ABSTRACT
Introduction: The progesterone receptor plays an essential role in uterine physiology and reproduction. Selective progesterone receptor modulators (SPRMs) have emerged as a valuable treatment option for hormone dependent conditions like uterine fibroids, which have a major impact on women’s quality of life. SPRMs offer potential for longer term medical treatment and thereby patients may avoid surgical intervention.
Areas covered: The authors have reviewed the functional role of the progesterone receptor and its isoforms and their molecular mechanisms of action via genomic and non-genomic pathways. The current knowledge of the interaction of the PR and different SPRMs tested in clinical trials has been reviewed. The authors focused on pharmacological effects of selected SPRMs on the endometrium, their anti-proliferative action, and their suppression of bleeding. Potential underlying molecular mechanisms and the specific histological changes in the endometrium induced by SPRMs (PAEC; Progesterone receptor modulator Associated Endometrial Changes) have been discussed. The clinical potential of this compound class including its impact on quality of life has been covered.
Expert Opinion: Clinical studies indicate SPRMs hold promise for treatment of benign gynecological complaints (fibroids, heavy menstrual bleeding; HMB). There however remains a knowledge gap concerning mechanism of action.
1. Introduction: the impact of progesterone and uterine function
Progesterone is a steroid hormone that plays a key role in development, differentiation, and normal functioning of female reproduction-related target tissues including the uterus (endometrium and myometrium), the ovary, and the mammary gland as well as in the regulation of the hypothalamic–pituitary–gonadal axis. Abnormal progesterone responses are implicated in a wide spectrum of benign human reproductive disorders, including fibroids, endometriosis and adenomyosis, abnormal uterine bleeding (AUB; including heavy menstrual bleeding [HMB]), and miscarriage.[Citation1–Citation4] From the onset of puberty to menopause, progesterone is mainly produced by the corpus luteum in the ovary with smaller amounts secreted by the adrenal glands.
Actions of progesterone on the female reproductive system are primarily mediated by progesterone receptors (PRs) synthesized from a single gene (PR) and expressed as two main protein isoforms (PR-A, PR-B).[Citation5,Citation6] Beyond its prominent function in reproductive tract tissues, progesterone is also involved in regulation of cellular functions in the central nervous system [Citation7] influencing reproductive behaviors. Progesterone also plays an important role during pregnancy and has striking impacts on the function of the breast.[Citation8]
The uterine endometrium comprises of epithelial cells (lining the luminal surface and glands), stromal cells, immune cells, and blood vessels and it is arranged in two morphologically and functionally distinct zones, the inner basal zone and the outer functional zone with the latter being shed at menstruation.[Citation6,Citation9] The human myometrium, localized between endometrium and perimetrium, is a heterogeneous tissue and can also be subdivided in two zones, namely the outer myometrium and a functionally distinct inner myometrial layer called the uterine junctional zone.[Citation10,Citation11] This ‘junctional’ zone can be visualized with magnetic resonance imaging (MRI) but is not histologically distinct. The myometrium is largely made up of smooth muscle cells but also contains connective tissue, blood vessels, and immune cells. The principal uterine cellular targets for progesterone, expressing PR-A and PR-B, are the epithelial and stromal/decidual cells in the endometrium [Citation12,Citation13] and smooth muscle cells in the myometrium.[Citation14]
During the menstrual cycle, the human endometrium undergoes dynamic changes including proliferation, differentiation, tissue breakdown, and shedding (menstruation) in response to fluctuating peripheral concentrations of ovarian-derived estrogen and progesterone. Estrogens, acting via their cognate receptors, play a key role in modulating tissue function in the follicular (proliferative) phase by inducing epithelial and stromal cell proliferation leading to a thickened functional zone. Estrogens also stimulate expression of PR, thus ensuring progesterone responsiveness in the post-ovulatory luteal (secretory) phase.[Citation6] Estrogen levels decline after ovulation [Citation15,Citation16] and rising concentrations of progesterone secreted by the corpus luteum initiate a differentiation program characterized by growth and coiling of the spiral arteries, secretory transformation of the glands, an influx of distinct immune cells, especially specialized uterine natural killer cells, and transformation of the stromal fibroblasts (decidualization) in preparation for blastocyst implantation.[Citation17,Citation18] Progesterone induces genes that allow the endometrium to permit embryo attachment and directly controls vascular permeability.[Citation6,Citation19]
2. PRs
2.1. Intracellular and membrane PR isoforms
The PR as a member of the nuclear hormone receptor superfamily is a ligand-dependent transcription factor [Citation20,Citation21] characterized by structural motifs like the N-terminal A/B region, a highly conserved DNA-binding domain (DBD), a hinge region, and a C-terminal ligand-binding domain (LBD). The DBD is composed of two conserved zinc fingers that distinguish nuclear receptors from other DNA-binding proteins.
