ABSTRACT
Introduction
Malnutrition and sarcopenia are common and impact the prognosis in patients with liver cirrhosis. The etiology is multifactorial and includes periods of reduced caloric intake, increased catabolism and direct molecular mechanisms that inhibit muscle synthesis. Although these conditions are widely acknowledged, and there is a growing interest in their diagnosis, robust evidence regarding the treatment and reversibility of these conditions is still lacking.
Areas covered
We have explored the current evidence on the pharmacological treatment of sarcopenia in patients with cirrhosis. Additionally, we have searched for drugs already in use and ongoing trials for other chronic diseases.
Expert opinion
The current guidelines recommend the use of a protein-adequate diet and moderate physical activity for treating sarcopenia in patients with cirrhosis. Currently, robust evidence is derived only from the supplementation of Branched-Chain Amino Acids, capable of increasing muscle mass and function. There are many drugs targeting various pathways that contribute to sarcopenia. However, evidence is sporadic and insufficient to suggest their use in clinical practice.Novel drugs specifically designed to enhance muscle mass and function should be developed. Finally, gender significantly influences the type of muscle alteration and therapeutic mechanisms; therefore, future studies should be designed taking gender differences into consideration.
1. Introduction
Malnutrition is common in liver cirrhosis. Sarcopenia, defined as a generalized loss of skeletal muscle mass, significantly affects the progression of liver disease, increasing the risk of hospitalizations, liver decompensations, hepatic encephalopathy, bacterial infections, and mortality. A recent meta-analysis, including 22 studies and almost 7000 patients, reported that the presence of sarcopenia reduces 1, 3, and 5-year survival rates in patients with liver cirrhosis [Citation1], although the quality of the studies was not sufficient to provide robust evidence.
Approximately 37% of cirrhotic patients are sarcopenic, and the prevalence progressively increases with the deterioration of liver function. It has also been proposed to include sarcopenia in the MELD score to better predict patients requiring liver transplantation (LT) [Citation2].
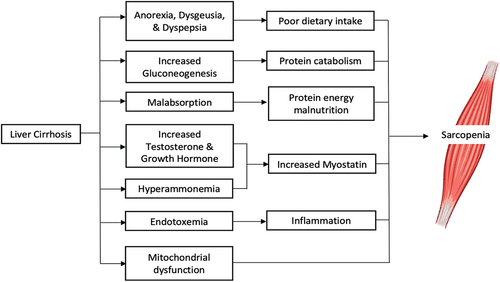
The origin of sarcopenia in chronic liver disease is multifactorial and not completely understood. Liver function deterioration and the presence of portal hypertension and hyperammonemia are undoubtedly main components contributing to sarcopenia. Furthermore, other common aspects present in liver cirrhosis can also impact this relationship, such as anorexia, reduced caloric intake, low protein diets, endocrine abnormalities, increased rate of catabolic events such as ascitic decompensation, infections, gastrointestinal bleeding. The main contributors to muscle depletion in cirrhotic patients are outlined in .
Table 1. Further pharmacological therapies proposed to improve sarcopenia in non-cirrhotic elderly individuals, along with their associated mechanisms of action and potential roles.
In recent decades, the epidemic of overweight and obesity following excessive food intake and unhealthy lifestyles in the overall population has raised a great concern. In this context, the coexistence of sarcopenia and overweight, commonly referred to as sarcopenic obesity, is increasing especially in patients with metabolic or post alcoholic cirrhosis, presenting additional challenges for interventions and therapeutic strategies [Citation3].
Given the significant impact of sarcopenia in cirrhosis, several studies have also explored the role of different pharmacological or non-pharmacological therapies. However, there are currently no defined recommendations regarding therapeutic strategies for sarcopenia in cirrhotic patients. Indeed, many studies are small, retrospective, or showed inconsistent results. In this article, we address the latest knowledge in therapeutic strategies, with a focus on emerging drugs.
2. Current therapy for sarcopenia and malnutrition in liver cirrhosis
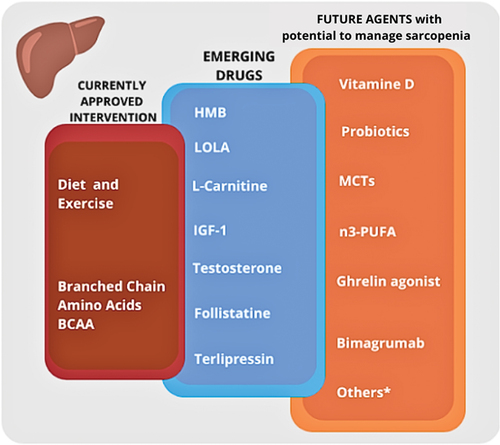
Both EASL (European Society for the Study of Liver Disease) and ESPEN (European Society of Parenteral and Enteral Nutrition) guidelines agree for the need of screening and carefully assessing nutritional status and muscular conditions in patients with liver cirrhosis [Citation4,Citation5]. Fewer evidence is available for the therapeutic strategy to adopt in case of malnutrition and sarcopenia in these patients. The severity of the disease and the frequent underestimation of nutritional problems have prevented clinicians from developing robust therapeutic tools for this condition. The main interventions and drugs in use or promising for nutritional managment of patients with liver disease are summarized in .
Figure 2. Interventions and drugs currently used for treatment of sarcopenia in patients with cirrhosis, and new agents in the study phase or already used in other disease settings.
Dietary and lifestyle approaches can represent a fundamental first step. Considering the frequent catabolic events and the presence of malnutrition, daily energy intake should be at least 35 kcal/kg IBW/day in non-obese cirrhotic patients, with adequate quantity of proteins to manage sarcopenia (1.2–1.5 g/Kg IBW/day). Care should also be taken to avoid prolonged periods of starvation. In cases of severe nutritional depletion, a period of enteral nutrition might be required [Citation4,Citation5]. Taking into account the frequent condition of frailty, cirrhotic patients should also be encouraged to avoid sedentary lifestyle and increase physical activity [Citation6]. This approach is now recommended in cirrhotic patients to improve muscle performance [Citation5] and for the correction of overweight [Citation7].
Among oral nutritional supplements, EASL and ESPEN guidelines have included branched chain amino acids (BCAA – Branched-Chain Amino Acids – leucine, isoleucine and valine) as a potential pharmacological approach in decompensated cirrhosis [Citation8,Citation9]. BCAA are essential amino acids [Citation10] and are fundamental for the synthesis of body proteins and for energy production [Citation11]. Metabolization of BCAA in glutamine allows ammonia detoxification, with potential benefits in hepatic encephalopathy management. In addition, hyperammonemia upregulates myostatin activity, a TGF-B superfamily member that has a key role in proteostasis, resulting in muscle loss [Citation12,Citation13].
