TBR-652) is an orally administered small molecule dual antagonist of CC-motif chemokine receptors 2 and 5 (CCR2/5), under development by AbbVie (Allergan before acquisition) for the treatment of NASH and liver fibrosis. The recruitment of inflammatory monocytes and macrophages via CCR2 and lymphocytes and HSCs via CCR5 promotes the progression of NASH to fibrosis. In preclinical models of chronic liver injury, cenicriviroc reduced monocyte/macrophage accumulation in the liver and ameliorated fibrosis [Citation73]. However, the observation that CCR2 deficient mice are protected from experimental fibrosis but are also unable to effectively resolve fibrosis [Citation74], likely reflects the importance of hepatic macrophages in remodeling scar and may indicate that anti-inflammatory treatments such as cenicriviroc are best applied during early/progressive fibrosis.
In a phase 2b trial (CENTAUR; NCT02217475) in patients with NASH and fibrosis stage 1–3, cenicriviroc improved fibrosis [Citation32], leading on to a large phase 3 trial (AURORA; NCT03028740) in patients with NASH and stage 2/3 fibrosis; topline results are imminent. Additionally, there is an open-label rollover study to assess the long-term safety of continued treatment with cenicriviroc in participants who completed CENTAUR or AURORA as a result of reaching an adjudicated liver-related clinical outcome (either histological progression to cirrhosis, MELD score >15, ascites needing treatment, or hospitalization for variceal bleed, encephalopathy or spontaneous bacterial peritonitis).
7.3.3. Pegbelfermin
Pegbelfermin (BMS‐986,036; ARX-618) is a SC administered polyethylene glycol‐modified (PEGylated) recombinant human fibroblast growth factor 21 (FGF21) analog, under development by Bristol-Myers Squibb, with a prolonged half‐life designed to support up to weekly dosing. FGF21 is a hormone involved in the regulation of glucose, lipids, and energy homeostasis. In rodents and primates, the half-life of recombinant FGF21 is approximately 1–2 h, therefore multiple approaches have been employed to engineer FGF21 to extend its duration of action. Preclinical studies suggest that FGF21 binds to the FGFR1/Klothoβ complex in adipose tissue, leading to elevated secretion of the insulin-sensitizing hormone adiponectin, although additional metabolic pathways may also be affected [Citation75]. The mechanism(s) behind the hepatic anti-inflammatory and anti-fibrotic effects of FGF21 are unclear but could be mediated via the strong increase in adiponectin. In a phase 2 trial (NCT02097277) in patients with obesity and type 2 diabetes, 12 weeks pegbelfermin treatment was associated with improved metabolic parameters and serum fibrosis markers [Citation76]. A subsequent phase 2a study (NCT02413372) of overweight/obese patients with NASH and fibrosis stage 1–3, showed a significant decrease in absolute hepatic fat fraction (MRI-PDFF) in the group receiving 10 mg pegbelfermin daily (−6·8% vs −1·3%) and in the group receiving 20 mg pegbelfermin weekly (−5·2% vs −1·3%) compared with the placebo group [Citation77]. Both pegbelfermin and efruxifermin (discussed below) are well tolerated. The most common side effects of FGF21 treatment are gastrointestinal (GI)-related (increased frequency of diarrhea and nausea). A potential effect of FGF21 on bone mineral density will require further studies of longer duration. Pegbelfermin also induces anti-drug antibodies, which can cross-react with the endogenous FGF21, so this may need to be carefully monitored. Pegbelfermin is currently being studied in phase 2b trials in pre-cirrhotic NASH (FALCON 1; NCT03486899) and in compensated NASH cirrhosis (FALCON 2; NCT03486912) with the primary endpoint of a one-stage reduction in fibrosis without worsening of NASH. However, since the antifibrotic effects of pegbelfermin are not well documented, its potential efficacy in NASH cirrhosis is uncertain.
7.3.4. Efruxifermin
Efruxifermin (AKR-001) is a SC administered human immunoglobulin 1 (IgG1) Fc-FGF21 fusion protein, engineered for sustained systemic pharmacologic exposure, under development by Akero Therapeutics for the treatment of NASH. In a phase 1 trial (NCT01856881) it showed sustained pharmacodynamic effects on insulin sensitivity and lipid metabolism in type-2 diabetes patients [Citation78]. The BALANCED study (NCT03976401) is an ongoing phase 2a dose-ranging trial of weekly SC efruxifermin treatment for up to 16 weeks in NASH patients with fibrosis stage 1–4. The primary endpoint is the change from baseline in hepatic fat fraction assessed by MRI-PDFF at week 12. In March 2020, the company reported data from the week 12 analysis, showing that all efruxifermin dose groups saw highly statistically significant absolute reductions in liver fat (12–15%, compared to 0% for placebo), relative reductions in liver fat (63–72%, compared to 0% for placebo) and reduction in ALT (24–32 U/L, compared to 6 U/L for placebo). In June 2020, the company reported data for the 40 treatment responders who had end-of-treatment biopsies at week 16, showing that 48% had fibrosis improvement of at least one stage without worsening of NAS across all dose groups, with a 62% response rate for the 50 mg dose group. Meaningful improvements in weight loss, dyslipidemia and glycemic control were also observed at week 16. A dose-dependent increase in plasma adiponectin was observed in all dose levels. Although results for efruxifermin have been encouraging, and among the strongest fibrosis changes reported in NASH so far, data from the compensated cirrhosis cohort is not yet available.