Some invasive procedures adopted during the natural history of liver cirrhosis may also impact on a patient’s nutritional status. TIPS placement is a radiological approach to manage portal hypertension related events by reducing the degree of portal hypertension. Several studies have demonstrated the potential role of TIPS placement in improving muscle mass and nutritional parameters [Citation14], even if the mechanisms are not completely understood. The resolution of portal hypertension may play a role by reducing protein-losing enteropathy, managing ascitic decompensation and consequent anorexia, improving pro-inflammatory status, and bacterial translocation reducing anabolic resistance. Other molecular mechanisms might also be involved, but future studies are needed to better understand this association. A recent systematic review compiled the key studies on the topic, demonstrating that overall, the placement of TIPS can improve lean muscle mass, although higher-quality evidence is essential to advance our knowledge in this field [Citation15].
Last but not the least, LT is the only definitive curative intervention for liver cirrhosis. In this setting, the causes leading to malnutrition in cirrhotic patients should also be removed. The presence of sarcopenia seems to be a predictor of higher costs [Citation16], lower survival and adverse outcomes after LT [Citation17]. Differently from what is expected, the available literature failed to clearly demonstrate a generalized reversal of sarcopenia in patients undergoing liver transplantation, while a persistent reduction in muscle mass was reported one year after LT [Citation18] in many patients.
Following the relevance of sarcopenia and malnutrition in the outcome of patients of liver cirrhosis, pharmacological or non-pharmacological tools have been explored for their correction, although the evidence is still limited to studies on animals or a small group of patients. We will delve into emerging studies on this topic in detail in the following paragraphs.
3. Emerging drugs for sarcopenia in liver cirrhosis
A variety of factors, involved as contributing causes of sarcopenia in liver cirrhosis, have been proposed as multiple targets for therapeutic intervention. The available therapies and promising drugs addressing these factors and thus sarcopenia are outlined.
3.1. Ammonia lowering therapies
In cirrhosis, hyperammonemia frequently occurs as a result of the liver urea cycle dysfunction, leading to heightened ammonia uptake by skeletal muscles and increased muscle ammonia concentrations [Citation19,Citation20]. Hyperammonemia causes an activation of myostatin, a negative regulator of muscle protein synthesis, which adversely affects skeletal muscle protein metabolism by hindering muscle protein synthesis and augmenting muscle autophagy [Citation21]. For these reasons, hyperammonemia has been considered a possible target to revert muscle depletion in liver cirrhosis [Citation22].
Lactulose and Rifaximin are the two most common drugs that are used to treat hyperammonemia in patients with liver cirrhosis. There have been no clinical studies showing the relationship between the chronic consumption of these drugs and changes in nutritional status or body composition. However, there are few experimental studies showing potential positive effects of rifaximin in combination with other drugs on sarcopenia in liver cirrhosis.
Rifaximin, in combination with L-ornithine L-aspartate (LOLA) has been shown to improve muscle mass in rats [Citation23]. Further, joint administration of rifaximin with L-carnitine has shown promising results in improving the inhibitory effects of L-carnitine on skeletal muscle wasting in cirrhotic rats with steatohepatitis [Citation24]. The details of these findings will be explored further in the subsequent paragraphs.
3.1.1. Lola
LOLA, a pharmaceutical nutrient which is composed of two non-essential amino acids- ornithine and aspartate, is an ammonia lowering agent which has been shown to improve both minimal and overt hepatic encephalopathy [Citation25].
A study delved into the impact of ammonia-lowering therapies on sarcopenia in a hyperammonemia animal model [Citation23]. The treatment group received a combination of rifaximin and LOLA orally and was compared to pair-fed controls. Rats subjected to the treatment exhibited a significant increase in lean body mass, forelimb grip strength, muscle mass, muscle fiber diameter, and type II muscle fiber compared to the control group (p < 0.001). This improvement was attributed to a reduction in intramuscular myostatin and pro-inflammatory cytokine levels. The authors report that the restoration of proteostasis leads to the reversal of sarcopenia. However, the use of LOLA or Rifaximin treatment alone were not investigated.
A study in patients with compensated liver cirrhosis and minimal HE examined oral administration of LOLA as a therapy for sarcopenia. A total of 34 patients were randomized into two groups: treatment group receiving oral LOLA at a dosage of 6 grams three times a day and control group receiving placebo for a period of 12 weeks. In the LOLA group, the average increase in biceps skinfold thickness was 1.5 mm, while the placebo group exhibited a mean decrease of 1.0 mm (p = 0.05). However, the study, which was only available in abstract form, could not find any difference in muscle performance (hand grip strength and 6-min walk test) between the two groups [Citation26].
3.1.2. L-Carnitine
L-Carnitine is a naturally occurring compound that plays a crucial role in the production of energy by transporting long chain fatty acids into mitochondria. It is synthesized in the body from amino acids lysine and methionine. It is found in various foods, particularly in meat and dairy products. In liver cirrhosis, L-carnitine has been studied for its potential role in reducing ammonia levels and improving symptoms of hepatic encephalopathy.
A study investigated the impact of combined rifaximin (100 mg/kg) and L-carnitine (200 mg/kg) treatment on skeletal muscle atrophy in cirrhotic rats with steatohepatitis. In a large cohort of Fischer rats fed a choline-deficient L-amino acid-defined (CDAA) diet, the 12-week intervention demonstrated significant outcomes. Notably the combined treatment prevented skeletal muscle mass atrophy and weakness by reducing intramuscular myostatin and pro-inflammatory cytokine levels [Citation24].
Ohara et al. conducted a retrospective study to evaluate psoas muscle index changes (measured by CT scans 6 months apart) among 35 cirrhotic patients who received L-carnitine supplementation for more than 6 months compared to a control group of 35 matched cirrhotics. The average L‐carnitine dose during the observational period was 1,018 mg/day. Loss of skeletal muscle mass was significantly prevented in patients receiving L‐carnitine supplementation as compared to the control group [Citation27]. Another study retrospectively assessed 52 patients with cirrhosis who were treated with L-carnitine for more than 3 months and studied the impact of carnitine on skeletal muscle index (SMI) measured by computed tomography at L3. Patients treated with higher doses of L-carnitine (>1274 mg/day) showed a higher increase in muscle mass compared to patients treated with lower doses (OR, 3.568; 95% CI, 1.138‐11.185; p = 0.027) [Citation28]. A further study evaluated the effect of exogenous L-carnitine on physical activity in patients with liver cirrhosis and a diagnosis of mild (HE1) or moderate (HE2) hepatic encephalopathy. Thirty-one patients with HE1 and 30 with HE2 received 2 g of acetyl-L-carnitine daily, while 30 patients with HE1 and 30 patients with HE2 received a placebo twice daily for a period of 3 months. Both HE1 and HE2 patients treated with L-carnitine supplementation showed a significant improvement in parameters of muscle function vs the control group [Citation29].
A recent meta-analysis including 5 studies and 334 individuals (cirrhotics and control) revealed however no significant effect of carnitine supplementation on body mass index, and/or body weight [Citation30].
At present, the results are controversial and future clinical trials are certainly needed to fully understand the potential of L-carnitine in the cirrhotic population. As a consequence, there are no clear dosage indications for administering L-carnitine in patients with cirrhosis in order to achieve improvement in body composition.
3.2. Hormonal therapies
Cirrhosis is linked to various endocrine abnormalities, such as reduced testosterone levels resulting from heightened aromatase activity [Citation31]. In cases where ethanol is the cause of cirrhosis, it directly impacts testicular function. Additionally, sarcopenia in cirrhosis is exacerbated by diminished concentrations and responsiveness of growth hormone in muscles [Citation32]. Both growth hormone and testosterone play roles in inhibiting myostatin expression and signaling responses, and their deficiency in cirrhosis is believed to contribute to impaired protein synthesis and increased myostatin expression [Citation33]. Testosterone therapy and Insulin-like growth factor-1 administration may therefore have the potential to ameliorate sarcopenia in cirrhotic patients.
3.2.1. Testosterone
Testosterone is an anabolic hormone that affects muscle growth, hematopoiesis, bone density, and metabolism. Serum testosterone levels are reduced in up to 90% of male patients with liver cirrhosis [Citation34].
Sinclair et al. conducted a study in 101 men with cirrhosis and low testosterone levels. At 12 months, intramuscular testosterone undecanoate significantly increased appendicular lean mass (p = 0.021) and total lean mass (p = 0.008), while reducing fat mass (p < 0.001) as evaluated by dual energy X-ray absorptiometry. Additionally, improvements were observed in bone mass and density [Citation35]. In another study involving 16 hypogonadal male cirrhotic patients, testogel 50 mg/day was administered for 6 months. Testosterone supplementation significantly increased muscle strength (p < 0.001) as assessed by hand grip dynamometer [Citation36].
Generally, testosterone therapy is not extensively used as a caution to the possible increased risk of hepatocellular carcinoma [Citation37]. Testosterone supplementation, based on a previous study [Citation38], was however suggested in cirrhotic males with hemochromatosis and hypogonadism [Citation4].
3.2.2. IGF-1
IGF-1 (Insulin-like Growth Factors-1) is another anabolic hormone whose synthesis is under the regulation of human growth hormone. IGF-1 deficiency in patients with liver cirrhosis has been closely linked to notable consequences such as a decline in muscle mass, diminished muscle strength, and impaired physical function [Citation39]. This deficiency operates through several mechanisms, including the inactivation of the mTOR pathway, augmentation of proteolysis, and the suppression of satellite cell proliferation [Citation40].
In a randomized, double-blind, placebo-controlled clinical trial, subcutaneous administration of IGF-I for 4 months was evaluated in patients with cirrhosis and subnormal IGF-I levels. The treatment group exhibited a tendency to enhance resting energy expenditure, particularly significant in alcoholic patients. While no significant differences were observed in body composition or muscle metrics, IGF-I therapy notably increased serum albumin [Citation41].
3.3. Myostatin antagonist
Myostatin is an inhibitor of protein synthesis and activates the ubiquitin proteasome and autophagy mediated proteolysis [Citation42]. As already mentioned, in cirrhosis there is impaired protein synthesis and increased myostatin expression [Citation43]. Hence, pharmaceutical drugs acting as myostatin antagonists could potentially contribute to improvement of sarcopenia.
3.3.1. Follistatin
Follistatin is a glycoprotein that plays a crucial role in regulating muscle growth. It functions by inhibiting the activity of myostatin. Myostatin is often referred to as a negative regulator of muscle mass because it limits the size and growth of muscles [Citation21].
Administration of follistatin in rats resulted in increased whole body weight, lean body mass, muscle weight, and grip strength [Citation44] Another study showed increase in body weight and skeletal muscle mass in mice administered with an engineered follistatin molecule (FST315-HBS-Fc) [Citation45].
In patients with end stage liver cirrhosis with transjugular intrahepatic portosystemic shunt (TIPS), follistatin blood levels were evaluated. Sarcopenia was assessed prior and after TIPS placement using fat free muscle area (FFMA) estimated from MRI. Follistatin was measured in a small cohort of 12 patients and exhibited a strong correlation with FFMA [Citation46]. In another study, infusion of glucagon and somatostatin, simulating the effects of exercise in 8 liver cirrhosis (LC) patients, led to an initial rise in follistatin levels. However, these levels rapidly declined in cirrhotic individuals compared to the healthy controls [Citation47].
Conflicting findings exist regarding the real involvement of follistatin in sarcopenia among patients with LC. In the study conducted by Boga et al., examining various myokines in LC, follistatin levels demonstrated no significant differences between sarcopenic and non-sarcopenic LC patients. Furthermore, follistatin levels did not vary significantly between patients and control subjects, nor did they show distinctions between early and late stages of LC [Citation48].
While there is theoretical promise, the translation of follistatin-based therapies into clinical practice is still in its early stages.
3.4. Therapies for portal hypertension
Portal hypertension, arising from elevated hepatic resistance and splanchnic hyperperfusion, plays a pivotal role in inducing severe complications in cirrhosis, including ascites, gastro-esophageal varices, hepatic encephalopathy due to portosystemic shunting, and hepatorenal syndrome. Portal hypertension contributes to impaired digestion and nutrient malabsorption. Studies indicate that managing portal vein pressure, either through TIPS placement or medication, has the potential to enhance the nutritional status of patients with cirrhosis [Citation49]. Additionally, it has been observed that sarcopenia can arise also in individuals without cirrhosis as in non-cirrhotic portal hypertension [Citation50]. This underscores the broader impact of portal hypertension on muscle health beyond its association with liver cirrhosis. Lastly, also portosystemic collaterals, often present in patients with portal hypertension, are responsible for shunting into the systemic circulation a variable proportions of portal-venous blood which escapes hepatic metabolism. In this way ammonia escapes hepatic metabolism and its disposal by ureagenesis, potentially contributing to sarcopenia [Citation51].
3.4.1. Terlipressin
A small study suggested that terlipressin may improve the nutritional status of patients with cirrhosis.
Terlipressin is an analog of vasopressin and is used for the treatment of bleeding from esophageal varices or hepatorenal syndrome. In patients with cirrhosis and portal hypertension, treatment with terlipressin increases mean arterial pressure and decreases portal flow and pressure.
In a prospective study, 19 cirrhotic patients awaiting liver transplant, 12 having hepatorenal syndrome and 7 refractory ascites, were treated with terlipressin therapy. All patients were initially malnourished as assessed by subjective global assessment and had a low hand grip strength. Dietary intake i.e. energy and protein consumption increased significantly after terlipressin treatment (17.94 ± 5.43 kcal/kg v/s 27.70 ± 7.48 kcal/kg, and 0.74 ± 0.28 g/kg v/s 1.16 ± 0.31 g/kg; p < 0.001). In addition, muscle strength also improved (25.36 ± 8.13 kg to 28.49 ± 7.63 kg; p = 0.001) and the degree of malnutrition was reduced in these patients [Citation52]. Unfortunately the study has many confounders and a control group is lacking.