7.3.5. Aldafermin
Aldafermin (NGM–282) is a SC administered engineered non-tumorigenic analog of human fibroblast growth factor 19 (FGF19) under development by NGM biopharmaceuticals for the treatment of NASH, PBC and PSC. Aldafermin acts on two receptor complexes, FGFR1c-KLB and FGFR4-KLB. FGFR1c-KLB activation reduces liver steatosis and increases insulin sensitivity, while FGFr4-KLB suppresses expression of CYP7A1, which encodes the rate limiting enzyme in de novo bile acid synthesis. Therefore, aldafermin may ameliorate dysregulated bile acid metabolism (and thereby attenuate hepatobiliary injury) as well as regulating metabolic homeostasis [Citation79]. In a phase 2 trial (NCT02704364) in patients with PSC, 6% of whom had compensated cirrhosis, NGM282 potently inhibited bile acid synthesis and decreased serum fibrosis markers (ELF score and Pro-C3), without significantly affecting alkaline phosphatase (ALP) levels [Citation80]. In contrast, aldafermin reduced ALP in a phase 2 trial (NCT02026401) in patients with PBC, although no assessment was made of its impact on fibrosis [Citation81]. In a phase 2 trial (NCT02443116) in patients with NASH, treatment with aldafermin for up to 24 weeks decreased absolute liver fat content (measured by MRI-PDFF), improved histological features of NASH and reduced ELF score and Pro-C3 levels [Citation31,Citation82]. Across studies, aldafermin has been generally well tolerated, but is associated with dose-related abdominal cramping and diarrhea. A significant observed increase in plasma LDL-C is a potential concern that may require counterregulatory treatment with statins. Further phase 2 trials evaluating its efficacy in patients with stage 2/3 fibrosis (ALPINE 2/3; NCT03912532) and compensated cirrhosis (ALPINE 4; NCT04210245) due to NASH are ongoing.
7.3.6. BMS-986,263
BMS-986,263 (ND-L02-s0201) is an IV administered vitamin A-coupled lipid nanoparticle (LNP) containing small interfering ribonucleic acid (siRNA) against HSP47, under development by Bristol-Myers Squibb. HSP47 is an ER-localized collagen-specific molecular chaperone indispensable for the correct folding of procollagen in the ER. Increased expression of HSP47 is associated with excessive accumulation of collagens in scar tissues of various experimental and human fibrotic diseases. BMS-986,263 is targeted to HSC via retinoid-containing moieties conjugated to the LNP surface and, by silencing HSP47, may halt or reverse liver fibrosis by disrupting collagen synthesis [Citation43]. In a phase 2 trial (NCT03420768) in 61 patients with advanced liver fibrosis due to chronic HCV who had achieved sustained virological response, once-weekly IV infusion of BMS-986,263 for 12 weeks was generally well tolerated, demonstrated target engagement by reducing liver HSP47 mRNA levels and reduced histological fibrosis, mostly in those with cirrhosis [Citation83]. A phase 2 study (NCT04267393) to evaluate the safety and efficacy of BMS-986,263 in patients with compensated NASH cirrhosis is now planned.
7.3.7. CC-90,001
CC-90,001 (CC-539) is an orally active JNK1 inhibitor (12.9-fold more potent for JNK1 inhibition than JNK2 in vitro) under development by Bristol-Myers Squibb (Celgene before acquisition) for the treatment of idiopathic pulmonary fibrosis (IPF), liver fibrosis and NASH. JNK activity regulates various pathophysiologic processes, including hepatocyte death, steatosis, inflammation and insulin resistance, which are associated with NASH, fibrosis and HCC. JNK is involved in HSC activation and fibrogenesis in animal models and in patients with liver fibrosis due to chronic HCV and NASH [Citation84]. Moreover, Jnk1 knockout mice are protected from liver fibrosis. Available pharmacodynamic and safety data on CC-90,001 are limited. In a phase 1b study in IPF (NCT02510937), CC-90,001 treatment caused a trend to reduction in plasma tenascin-C levels [Citation85]. The most common side effects were GI in nature (all mild to moderate). CC-90,001 is currently being investigated in a phase 2 dose-finding study (NCT04048876) in patients with NASH and fibrosis stage 3 or 4 (cirrhosis), to evaluate its safety and efficacy, with a primary endpoint of a ≥ 1 stage improvement in liver fibrosis after one year of treatment.
7.3.8. Semaglutide
Semaglutide (NN-9535) is a long-acting once-weekly SC administered human glucagon-like peptide 1 (GLP-1) receptor agonist, under development by Novo Nordisk. The discovery of GLP-1, an incretin hormone with important effects on glycemic control and body weight regulation, led to efforts to synthesize GLP-1 analogs with increased half-life for the treatment of type-2 diabetes, obesity and NASH [Citation86]. Semaglutide is approved for the treatment of type-2 diabetes, whilst different formulations of liraglutide are approved for the treatment of type-2 diabetes and chronic weight management. Encouragingly, GLP-1 analogs also positively affect cardiovascular outcomes in patients with type-2 diabetes, probably through modified atherosclerotic progression by an anti-inflammatory mechanism [Citation87]. In a recent phase 2 trial (NCT02970942) of 320 patients with NASH and stage 2/3 fibrosis, 72 weeks of semaglutide treatment at the highest dose resulted in a significantly higher percentage of patients with NASH resolution than placebo (59% versus 17%) [Citation88]. The trial did not show a significant between-group difference in the percentage of patients with an improvement in fibrosis stage, although improvements in noninvasive markers of fibrosis were observed with semaglutide treatment. Semaglutide has no direct liver effects (lack of GLP1 receptor in the liver) so all the benefits are driven by weight loss. The drug is associated with less hunger and food cravings, better control of eating and a lower preference for high-fat foods [Citation89]. The amount of weight loss achieved is greater than that seen with any licensed anti-obesity drug. Semaglutide also has a favorable effect on both TG and LDL-C. Dose-related GI side effects (nausea, constipation, and vomiting) have been reported across trials of semaglutide. It is currently in a phase 2 trial (NCT03987451) in 65 patients with NASH and compensated liver cirrhosis, to evaluate its safety and efficacy compared with placebo. Importantly, the development of an oral formulation of semaglutide may help to improve treatment adherence in the future.
7.3.9. Firsocostat
Firsocostat (ND-630; GS-0976) is an acetyl CoA carboxylase (ACC) allosteric inhibitor, under development by Nimbus Apollo (part of Gilead Sciences). Inhibition of ACC reduced hepatic lipotoxicity, blocked the activation of TGF-β-induced collagen production in HSCs by inhibiting de novo lipogenesis (DNL), and significantly reduced fibrosis in 4 models of NASH [Citation90]. In a recent phase 2 trial in patients with NASH and fibrosis (F1-3), GS-0976 20 mg daily for 12 weeks decreased liver fat (MRI-PDFF) by 29% and reduced levels of the serum fibrosis marker TIMP-1 [Citation91]. However, increases in circulating TG are a known mechanistic consequence of hepatic ACC inhibition so long-term cardiovascular effects require further investigation. Although the phase 2b ATLAS trial (NCT03449446) was unsuccessful, the firsocostat-cilofexor combination was superior to placebo in reducing liver stiffness and serum markers of fibrosis in patients with bridging fibrosis and cirrhosis due to NASH [Citation92]. Gilead are continuing to evaluate firsocostat in combination regimens (NCT02781584).
7.3.10. Cilofexor
Cilofexor (GS-9674) is an orally administered gut-restricted nonsteroidal FXR agonist under development by Gilead Sciences for the treatment of NASH, PBC, and PSC. Intestinal FXR agonism by cilofexor augments the physiological release of FGF19, and this could mitigate potential deleterious effects of systemic FXR activation (as seen with OCA), including dyslipidemia, pruritus, and hepatotoxicity. Cilofexor has demonstrated anti‐inflammatory and antifibrotic effects and reduced portal pressure in a rat model of NASH [Citation93]. In a phase 2 trial (NCT02854605) in patients with NASH, cilofexor for 24 weeks was generally well-tolerated and caused significant reductions in hepatic steatosis and liver biochemistry [Citation94]. Moderate to severe pruritus was more common in patients receiving cilofexor 100 mg (14%) than in those receiving cilofexor 30 mg (4%) and placebo (4%). However, cilofexor did not cause significant changes in lipid parameters. As mentioned previously, cilofexor is being evaluated in combination with firsocostat in patients with advanced fibrosis/cirrhosis due to NASH. In addition, there is a phase 1 open label study (NCT04060147) to assess the safety and tolerability of escalating doses of cilofexor in patients with PSC and compensated cirrhosis.
7.3.11. Tocotrienol
Tocotrienol is a natural vitamin E supplement. Vitamin E has potent anti-inflammatory and antioxidant properties which may reduce liver injury in NAFLD. Gamma‐tocotrienol supplementation attenuated hepatic inflammation and fibrosis in experimental NASH models, through a synergistic mechanism of decreased de novo lipogenesis and hepatic ER stress [Citation95]. In a previous study, oral tocotrienol treatment increased hepatic tocotrienol content and attenuated the time-dependent rise in MELD score in patients with end-stage liver disease/cirrhosis [Citation96]. A current phase 2 randomized placebo-controlled trial (NCT02581085) of daily tocotrienol treatment for 3 years is being undertaken to validate the observed effect on Model For End-Stage Liver Disease (MELD) score in patients with cirrhosis.
7.3.12. PRI-724
PRI-724 is a small-molecule cAMP-response element-binding protein (CBP)/β-catenin inhibitor under development by Prism Pharma and Ohara Pharmaceutical for the treatment of liver fibrosis/cirrhosis, solid tumors and leukemia. Wingless-related integration site (Wnt)/β-catenin signaling is a highly conserved evolutionary pathway that regulates key cellular functions including proliferation, differentiation, migration, genetic stability, apoptosis, and stem cell renewal. Aberrant Wnt/β-catenin signaling has been implicated in fibrosis in a number of organs including the lungs, kidneys, skin, and liver. In liver, CBP/β-catenin inhibitors mediate antifibrotic effects through inhibition of HSC activation and increased resolution of inflammation by macrophages [Citation97]. PRI-724 has shown antifibrotic efficacy in various experimental liver fibrosis models including HCV transgenic (HCV-Tg) mice, carbon tetrachloride toxicity, bile-duct ligation and NASH related liver injury [Citation98]. A phase 1 clinical trial (NCT02195440) of IV administered PRI-724 demonstrated its safety, tolerability, and preliminary efficacy in patients with HCV-induced cirrhosis. Currently, a phase 1/2a trial (NCT03620474) is investigating the pharmacokinetics, safety and antifibrotic efficacy of twice-weekly IV PRI-724 for 12 weeks in patients with cirrhosis due to chronic hepatitis B or C.