3.5. Nutritional supplements
Cirrhosis represents a state of accelerated starvation. During an overnight fast, individuals with cirrhosis experience a gradual shift to utilizing fatty acids as the primary substrate for oxidation, reflected in a reduced respiratory quotient (RQ). While many tissues can adapt to fatty acid oxidation as energy supply, certain tissues necessitate glucose, thereby escalating the demand for gluconeogenesis. Given that fatty acids cannot directly contribute to gluconeogenesis, amino acids become the primary source for this process. Consequently, frequent fasting leads to a progressive loss of protein. Additionally, cirrhotic patients commonly exhibit poor oral intake due to factors such as decreased appetite linked to elevated circulating inflammatory cytokines, alcohol-induced anorexia, early satiety attributable to clinically evident ascites, and abdominal discomfort, nausea, and bloating.
3.5.1. Beta-hydroxy-beta-methylbutyrate (HMB)
Beta‐hydroxy‐beta‐methylbutyrate (HMB) is a naturally occurring metabolite of leucine that has been reported to have anabolic effects on protein metabolism. HMB is often used as a dietary supplement, particularly in the realm of sports and fitness, due to its potential effects on muscle.
HMB potentially enhances protein synthesis by activating the mTOR pathway and promoting the production of IGF-1. Simultaneously, it may mitigate proteolysis by inhibiting the ubiquitin-proteasome system. Moreover, HMB can stimulate the proliferation of satellite cells through the activation of myogenic refractory factors [Citation53].
In a 12-week pilot randomized single blind trial, 24 patients with liver cirrhosis were treated with either HMB or placebo supplementation. Fourteen patients in the intervention group received 3 g/day of HMB and showed significant improvements: chair stand test reduced from 14.2 ± 5s to 11.7 ± 2.6s (p < 0.05); 6-minute walk test from 361.8 ± 68 m to 409.4 ± 58 m (p < 0.05), and quadriceps muscle mass measured by ultrasound increased from 4.9 ± 1.8 mm to 5.4 ± 1.8 mm (p < 0.05) [Citation54]. In another randomized trial involving, 43 cirrhotic patients with malnutrition were randomized to receive either a HMB-enriched (1.5 g of calcium HMB twice a day) or a HMB-devoid supplement for 12 weeks. Both groups showed an increase in fat mass index (p = 0.014) measured by BIA. However, only the HMB group demonstrated an upward trend in handgrip strength (p = 0.095) [Citation55].
While some studies suggest that HMB supplementation may have benefits in improving muscle mass and function, the safety and effectiveness of HMB in cirrhotic patients need further investigation.
4. Future directions and ongoing studies in sarcopenic patients
Sarcopenia and malnutrition are intriguing research topics, given their negative prognostic role in the context of both neoplastic and non-neoplastic chronic diseases and the aim of improving survival rates. For these compelling reasons, research is flourishing on this topic in different diseases.
When searching for ‘clinical trials,’ ‘randomized clinical trials’ and ‘sarcopenia’ on electronic databases (PubMed), over the last 5 years, 432 results are identified. After excluding trials focusing on exercise, dietary intake, supplementation with HMB and leucine (which were previously discussed) or non-pharmacological therapies, 21 articles can be found.
Vitamin D is one of the most studied elements, thanks to its ability to improve muscle protein synthesis and strength [Citation56]. Vitamin D is able to enhance cell proliferation and differentiation, has anti-inflammatory properties [Citation57], and is involved in neuromodulation [Citation58]. These pleiotropic properties are linked to the regulation of the vitamin D receptor (VDR) [Citation59], which is a member of the nuclear receptor family of transcription factors, strongly expressed in skeletal muscle and in other tissues. Low vitamin D levels are associated with higher risk of developing sarcopenia, both in elderly [Citation60] and in cirrhotic patients [Citation61]. Lots of studies have explored the role of supplementation of vitamin D in different settings: in association with whey protein and vitamin E [Citation62], whey protein and leucine in elderly [Citation63] and BCAA in cirrhotic patients [Citation64]. All these studies have shown significant results in terms of improvement in muscle mass, strength and balance. The study carried out in cirrhotic patients demonstrated a significant improvement in skeletal muscle index (SMI) (p = 0.002) and prevalence of sarcopenia (p = 0.02) after 12 months of vitamin D supplementation.
Ghrelin, an amino peptide, is acknowledged as a versatile hormone with the ability to stimulate food consumption, facilitate weight gain, and enhance gastric emptying. While there is a lack of current research investigating the impact of ghrelin on body composition in liver cirrhotic patients, studies conducted in non-cirrhotic populations have explored the positive effects of ghrelin administration. In an open-label pilot study, ghrelin was intravenously administered to seven cachectic patients with chronic obstructive pulmonary disease (COPD) for three weeks. The treatment led to a significant increase in body weight, food intake, lean body mass, and muscle strength [Citation65]. RC-1291, a novel oral ghrelin mimetic and growth hormone secretagogue, demonstrated significant dose-related weight gain in a randomized, double-blind, placebo-controlled phase 1 study involving healthy volunteers [Citation66].
Many diseases find a fundamental and promising etiopathogenic role in intestinal dysbiosis and increased bacterial translocation [Citation67]. From this perspective, probiotics improve bacterial diversity, normalize intestinal permeability and reduce the translocation of inflammatory molecules [Citation67]. An RCT has demonstrated improvement in handgrip test performance and gait speed in patients with chronic heart failure treated with multistrain probiotics for 12 weeks, compared to the placebo group. In addition, probiotics supplementation was able to reduce gut permeability (measured through zonulin determination). Similar results were obtained in another RCT in patients with COPD [Citation68] or, more recently, in a cohort of patients with prolonged hospitalizations due to COVID-19 infection [Citation69]. The relationship between increased bacterial translocation and dysbiosis with sarcopenia, liver decompensations and progression in cirrhosis, has adequate evidence [Citation70]. Less is known about the nutritional effects of therapeutic strategies based on probiotics in cirrhotic patients. A recent RCT has demonstrated a role in improving nutritional parameters in patients with cirrhosis treated with multistrain probiotics for six weeks compared to a placebo group [Citation71]. The authors also reported an improvement in muscle strength and triceps skin fold thickness in patients treated with probiotics, as well as better results in subjective global nutritional assessment scores. In another RCT, a special medical-purpose food composed of a probiotic (Lactobacillus paracasei PS23) with leucine and omega-3 fatty acids, supplemented for 2 months in sarcopenic elderly patients, was able to induce an improvement in handgrip strength, appendicular lean mass and in the short physical performance battery score, compared to the placebo group [Citation72].