7.3.13. Carbamazepine
Carbamazepine (Tegretol®; Novartis) is a sodium channel blocker that is FDA approved for the treatment of epilepsy, trigeminal neuralgia and bipolar disorder. However, it was also shown to act as an autophagy-enhancing drug that decreased the hepatic load of mutant alpha1-antitrypsin Z (ATZ) and hepatic fibrosis in a mouse model of AT deficiency-associated liver disease [Citation99]. The drug is now being investigated in a phase 2 trial (NCT01379469) in patients with severe liver disease and portal hypertension caused by AT deficiency to evaluate effects on ATZ load (primary outcome) and hepatic fibrosis, HVPG, and MELD score (secondary outcomes).
7.3.14. Erlotinib
Erlotinib hydrochloride (Tarceva®; Genentech) is an orally administered inhibitor of the intracellular phosphorylation of tyrosine kinase associated with the epidermal growth factor receptor (EGFR). It is FDA approved for the treatment of non-small cell lung cancer. In three different rodent models of progressive cirrhosis, erlotinib reduced the total number of activated HSCs, decreased hepatocyte proliferation and, consequently, attenuated fibrosis and the development of HCC [Citation42,Citation100]. A phase 1/2 academic-sponsored pilot study (NCT02273362) of erlotinib in patients with Child-Pugh class A cirrhosis, to evaluate effects on fibrogenesis and development of HCC, is closed to enrollment but results are not yet available.
7.4. Synthetic drugs currently being investigated in phase 2 or phase 3 trials for cirrhosis: combination therapy landscape
As the number of headstones in the ‘NASH graveyard’ continues to grow, drug developers are shifting focus to combination regimens. The rationale is that the efficacy of treatment for conditions such as NASH and fibrosis, with complex inter-related pathogenic pathways, may be enhanced by using combinations of drugs that target different and complementary mechanisms. Indeed, the impact of an antifibrotic or anti-inflammatory therapy may be attenuated if unrestrained upstream metabolic drivers remain unchecked. Ideally, combinations should also have positive effects beyond the liver such as weight loss, cardiovascular protection, insulin sensitization, and lipid reduction. However, there are potential downsides such as safety, tolerability (although drug combination could actually decrease side effects), cost and a more challenging route to regulatory approval. Important topics include the optimal selection of drugs for combination (possibly using computational methods), chronology (i.e., overlapping, outlasting or additive), identification of patient populations that might benefit from combination regimens as first-line treatment, and trial design. Although the phase 2 ATLAS trial of firsocostat, cilofexor and selonsertib () did not meet its primary endpoint (possibly due to a small sample size), the complex interactions between drugs in different combination regimens will continue to inform future development [Citation101]. For example, data from the phase 2a proof-of-concept trial of firsocostat, cilofexor, and semaglutide in pre-cirrhotic NASH (NCT03987074) was recently reported [Citation102]. This combination seems logical. Whilst firsocostat increases TG and cilofexor increases LDL-C, semaglutide has a favorable effect on both TG and LDL-C; in addition, firsocostat/cilofexor may boost the therapeutic effects of semaglutide. The positive safety/tolerability data and exploratory biomarker improvements observed for hepatic steatosis and liver injury will be investigated further in a phase 2b trial in compensated NASH cirrhosis patients. Theoretically, a palette of different combinations would allow tailoring of treatment according to the predominant pathophysiological drivers, stage of NASH and existing co-morbidities (i.e., personalized therapy).
8. Potential development issues
8.1. Placebo response rate in NASH trials
One key observation from recent trials in NASH is the striking placebo response rate. In the recently published phase 2 trial (NCT02970942) of semaglutide in patients with NASH and stage 1–3 fibrosis, an improvement in fibrosis was reported in 33% of placebo-treated patients [Citation88]. This raises concern that biopsy staging of fibrosis is not a sufficiently accurate longitudinal surrogate biomarker and may obscure the detection of a beneficial drug effect. A high placebo response rate also necessitates larger trials to guarantee adequate power to detect a significant difference between placebo and intervention arms. Application of AI-augmented histological quantification methods or, ideally, accurate noninvasive surrogate biomarkers may help to parse out truly dynamic changes from sampling variability. In addition, standardization of lifestyle management plans within and across therapeutic trials in NASH may also help to reduce the placebo response, as recently recommended by the Liver Forum’s Standard of Care Working Group [Citation103].
8.2. Regulatory considerations
The FDA currently supports commercial drug development in patients with NASH and significant fibrosis (≥ F1 but ≤ F4) and with NASH and compensated cirrhosis, as these individuals are at higher risk for liver-related adverse clinical outcomes. However, the latest FDA draft guidance (June 2019) [Citation104] has important implications for drug development and current trials in compensated cirrhosis related to NASH. Crucially, only a composite clinical outcomes endpoint is deemed acceptable for approval. At present, the FDA questions the feasibility of pharmacological reversal of cirrhosis in NASH and is unconvinced about the relationship between histological changes in cirrhosis and clinical outcomes. Ongoing trials in compensated NASH cirrhosis include the phase 3 trial of obeticholic acid (REVERSE; NCT03439254) and the phase 2b/3 trial of belapectin (NCT04365868) that have primary endpoints of a one-stage reduction in fibrosis and prevention of esophageal varices, respectively. The current draft FDA guidance indicates that these endpoints would be insufficient for accelerated approval in compensated NASH cirrhosis. This draft guidance provides a clear roadmap for the future and should expedite research to validate surrogate endpoints in cirrhosis populations.
9. Conclusion
Although there are no approved antifibrotic drugs for cirrhosis, there is significant investment and drug development activity in the NASH space. As a therapeutic indication, cirrhosis poses many challenges. Ultimately, optimal therapies in cirrhosis need to go beyond fibrosis regression and impact meaningful clinical endpoints. Accordingly, parallel development of noninvasive biomarkers that accurately correlate with therapeutic response and that are predictive of outcomes are priorities for the field.
10. Expert opinion
This review of emerging synthetic drugs for the treatment of cirrhosis, overwhelmingly in NASH, indicates a continued search for efficacious pharmacotherapies targeting diverse mechanisms. However, recent prominent terminated programs attest to the challenge of cirrhosis as an indication. The new FDA guidance provides a framework for future studies on NASH-related compensated cirrhosis, with the objective of preventing decompensation events and subsequent liver transplant and liver-related mortality. The current NASH-centric approach has drawbacks, however, for the wider cirrhosis population. Among all cirrhosis deaths in 2017, 50% were alcohol related. Alcohol-related cirrhosis remains an under-explored indication, but the disease burden reinforces the importance of developing more generic antifibrotic medicines.
The coming years will see increasing use of next-generation technologies, such as ‘omics-driven target and drug discovery and the evolution of precision medicine to determine patient subpopulations with different risk of disease progression and drug response profiles. In NASH, genome-wide association studies have identified variants in genes including patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2) and hydroxysteroid 17β dehydrogenase 13 (HSD17B13) that may predict individual risk of developing advanced disease [Citation105]. Novel prognostic genetic markers are also potential drug targets. For example, a loss-of-function variant in the HSD17B13 gene is protective against NASH and adverse outcomes among NASH patients. Alnylam Pharmaceuticals have developed an siRNA therapeutic approach designed to mimic the genetic loss of HSD17B13 that is currently being evaluated in a phase 1 trial (NCT04565717).
The identification of optimal participants for pharmacotherapy trials is particularly relevant in cirrhosis. Even ‘compensated cirrhosis’ could encompass a very heterogeneous cohort (i.e. extending from some patients at the F3/F4 interface to others with clinically significant portal hypertension and varices; ). Early cirrhosis without varices might be a better disease stage, theoretically, to demonstrate cirrhosis reversal.
Marketing approval for a synthetic drug in cirrhosis does not seem imminent and there are relatively few agents in active phase 2/3 trials (). Although results from the phase 3 trial of obeticholic acid in NASH cirrhosis (REVERSE; NCT03439254) are keenly anticipated, hopes should be tempered by the fact that efficacy was only modest in pre-cirrhotic NASH and fibrosis in cirrhosis appears, so far, recalcitrant to therapy. Indeed, companies may avoid cirrhosis as an indication and focus on pre-cirrhotic NASH, or even target an alternative organ altogether (e.g., IPF) where the path to registration is better defined and potentially easier to achieve.
Of course, drugs that are shown to be effective at earlier stages of disease may also be beneficial in cirrhosis. Promising agents continue to emerge in pre-cirrhotic NASH, such as the pan-peroxisome proliferator-activated receptor (pan-PPAR) agonist lanifibranor, which showed encouraging effects against fibrosis and portal hypertension in preclinical models [Citation106]. Furthermore, data was recently reported from Inventia’s phase 2b trial in non-cirrhotic NASH (NATIVE; NCT03008070) showing positive effects of lanifibranor (IVA337) on histological endpoints (NASH resolution and fibrosis regression) [Citation107]. Nevertheless, for pre-cirrhotic and cirrhotic NASH, the field appears to be moving inexorably toward drug combination therapy, for example a metabolic focused drug (e.g. insulin sensitizer or FXR agonist) plus an anti-inflammatory or antifibrotic drug to shut down key drivers of NASH synergistically.
Liver-targeting technologies (e.g., using nanoparticles) may allow testing of more potent and specific approaches such as siRNA mediated knockdown of core pathogenic molecules/pathways, with a reduced risk of off-target liabilities. Alternatively, well-established drugs could be repositioned as antifibrotic therapies, following identification using novel systems biology or phenotypic screening strategies. For example, nitazoxanide is an approved anti-parasitic agent that has shown promising antifibrotic activity in preclinical disease models and is currently being evaluated by Genfit in a phase 2 trial (NCT03656068) of patients with NASH and significant (F2/3) fibrosis.