Nutritional integration of medium chain fatty acids (MCTs) is promising in elderly sarcopenic individuals, given their ability to increase energy intake in patients with decreased appetite [Citation73]. The addition of different amounts of octanoic and decanoic acid to an exercise program in healthy and sedentary people with a low body mass index demonstrated better results in knee extension strength and grip strength after 12 weeks of treatment compared to the placebo group [Citation74]. Another randomized single blinded, parallel group intervention trial, demonstrated an increase in muscle mass, muscle function and cognition in frail elderly [Citation75]. Also, long-chain omega-3 polyunsaturated fatty acids (n3-PUFA) have shown encouraging results in improving the nutritional status of elderly individuals. This was attributed to their anti-inflammatory properties, which mitigate the impact of inflammation on proper anabolic signaling in skeletal muscle [Citation76–78].
Bimagrumab is a fully human monoclonal antibody that blocks both receptor subtypes, ActRIIA and ActRIIB, thereby promoting the differentiation of myoblasts [Citation79]. A double-blind, placebo-controlled, multicenter, phase II randomized clinical trial has explored the additive role of Bimagrumab with personalized exercise program, diet counseling, an oral nutritional supplement, and vitamin D in a cohort of 180 elderly with sarcopenia [Citation80]. The authors have demonstrated an improvement in lean muscle mass, as evaluated with DXA; however, there is no additive effect observed on SPPB and physical tests in patients treated with Bimagrumab.
Other therapeutic interventions tested to improve nutritional status are reported in .
The great interest in the field can also be inferred from the analysis of ongoing studies registered at ClinicalTrials.gov. Searching for ‘sarcopenia’ in adults or elderly, 102 registered researchers in different study phases (from 1 to 4) were identified. Of these, 9 were focused on liver cirrhosis and 4 concerned pharmacological therapies. Two of these studies analyzed the effect of oral BCAA and one the efficacy of growth hormone supplementation. An oral prodrug of bioidentical testosterone (LPCN 1148) is under study in a multicenter, double-blind, placebo-controlled study (NCT04874350) for the assessment of the efficacy, safety, and tolerability of this product in sarcopenic patients with liver cirrhosis on the liver transplant waitlist.
Moreover, there is distinct prevalence in muscle alterations, with myosteatosis prevailing over sarcopenia in female cirrhotics as opposed to males [Citation88]. Similarly, the physiopathological mechanisms between the two genders could differ in certain aspects. It is reasonable to assume that the efficacy of therapeutic interventions may differ between genders, and remarkably, this aspect has not been thoroughly explored in prior studies. Consequently, it is crucial for future studies to take into account the influence of gender differences on these aspects.
The evidence from the literature on pharmacological therapy in non-cirrhotic sarcopenic patients can assist specialists in identifying additional therapeutic strategies in liver disease, where current treatment options are limited, poorly established, and infrequently utilized in the field.
5. Conclusion
Malnutrition and sarcopenia pose significant challenges in the management of liver cirrhosis, impacting patient outcomes and complicating therapeutic strategies. The prevalence of sarcopenia in cirrhotic patients is substantial, with a clear association with liver function deterioration, portal hypertension, and hyperammonemia. The coexistence of sarcopenia with overweight, known as sarcopenic obesity, further complicates interventions.
While LT remains the only curative intervention for cirrhosis, the presence of sarcopenia is associated with worse outcomes post-LT, challenging the notion of sustained improvement in muscle mass. TIPS placement emerges as an alternative with potential benefits in improving muscle mass and nutritional parameters, though further research is needed to elucidate the underlying mechanisms.
The current therapeutic landscape involves dietary and lifestyle interventions, with BCAA being the only pharmacological approach recommended in decompensated cirrhosis.
Emerging drugs and therapies show promise, with studies exploring LOLA, L-carnitine, testosterone, IGF-1, HMB, follistatin, and terlipressin.
Follistatin, which inhibits myostatin and promotes muscle growth, has shown positive outcomes in animal studies but awaits further translation into clinical practice. LOLA, known for ammonia-lowering properties, demonstrated mixed results in human studies, highlighting the need for more comprehensive investigations. L-carnitine, testosterone, IGF-1, and terlipressin, present varied potential in improving muscle mass, strength, and overall nutritional status, though larger and more rigorous trials are warranted.
Ongoing research is exploring additional avenues, with a particular focus on vitamin D supplementation, showing promise in enhancing muscle protein synthesis and strength. The multifaceted nature of vitamin D, influencing cell proliferation, inflammation, and neuromodulation, underscores its potential as a therapeutic target for sarcopenia in diverse clinical settings. Ghrelin, probiotics, and fatty acids are also currently under investigation across various patient groups, demonstrating promising potential as interventions for addressing sarcopenia. These areas of investigation aim to enhance our understanding of nutritional interventions and pharmacological strategies, providing new insights into the complex interplay between nutrition, muscle health, and liver cirrhosis.
In conclusion, the evolving landscape of therapeutic strategies for sarcopenia in liver cirrhosis reflects the complexity of the condition. While advancements in understanding etiopathogenesis and exploring novel drugs offer hope, the field necessitates further robust clinical trials and evidence-based recommendations to guide clinicians in optimizing care for cirrhotic patients with sarcopenia.
6. Expert opinion
Sarcopenia is a common complication in patients with liver cirrhosis, associated with a range of clinical issues. Pharmacological therapy is an evolving field addressing this challenge, but there are still many avenues of research to explore.
To date, research on pharmacological therapy for sarcopenia in cirrhotic patients is still in its early stages; however, a number of new proposals are in the pipeline and some clinical trials are ongoing. Studies will require adequate sample sizes and suitable methodologies to drive definitive conclusions. Still, the mechanisms inducing sarcopenia in liver cirrhosis are not completely clarified, complicating the search for the best target for therapies. Indeed, in some clinical settings, chronic inflammation and elevated cytokines levels may be relevant triggers for sarcopenia, while in other patients, the axis liver-ammonia-myostatin-muscle tissue may prevail.
The potential of this research is significant, as effectively treating sarcopenia could greatly improve the prognosis and clinical outcome for patients with cirrhosis.
Future research should focus on identifying early biomarkers of sarcopenia in cirrhotic patients and on evaluating pharmacological therapies by monitoring these markers. Additionally, it is essential to explore the interactions between sarcopenia and other complications of liver cirrhosis, such as hepatic encephalopathy, to develop a holistic approach to treatment.
The greatest challenge in this endeavor is the complexity of the physiological and pathological context of liver cirrhosis. Patient heterogeneity and variability in disease severity require personalized approaches to therapy. Research must address this task by developing therapies that can be tailored to the specific needs of each patient, taking into account associated comorbidities.
In the coming years, there is expected to be increased attention on sarcopenia research in liver cirrhosis, with the development of more targeted therapies and personalized management strategies. Advanced technologies, such as artificial intelligence, may play a crucial role in analyzing complex data and identifying specific patterns of sarcopenia in cirrhotic patients.