At present, there is a paucity of fibrolytic agents in development that may be effective at degrading mature hepatic scar. Even a modest effect on fibrosis remodeling may be sufficient to reduce portal hypertension and/or allow liver regeneration to occur endogenously. Alternatively, cell-based therapies may have potential for augmenting liver function in cirrhosis across the etiological spectrum [Citation108]. Indeed, perhaps the field should focus more intently on approaches that target liver regeneration, rather than scarring per se, as a better strategy to improve how a patient with cirrhosis feels, functions and survives.
Declaration of interest
JA Fallowfield has served as a consultant or advisory board member for Novartis, Ferring Pharmaceuticals, Macrophage Pharma, Aquilla BioMedical, Galecto Biotech, Caldan Therapeutics, Cypralis Ltd, Rallybio, Tectonic, Gilde Healthcare, Guidepoint, Techspert.io and has received research grant funding from Novartis and Intercept Pharmaceuticals. M Jimenez-Ramos is an iCASE PhD student funded by the Medical Research Council and Galecto Biotech. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgement
The authors would like to thank Aurora Piñas-Fernández (Edinburgh Innovations, University of Edinburgh, UK) for assistance in searching Pharma market intelligence databases.
Additional information
Funding
References
- Paik JM, Golabi P, Younossi Y, et al. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72(5):1605–1616.
- Estes C, Razavi H, Loomba R. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133.
- Sepanlou SG, Safiri S, Bisignano C; GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–266.
- Younossi Z, Henry L. Overall health-related quality of life in patients with end-stage liver disease. Clin Liver Dis. 2015;6(1):9–14.
- D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231.
- Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63(5):1272–1284.
- Sanyal AJ, Friedman SL, McCullough AJ, et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American association for the study of liver diseases-U.S. Food and drug administration joint workshop. Hepatology. 2015;61(4):1392–1405.
- Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2(2):100067.
- Ripoll C, Groszmann R, Garcia-Tsao G. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481–488.
- Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56(5):1171–1180.
- Mallet V, Gilgenkrantz H, Serpaggi J, et al., Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149(6):399–403.
- Afdhal N, Everson GT, Calleja JL, et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat. 2017;24(10):823–831.
- Berzigotti A, Albillos A, Villanueva C, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the sportdiet study. Hepatology. 2017;65(4):1293–1305.
- Lampertico P, Invernizzi F, Viganò M, et al. The long-term benefits of nucleos(t)ide analogs in compensated HBV cirrhotic patients with no or small esophageal varices: a 12-year prospective cohort study. J Hepatol. 2015;63(5):1118–1125.
- Lackner C, Spindelboeck W, Haybaeck J, et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66(3):610–618.
- Shim JH, Lee HC, Kim KM, et al. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010;52(2):176–182.
- Lens S, Baiges A, Alvarado-Tapias E, et al. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J Hepatol. 2020;73(6):1415–1424.
- Alvarez MA, Cirera I, Solà R, et al. Long-term clinical course of decompensated alcoholic cirrhosis: a prospective study of 165 patients. J Clin Gastroenterol. 2011;45(10):906–911.
- Lassailly G, Caiazzo R, Ntandja-Wandji L-C, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290–1301.e5.
- Chang -T-T, Liaw Y-F, Wu -S-S, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886–893.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460.
- Bosch J, Gracia-Sancho J, Abraldes JG. Cirrhosis as new indication for statins. Gut. 2020;69(5):953–962.
- Schierwagen R, Maybüchen L, Hittatiya K, et al. Statins improve NASH via inhibition of RhoA and Ras. Am J Physiol Gastrointest Liver Physiol. 2016;311(4):G724–G733.
- Trebicka J, Hennenberg M, Laleman W, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46(1):242–253.
- Milliman Insight. 2020 U.S. organ and tissue transplants: cost estimates, discussion, and emerging issues; 2020 Feb 18 [cited 2020 Dec 14]; Available from: https://www.milliman.com/en/insight/2020-us-organ-and-tissue-transplants
- NHS Blood and Transplant. ANNUAL REPORT ON LIVER TRANSPLANTATION; 2020 Aug [cited 2020 Dec 14; Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/16782/nhsbt-liver-transplantation-annual-report-2018-19.pdf
- Younossi ZM, Stepanova M, Ong J, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2020;S1542-3565(20):30775–30778.
- Hicks DF, Goossens N, Blas-García A, et al. Transcriptome-based repurposing of apigenin as a potential anti-fibrotic agent targeting hepatic stellate cells. Sci Rep. 2017;7(1):42563.
- Deng J, Wei W, Chen Z. Engineered liver-on-a-Chip platform to mimic liver functions and its biomedical applications: a review. Micromachines. 2019;10(10):676.
- Paish HL, Reed LH, Brown H, et al. Technology for modeling fibrosis in human and rodent precision-Cut liver slices. Hepatology. 2019;70(4):1377–1391.
- Harrison SA, Rossi SJ, Paredes AH, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71(4):1198–1212.
- Ratziu V, Sanyal A, Harrison SA, et al., Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology. 2020;72(3):892–905.
- Harrison SA, Dennis A, Fiore MM, et al. Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS One. 2018;13(9):e0203054.
- Jayakumar S, Middleton MS, Lawitz EJ, et al., Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J Hepatol. 2019;70(1):133–141.
- Liu F, Goh G-B-B, Tiniakos D, et al., qFIBS: an automated technique for quantitative evaluation of fibrosis, inflammation, ballooning, and steatosis in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71(6):1953–1966.
- Burton JR Jr, Helmke S, Lauriski S, et al. The within-individual reproducibility of the disease severity index from the HepQuant SHUNT test of liver function and physiology. Transl Res. 2021;S1931-5244(20):30321–30322.
- Qi X, Berzigotti A, Cardenas A, et al. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol. 2018;3(10):708–719.
- Ramachandran P, Iredale JP, Fallowfield JA. Resolution of liver fibrosis: basic mechanisms and clinical relevance. Semin Liver Dis. 2015;35(2):119–131.
- Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–194.
- Nakano Y, Kamiya A, Sumiyoshi H, et al. A deactivation factor of fibrogenic hepatic stellate cells induces regression of liver fibrosis in mice. Hepatology. 2020;71(4):1437–1452.
- Fallowfield JA, Hayden AL, Snowdon VK, et al. Relaxin modulates human and rat hepatic myofibroblast function and ameliorates portal hypertension in vivo. Hepatology. 2014;59(4):1492–1504.
- Fuchs BC, Hoshida Y, Fujii T, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59(4):1577–1590.
- Sato Y, Murase K, Kato J, et al., Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26(4):431–442.
- Oakley F, Meso M, Iredale JP, et al. Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology. 2005;128(1):108–120.
- DeLeve LD, Maretti-Mira AC. Liver sinusoidal endothelial cell: an update. Semin Liver Dis. 2017;37(4):377–387.
- Gracia-Sancho J, Caparrós E, Fernández-Iglesias A, et al. Role of liver sinusoidal endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol. 2021. 10.1038/s41575-020-00411-3.
- Duffield JS, Forbes SJ, Constandinou CM, et al., Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65.
- Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–274.
- Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(46):E3186–95.
- Ramachandran P, Dobie R, Wilson-Kanamori JR, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575(7783):512–518.
- Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67(5):1754–1767.
- Harrison SA, Marri SR, Chalasani N, et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther. 2016;44(11–12):1183–1198.
- Bartneck M, Scheyda KM, Warzecha KT, et al. Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials. 2015;37:367–382.
- Abe H, Kamimura K, Kobayashi Y, et al. Effective prevention of liver fibrosis by liver-targeted hydrodynamic gene delivery of matrix metalloproteinase-13 in a rat liver fibrosis model. Mol Ther Nucleic Acids. 2016;5(1):e276.
- Kallis YN, Robson AJ, Fallowfield JA, et al. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut. 2011;60(4):525–533.
- Nishikawa T, Bell A, Brooks JM, et al. Resetting the transcription factor network reverses terminal chronic hepatic failure. J Clin Invest. 2015;125(4):1533–1544.
- Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922.
- Yuan J, Chen C, Cui J, et al. Fatty liver disease caused by high-Alcohol-Producing klebsiella pneumoniae. Cell Metab. 2019;30(4):675–688.e7.
- Barry-Hamilton V, Spangler R, Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–1017.
- Harrison SA, Abdelmalek MF, Caldwell S, et al., Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155(4):1140–1153.
- Muir AJ, Levy C, Janssen HLA, et al. Simtuzumab for primary sclerosing cholangitis: phase 2 study results with insights on the natural history of the disease. Hepatology. 2019;69(2):684–698.
- Yamamoto E, Dong Y-F, Kataoka K, et al. Olmesartan prevents cardiovascular injury and hepatic steatosis in obesity and diabetes, accompanied by apoptosis signal regulating kinase-1 inhibition. Hypertension. 2008;52(3):573–580.
- Harrison SA, Wong VW-S, Okanoue T, et al., Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73(1):26–39. .
- Gracia-Sancho J, Manicardi N, Ortega-Ribera M, et al. Emricasan ameliorates portal hypertension and liver fibrosis in cirrhotic rats through a hepatocyte-Mediated paracrine mechanism. Hepatol Commun. 2019;3(7):987–1000.
- Harrison SA, Goodman Z, Jabbar A, et al. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol. 2020;72(5):816–827.
- Garcia-Tsao G, Bosch J, Kayali Z, et al. Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J Hepatol. 2020;72(5):885–895.
- Frenette C, Kayali Z, Mena E, et al. Emricasan to prevent new decompensation in patients with NASH-related decompensated cirrhosis. J Hepatol. 2020;S0168-8278(20):33673–33674.
- Traber PG, Chou H, Zomer E, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8(10):e75361.
- Chalasani N, Abdelmalek MF, Garcia-Tsao G, et al., Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158(5):1334–1345.e5.
- Shah RA, Alkhouri N, Kowdley KV. Emerging drugs for the treatment of non-alcoholic steatohepatitis: a focused review of farnesoid X receptor agonists. Expert Opin Emerg Drugs. 2020;25(3):251–260.
- Younossi ZM, Ratziu V, Loomba R, et al., Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–2196. .
- Siddiqui MS, Van Natta ML, Connell MA, et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol. 2020;72(1):25–33.
- Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016;11(6):e0158156.
- Mitchell C, Couton D, Couty J-P, et al. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174(5):1766–1775.
- Lin Z, Tian H, Lam KSL, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17(5):779–789.
- Charles ED, Neuschwander-Tetri BA, Frias JP, et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity. 2019;27(1):41–49.