An area of particular current interest could involve exploring combined therapies, incorporating both pharmacological approaches and non-pharmacological interventions such as targeted exercise, personalized nutrition, and TIPS placement. This integrated approach might yield more robust results in improving sarcopenia and muscle function in patients with liver cirrhosis.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/14728214.2024.2342646).
Additional information
Funding
References
- Tantai X, Liu Y, Yeo YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022 Mar;76(3):588–599.
- Lattanzi B, Nardelli S, Pigliacelli A, et al. The additive value of sarcopenia, myosteatosis and hepatic encephalopathy in the predictivity of model for end-stage liver disease. Dig Liver Dis. 2019 Nov;51(11):1508–1512.
- Bischoff SC, Barazzoni R, Busetto L, et al. European guideline on obesity care in patients with gastrointestinal and liver diseases - joint European society for clinical nutrition and metabolism/united European gastroenterology guideline. United Eur Gastroenterol J. 2022 Sep;10(7):663–720.
- EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol. 2018. doi: 10.1016/j.jhep.2018.06.024
- Plauth M, Bernal W, Dasarathy S, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38(2):485–521.
- Williams FR, Berzigotti A, Lord JM, Lai JC, Armstrong MJ. Review article: impact of exercise on physical frailty in patients with chronic liver disease. Aliment Pharmacol Ther. 2019 Nov;50(9):988–1000. doi: 10.1111/apt.15491. Epub 2019 Sep 9. PMID: 31502264.
- Bischoff SC, Ockenga J, Eshraghian A, et al. Practical guideline on obesity care in patients with gastrointestinal and liver diseases - joint ESPEN/UEG guideline. Clin Nutr. 2023 Jun;42(6):987–1024. Epub 2023 Apr 10. doi: 10.1016/j.clnu.2023.03.021 PMID: 37146466.
- Nakaya Y, Harada N, Kakui S, et al. Severe catabolic state after prolonged fasting in cirrhotic patients: effect of oral branched-chain amino-acid-enriched nutrient mixture. J Gastroenterol. 2002;37(7):531–536. doi: 10.1007/s005350200082
- Yoshida T, Muto Y, Moriwaki H, Yamato M. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol Jpn. 1989 Dec;24(6):692–698. doi: 10.1007/BF02774169
- Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013 Oct;28(5):580–588. doi: 10.1177/0884533613496432
- Paul HS, Adibi SA. Activation of hepatic branched chain alpha-keto acid dehydrogenase by a skeletal muscle factor. J Biol Chem. 1982 Nov 10;257(21):12581–12588. doi: 10.1016/S0021-9258(18)33550-6
- Di Cola S, Nardelli S, Ridola L, et al. Ammonia and the muscle: an emerging point of view on hepatic encephalopathy. J Clin Med. 2022;11(3):611. doi: 10.3390/jcm11030611
- Dasarathy S. Myostatin and beyond in cirrhosis: all roads lead to sarcopenia. J Cachexia Sarcopenia Muscle. 2017 Dec;8(6):864–869. doi: 10.1002/jcsm.12262
- de Felice I, Ridola L, Riggio O, et al. Transjugular intrahepatic portosystemic shunt placement: effects on nutritional status in cirrhotic patients. J Clin Med. 2023;12(22):7029. doi: 10.3390/jcm12227029
- Gazda J, Di Cola S, Lapenna L, et al. The impact of transjugular intrahepatic portosystemic shunt on nutrition in liver cirrhosis patients: a systematic review. Nutrients. 2023 Mar 27;15(7):1617. doi: 10.3390/nu15071617
- van Vugt JL, Levolger S, de Bruin RW, et al. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016 Aug;16(8):2277–2292. doi: 10.1111/ajt.13732
- Zhou D, Zhang D, Zeng C, et al. Impact of sarcopenia on the survival of patients undergoing liver transplantation for decompensated liver cirrhosis. J Cachexia Sarcopenia Muscle. 2023 Dec;14(6):2602–2612.
- Brown S, Richardson B, Bouquet E, et al. Cirrhosis-related sarcopenia may not resolve after liver transplantation. JHEP Rep. 2023 Nov;5(11). doi: 10.1016/j.jhepr.2023.100881
- Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009 Mar;24(1):169–181. doi: 10.1007/s11011-008-9122-5
- Jindal A, Jagdish RK. Sarcopenia: ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. 2019 Sep;25(3):270–279. doi: 10.3350/cmh.2019.0015
- Dasarathy S, Hatzoglou M. Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care. 2018 Jan;21(1):30–36. doi: 10.1097/MCO.0000000000000426
- Ebadi M, Burra P, Zanetto A, Montano-Loza AJ. Current treatment strategies and future possibilities for sarcopenia in cirrhosis. J Hepatol. 2023 May;78(5):889–892. doi: 10.1016/j.jhep.2023.01.031
- Kumar A, Davuluri G, Silva RNE, et al. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology. 2017 Jun;65(6):2045–2058. doi: 10.1002/hep.29107
- Murata K, Kaji K, Nishimura N, et al. Rifaximin enhances the L‑carnitine‑mediated preventive effects on skeletal muscle atrophy in cirrhotic rats by modulating the gut‑liver‑muscle axis. Int J Mol Med. 2022 Aug;50(2):101. doi: 10.3892/ijmm.2022.5157
- Butterworth RF, Kircheis G, Hilger N, et al. Efficacy of l-ornithine l-aspartate for the treatment of hepatic encephalopathy and hyperammonemia in cirrhosis: systematic review and meta-analysis of randomized controlled trials. J Clin Exp Hepatol. 2018 Sep;8(3):301–313. doi: 10.1016/j.jceh.2018.05.004
- Pasha Y, Taylor-Robinson S, Leech R, et al. PWE-091 L-ornithine L-aspartate in minimal hepatic encephalopathy: possible effects on the brain-muscle axis? Gut. 2018;67:A117–A118. doi: 10.1136/gutjnl-2018-BSGAbstracts.233
- Ohara M, Ogawa K, Suda G, et al. L-Carnitine suppresses loss of skeletal muscle mass in patients with liver cirrhosis. Hepatol Commun. 2018 Aug 6;2(8):906–918. doi: 10.1002/hep4.1207
- Hiramatsu A, Aikata H, Uchikawa S, et al. Levocarnitine use is associated with improvement in Sarcopenia in patients with liver cirrhosis. Hepatol Commun. 2019 Jan 22;3(3):348–355. doi: 10.1002/hep4.1309
- Michele M, Marco V, Maria G, et al. Oral acetyl-l-carnitine therapy reduces fatigue in overt hepatic encephalopathy: a randomized, double-blind, placebo-controlled study1,2,3. Am J Clin Nutr. 2011 Apr;93(4):799–808. doi: 10.3945/ajcn.110.007393
- Mohammad A, Mohd-Yusof BN, Hanipah ZN, et al. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2020 Jan; 48;48:102273. doi: 10.1016/j.ctim.2019.102273
- Dasarathy S, Mullen KD, Dodig M, et al. Inhibition of aromatase improves nutritional status following portacaval anastomosis in male rats. J Hepatol. 2006 Aug;45(2):214–20.