- Sanyal A, Charles ED, Neuschwander-Tetri BA, et al., Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392(10165):2705–2717.
- Kaufman A, Abuqayyas L, Denney WS, et al., AKR-001, an Fc-FGF21 analog, showed sustained pharmacodynamic effects on insulin sensitivity and lipid metabolism in type 2 diabetes patients. Cell Rep Med. 2020;1(4):100057.
- Zhou M, Learned RM, Rossi SJ, et al. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun. 2017;1(10):1024–1042.
- Hirschfield GM, Chazouillères O, Drenth JP, et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70(3):483–493.
- Mayo MJ, Wigg AJ, Leggett BA, et al. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun. 2018;2(9):1037–1050.
- Harrison SA, Neff G, Guy CD, et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2020;S0016-5085(20):35018.
- Lawitz E, Shevell D, Revankaret R, et al. BMS-986263 (novel targeted lipid nanoparticle delivering HSP47 siRNA) safety and target engagement in patients with advanced hepatic fibrosis: week 36 results from a phase 2 randomised, double-blind, placebo-controlled trial [abstract]. J Hepatol. 2020;73:S123–S400.
- Kluwe J, Pradere J-P, Gwak G-Y, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138(1):347–359.
- Greenberg S, Horan G, Bennett B, et al. Evaluation of the JNK inhibitor, CC-90001, in a phase 1b pulmonary fibrosis trial [abstract]. Eur Respir J. 2017;50:OA474.
- Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol. 2019;10:155.
- Rakipovski G, Rolin B, Nøhr J, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE -/- and LDLr -/- Mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3(6):844–857.
- Newsome PN, Buchholtz K, Cusi K, et al. A placebo-Controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2020;384(12):1113–1124.
- Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–1251.
- Bates J, Vijayakumar A, Ghoshal S, et al. Acetyl-CoA carboxylase inhibition disrupts metabolic reprogramming during hepatic stellate cell activation. J Hepatol. 2020;73(4):896–905.
- Loomba R, Kayali Z, Noureddin M, et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(5):1463–1473.
- Loomba R, Noureddin M, Kowdley KV, et al. Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis due to NASH. Hepatology. 2020;73:S116–S117.
- Schwabl P, Hambruch E, Budas GR, et al. The non-Steroidal FXR agonist cilofexor improves portal hypertension and reduces hepatic fibrosis in a rat NASH model. Biomedicines. 2021;9(1):60.
- Patel K, Harrison SA, Elkhashab M, et al., Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72(1):58–71.
- Kim Y, Natarajan SK, Chung S. Gamma-Tocotrienol ATTENUATES THE HEPATIC INflAMMATION AND FIBROSIS BY SUPPRESSING ENDOPLASMIC RETICULUM STRESS IN MICE. Mol Nutr Food Res. 2018;62(21):1800519.
- Patel V, Rink C, Gordillo GM, et al. Oral tocotrienols are transported to human tissues and delay the progression of the model for end-stage liver disease score in patients. J Nutr. 2012;142(3):513–519.
- Osawa Y, Oboki K, Imamura J, et al. Inhibition of Cyclic Adenosine Monophosphate (cAMP)-response element-binding protein (CREB)-binding protein (CBP)/beta-Catenin reduces liver fibrosis in mice. EBioMedicine. 2015;2(11):1751–1758.
- Nishikawa K, Osawa Y, Kimura K. Wnt/β-Catenin signaling as a potential target for the treatment of liver cirrhosis using antifibrotic drugs. Int J Mol Sci. 2018;19(10):3103.
- Hidvegi T, Ewing M, Hale P, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329(5988):229–232.
- Liang D, Chen H, Zhao L, et al. Inhibition of EGFR attenuates fibrosis and stellate cell activation in diet-induced model of nonalcoholic fatty liver disease. Biochim Biophys Acta Mol Basis Dis. 2018;1864(1):133–142.
- Dufour J-F, Caussy C, Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020;69(10):1877–1884.
- Alkhouri N, Herring RW Jr, Kabler H, et al. Safety and efficacy of combination therapies including semaglutide, cilofexor, and firsocostat in patients with NASH [abstract]. Hepatology. 2020;72(S1):L02A.
- Glass O, Filozof C, Noureddin M, et al. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: recommendations from the liver forum. J Hepatol. 2020;73(3):680–693.
- US Food and Drug Administration (FDA). Nonalcoholic steatohepatitis with compensated cirrhosis: developing drugs for treatment guidance for industry; 2019 June [cited 2020 Dec 14; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/nonalcoholic-steatohepatitis-compensated-cirrhosis-developing-drugs-treatment-guidance-industry
- Anstee QM, Darlay R, Cockell S, et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J Hepatol. 2020;73(3):505–515.
- Boyer-Diaz Z, Aristu-Zabalza P, Andrés-Rozas M, et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol. 2020;S0168-8278(20):33832.
- Francque SM, Bedossa P, Ratziu V, et al. The panPPAR agonist lanifibranor induces both resolution of NASH and regression of fibrosis after 24 weeks of treatment in non-cirrhotic NASH: results of the NATIVE phase 2b trial [abstract]. Hepatology. 2020;72(S1):9A.
- Moroni F, Dwyer BJ, Graham C, et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat Med. 2019;25(10):1560–1565.