- Møller S, Becker U, Grønbaek M, et al. Short-term effect of recombinant human growth hormone in patients with alcoholic cirrhosis. J Hepatol. 1994 Nov;21(5):710–717.
- Liu W, Thomas SG, Asa SL, Gonzalez-Cadavid N, Bhasin S, Ezzat S. Myostatin is a skeletal muscle target of growth hormone anabolic action. J Clin Endocrinol Metab. 2003 Nov;88(11):5490–6. doi: 10.1210/jc.2003-030497
- Sinclair M, Grossmann M, Gow PJ, Angus PW. Testosterone in men with advanced liver disease: abnormalities and implications. J Gastroenterol Hepatol. 2015 Feb;30(2):244–51. doi: 10.1111/jgh.12695
- Sinclair M, Grossmann M, Hoermann R, et al. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016 Nov;65(5):906–913.
- Yurci A, Yucesoy M, Unluhizarci K, et al. Effects of testosterone gel treatment in hypogonadal men with liver cirrhosis. Clin Res Hepatol Gastroenterol. 2011 Dec;35(12):845–54.
- Nagasue N, Yukaya H, Chang YC, et al. Active uptake of testosterone by androgen receptors of hepatocellular carcinoma in humans. Cancer. 1986 Jun 1;57(11):2162–2167. doi: 10.1002/1097-0142(19860601)57:11<2162:aid-cncr2820571114>3.0.co;2-6
- Diamond T, Stiel D, Posen S. Effects of testosterone and venesection on spinal and peripheral bone mineral in six hypogonadal men with hemochromatosis. J Bone Miner Res. 1991 Jan;6(1):39–43. doi: 10.1002/jbmr.5650060108
- de la Garza RG, Morales-Garza LA, Martin-Estal I, et al. Insulin-like growth factor-1 deficiency and cirrhosis establishment. J Clin Med Res. 2017 Apr;9(4):233–247. doi: 10.14740/jocmr2761w
- Geladari E, Alexopoulos T, Kontogianni MD, et al. Mechanisms of sarcopenia in liver cirrhosis and the role of myokines. Ann Gastroenterol. 2023 Jul;36(4):392–404.
- Naseer M, Turse EP, Syed A, et al. Interventions to improve sarcopenia in cirrhosis: a systematic review. World J Clin Cases. 2019 Jan 26;7(2):156–170. doi: 10.12998/wjcc.v7.i2.156
- Han HQ, Zhou X, Mitch WE, Goldberg AL. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013 Oct;45(10):2333–47. doi: 10.1016/j.biocel.2013.05.019
- Anand AC. Nutrition and muscle in cirrhosis. J Clin Exp Hepatol. 2017 Dec;7(4):340–357. doi: 10.1016/j.jceh.2017.11.001
- Dasarathy S, McCullough AJ, Muc S, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011 May;54(5):915–21.
- Yaden BC, Croy JE, Wang Y, et al. Follistatin: a novel therapeutic for the improvement of muscle regeneration. J Pharmacol Exp Ther. 2014 May;349(2):355–71. doi: 10.1124/jpet.113.211169
- Praktiknjo M, Book M, Luetkens J, et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology. 2018 Mar;67(3):1014–1026. doi: 10.1002/hep.29602
- Rinnov AR, Plomgaard P, Pedersen BK, Gluud LL. Impaired follistatin secretion in cirrhosis. J Clin Endocrinol Metab. 2016 Sep;101(9):3395–400. doi: 10.1210/jc.2016-1923
- Boga S, Yildirim AE, Ucbilek E, et al. The effect of sarcopenia and serum myokines on prognosis and survival in cirrhotic patients: a multicenter cross-sectional study. Eur J Gastroenterol Hepatol. 2022 Dec 1;34(12):1261–1268. doi: 10.1097/MEG.0000000000002461
- Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol. 2008 Apr;23(4):527–33. doi: 10.1111/j.1440-1746.2008.05369.x
- Lattanzi B, Gioia S, Di Cola S, et al. Prevalence and impact of sarcopenia in non-cirrhotic portal hypertension. Liver Int. 2019 Jun;39(10):1937–1942.
- ) Shangraw RE, Jahoor F Effect of liver disease and transplantation on urea synthesis in humans: relationship to acid-base status. Am J Physiol. 1999;276:G1145–G1152. 5. doi: 10.1152/ajpgi.1999.276.5.G1145
- Chapman B, Gow P, Sinclair M, et al. Continuous terlipressin infusion is associated with improved diet intake and muscle strength in patients awaiting liver transplant. JHEP Rep. 2019 May 17;1(2):107–113. doi: 10.1016/j.jhepr.2019.05.002
- Lattanzi B, Giusto M, Albanese C, et al. The effect of 12 weeks of β-hydroxy-β-methyl-butyrate supplementation after liver transplantation: a Pilot randomized controlled study. Nutrients. 2019 Sep 19;11(9):2259. doi: 10.3390/nu11092259
- Lattanzi B, Bruni A, Di Cola S, et al. The effects of 12-week beta-hydroxy-beta-methylbutyrate supplementation in patients with liver cirrhosis: results from a randomized controlled single-blind pilot study. Nutrients. 2021 Jul;13(7):2296. doi: 10.3390/nu13072296
- Espina S, Sanz-Paris A, Gonzalez-Irazabal Y, et al. Randomized clinical trial: effects of β-hydroxy-β-methylbutyrate (HMB)-enriched vs. HMB-Free oral nutritional supplementation in malnourished cirrhotic patients. Nutrients. 2022 Jun;14(11):2344. doi: 10.3390/nu14112344
- Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011 Dec;59(12):2291–2300. doi: 10.1111/j.1532-5415.2011.03733.x
- Dattola A, Silvestri M, Bennardo L, et al. Role of vitamins in skin health: a Systematic Review. Curr Nutr Rep. 2020 Jun;9:226–235. doi: 10.1007/s13668-020-00322-4
- Ceglia L, Harris SS. Vitamin D and its role in skeletal muscle. Calcif Tissue Int. 2013;92:151–162. doi: 10.1007/s00223-012-9645-y
- De Luca HF. Overview of general physiologic features and functions of vitamin D23.The. Am J Clin Nutr. 2004 Dec;80(6):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S
- Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D deficiency and Sarcopenia in older persons. Nutrients. 2019 Nov 21;11(12):2861. doi: 10.3390/nu11122861
- Saeki C, Kanai T, Nakano M, et al. Low serum 25-hydroxyvitamin D levels are related to frailty and Sarcopenia in patients with chronic liver disease. Nutrients. 2020 Dec;12(12):3810. doi: 10.3390/nu12123810
- Bo Y, Liu C, Ji Z, et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: a double-blind randomized controlled trial. Clin Nutr. 2019 Feb;38(1):159–164.
- Lin CC, Shih MH, Chen CD, Yeh SL. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: an open-label, parallel-group study. Clin Nutr. 2021 Mar;40(3):1323–1329. doi: 10.1016/j.clnu.2020.08.017
- Okubo T, Atsukawa M, Tsubota A, et al. Effect of vitamin D supplementation on skeletal muscle volume and strength in patients with decompensated liver cirrhosis undergoing branched chain amino acids supplementation: a prospective, randomized, controlled Pilot trial. Nutrients. 2021;13(6):1874. doi: 10.3390/nu13061874
- Nagaya N, Itoh T, Murakami S, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005 Sep;128(3):1187–1193. doi: 10.1378/chest.128.3.1187
- Garcia JM, Polvino WJ. Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple-dose study in healthy volunteers. Oncology. 2007 May;12(5):594–600. doi: 10.1634/theoncologist.12-5-594
- Camilleri M. Human intestinal barrier: effects of stressors, diet, prebiotics, and probiotics. Clin Transl Gastroenterol. 2021 Jan;12(1):e00308. doi: 10.14309/ctg.0000000000000308
- Karim A, Muhammad T, Iqbal MS, et al. A multistrain probiotic improves handgrip strength and functional capacity in patients with COPD. A randomized controlled trial. Arch Gerontol Geriatr. 2022;102:104721. doi: 10.1016/j.archger.2022.104721
- Nistor-Cseppento CD, Moga TD, Bungau AF, et al. The contribution of diet therapy and probiotics in the treatment of sarcopenia induced by prolonged immobilization caused by the COVID-19 pandemic. Nutrients. 2022;14(21):4701. doi: 10.3390/nu14214701
- Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Liver cirrhosis and sarcopenia from the viewpoint of dysbiosis. Int J Mol Sci. 2020 Jul 21(15):5254. doi: 10.3390/ijms21155254
- Ramachandram G, Pottakkat B, Basu S, et al. Effect of probiotics on nutritional status, biochemical parameters, and disease severity in cirrhotic patients referred for liver transplantation–a randomised double blind, placebo-controlled trial. Clin Nutr. 2023 Oct;57:703–710. doi: 10.1016/j.clnesp.2023.08.021
- Rondanelli M, Gasparri C, Barrile GC, et al. Effectiveness of a novel food composed of leucine, omega-3 fatty acids and probiotic lactobacillus paracasei PS23 for the treatment of Sarcopenia in elderly subjects: a 2-month randomized double-blind placebo-controlled trial. Nutrients. 2022;14(21):4566. doi: 10.3390/nu14214566
- Papamandjaris AA, Macdougall DE, Jones PJH. Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci. 1998 Feb;14(14):1203–1215. doi: 10.1016/S0024-3205(97)01143-0
- Kojima K, Ishikawa H, Watanabe S, et al. A randomized, double-blind, controlled trial assessing if medium-chain triglycerides in combination with moderate-intensity exercise increase muscle strength in healthy middle-aged and older adults. Nutrients. 2023;15(14):3275.
- Abe S, Ezaki O, Suzuki M. Effects of timing of medium-chain triglycerides (8: 0 and 10: 0) supplementation during the Day on muscle mass, function and cognition in frail elderly adults. J Frailty Aging. 2022;11:1–9. doi: 10.14283/jfa.2021.33
- Rodacki CL, Rodacki AL, Pereira G, et al. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr. 2012 Feb;95(2):428–436.
- Lalia AZ, Dasari S, Robinson MM, et al. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging (Albany NY). 2017 Apr;9(4):1096–1129. doi: 10.18632/aging.101210
- Kunz HE, Michie KL, Gries KJ, et al. A randomized trial of the effects of dietary n3-PUFAs on skeletal muscle function and acute exercise response in healthy older adults. Nutrients. 2022;14(17):3537. doi: 10.3390/nu14173537
- Lach-Trifilieff E, Minetti GC, Sheppard K, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014 Feb;34(4):606–618.
- Rooks D, Swan T, Goswami B, et al. Bimagrumab vs optimized standard of care for treatment of Sarcopenia in community-dwelling older adults: a randomized clinical trial. JAMA Netw Open. 2020 Oct;3(10):e2020836. doi: 10.1001/jamanetworkopen.2020.20836
- Zhang YJ, Wang JX, Fu SH, Li XY. Trimetazidine in angina and poor muscle function: protocol for a randomized controlled study. Chin Med J (Engl). 2019 Jun 20;132(12):1461–1466. doi: 10.1097/CM9.0000000000000267. PMID: 31205105; PMCID: PMC6629326.
- Vinel C, Lukjanenko L, Batut A, et al. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 2018 Sep;24(9):1360–1371. doi: 10.1038/s41591-018-0131-6. Epub 2018 Jul 30. PMID: 30061698.
- Evans W, Shankaran M, Nyangau E, et al. Effects of fortetropin on the rate of muscle protein synthesis in older men and women: a randomized, double-blinded, placebo-controlled study. J Gerontol A Biol Sci Med Sci. 2021 Jan 1;76(1): 108–114. doi: 10.1093/gerona/glaa162. PMID: 32598445; PMCID: PMC7756695.
- Espinoza SE, Lee JL, Wang CP, et al. Intranasal oxytocin improves lean muscle mass and lowers LDL Cholesterol in older adults with sarcopenic obesity: a Pilot randomized controlled trial. J Am Med Dir Assoc. 2021 Sep;22(9):1877–1882.e2. doi: 10.1016/j.jamda.2021.04.015. Epub 2021 May 21. PMID: 34029521; PMCID: PMC8567747.
- Caballero-García A, Pascual-Fernández J, Noriega-González DC, et al. L-Citrulline supplementation and exercise in the management of sarcopenia. Nutrients. 2021 Sep 8;13(9):3133. doi: 10.3390/nu13093133. PMID: 34579009; PMCID: PMC8465698.
- Rheu KM, Lee BJ, Son WH, et al. Effect of fermented sarco oyster (Crassostrea gigas) extract on muscle strength enhancement in postmenopausal females: a randomized, double-blind, placebo-controlled trial. Int J Environ Res Public Health. 2022 Dec 8;19(24):16450. doi: 10.3390/ijerph192416450. PMID: 36554328; PMCID: PMC9779144.
- Dioh W, Tourette C, Del Signore S, et al. A phase 1 study for safety and pharmacokinetics of BIO101 (20-hydroxyecdysone) in healthy young and older adults. J Cachexia Sarcopenia Muscle. 2023 Jun;14(3):1259–1273. doi: 10.1002/jcsm.13195. Epub 2023 Apr 13. PMID: 37057316; PMCID: PMC10235879.
- Tachi Y, Kozuka A, Hirai T, et al. Impact of myosteatosis on skeletal muscle volume loss in patients with chronic liver disease. J Gastroenterol Hepatol. 2018 Feb 27;33(9): 1659–1666. doi: 10.1111/jgh.14133. Epub ahead of print. PMID: 29486094.