The PR-A and PR-B mRNA isoforms are both transcribed from the same PR gene and the proteins they encode are identical in their DNA-binding and ligand-binding properties; it is likely that PR A/B homodimers and heterodimers exist.[Citation22] PR-B (116 kDa) differs from PR-A (94 kDa) only by an additional stretch of 165 AA at the N-terminus of the protein. A marked physiological difference is the action of PR-A as a trans-dominant inhibitor of PR-B [Citation23] and it even exerts this inhibitory action onto other members of the NR superfamily including ER, androgen receptor (AR), MRI, and GR.[Citation24]
Differential recruitment of PR [Citation25,Citation26] and associated transcriptional co-regulators to gene promoters are critical to tissue selective impacts of progesterone (details see below) for example, whereas in the uterus progesterone stimulates growth of leiomyomas, it inhibits growth of the endometrium.[Citation6]
The ratio of PR-A and -B expression varies from tissue to tissue and is dependent on the hormonal status of the cell.[Citation27] In full thickness sections of the human uterus, parallel expression of ERα and PR can be detected using immunofluorescence (; antibody for PR, recognizing both A and B isoforms), demonstrating intense immunoexpression in glandular epithelium at the start of the secretory phase following induction during the follicular phase with subsequent downregulation in the mid-secretory phase. Whereas PR-A levels in epithelial cells decline in late secretory phase, PR-B levels remain constant, suggesting that this subtype may be involved in the control of glandular secretion.[Citation6] Studies to assess the cellular localization of PR-A and PR-B have to be interpreted with caution as they are technically limited due to the common sequence of PR-A and PR-B and the abundance of PR-A and its relative level to PR-B may only be determined in a semiquantitative manner at best. PR-A appears to be predominant subtype in the stromal cells, with a less obvious decline in expression during the luteal phase than in the epithelium, which might reflect the need for prolonged progesterone PR-A signaling in this compartment to support the establishment of pregnancy.[Citation12,Citation28] Both receptors have been detected in leiomyoma (fibroid) tissue [Citation29] and seem to be increased in leiomyoma compared with normal myometrium from the same patients.[Citation30,Citation31]
Figure 1. Immunolocalisation of ERα and PR in full thickness sections of human endometrium.
Images shown from four samples of uterine tissue recovered during the early (ES) or mid (MS) secretory phases of the cycle: each section represents the full thickness of the uterine wall with the lumen at the top and myometrium at the bottom. Sections were co-stained for ERα (red) and PR (green) using standard protocols [Citation32] for clarity the images recorded in the different channels (red, green) are shown side-by-side rather than overlaid. Note that during the ES there is intense immunopositive staining for both ERα and PR-A in the glandular epithelium in both the functional (arrows) and basal (white asterisks) layers. During the MS immunoexpression in the epithelium is down-regulated but expression of PR in stromal fibroblasts is maintained (green asterisks). Full color available online.
![Figure 1. Immunolocalisation of ERα and PR in full thickness sections of human endometrium.Images shown from four samples of uterine tissue recovered during the early (ES) or mid (MS) secretory phases of the cycle: each section represents the full thickness of the uterine wall with the lumen at the top and myometrium at the bottom. Sections were co-stained for ERα (red) and PR (green) using standard protocols [Citation32] for clarity the images recorded in the different channels (red, green) are shown side-by-side rather than overlaid. Note that during the ES there is intense immunopositive staining for both ERα and PR-A in the glandular epithelium in both the functional (arrows) and basal (white asterisks) layers. During the MS immunoexpression in the epithelium is down-regulated but expression of PR in stromal fibroblasts is maintained (green asterisks). Full color available online.](/cms/asset/a1ca4e90-6012-4bcb-86bc-87e8f2696491/iett_a_1180368_f0001_oc.jpg)
To delineate the individual roles of the receptor subtypes in vivo, PR isoform-specific knockout (KO) mice have been generated.[Citation33,Citation34] In these studies, female mice with a global ablation of both receptor subtypes failed to reproduce due to defects in both ovulation and implantation.[Citation33,Citation34] Specific ablation of PR-A alone also resulted in severe abnormalities in ovarian and uterine function leading to female infertility.[Citation35] In contrast, PR-B-specific KO mice had normal ovarian and uterine responses to progesterone but exhibited reduced mammary ductal morphogenesis and alveologenesis during pregnancy. Both PR subtypes of receptor appear to mediate anti-inflammatory actions of progesterone on the endometrium.[Citation36] Notably, both subtypes mediate progesterone-dependent responses through activation of different subsets of genes with those regulated by PR-A being both necessary and sufficient for reproductive fertility functions, while PR-B-dependent gene activation, at least in mice, plays a key role in mammary development.[Citation24,Citation36] Although some additional variant PR mRNAs (PR-C, -M, -S, and -T) have been described, a recent comprehensive protein analysis did not support their translation in vivo.[Citation37]
In addition to the PR receptors that are members of the superfamily of transcription factors, a membrane-bound PR (mPR) first described in fish has been implicated in rapid non-genomic actions (see below) of progesterone.[Citation38,Citation39] Upon progesterone binding, mPRs may influence the activity of several signaling pathways, including mobilization of intracellular Ca2+, activation of mitogen-activated protein kinase (MAPK) cascades, and inhibition of cAMP production.[Citation40] The physiologic relevance of the membrane PR is still unclear since their capacity to bind progesterone is relatively low compared to nuclear PRs and some studies could not even detect evidence of activation by progesterone.[Citation41]
2.2. Genomic and non-genomic activation of PR
In the reproductive tract, it is likely that progesterone exerts its effects via both genomic and non-genomic actions mediated via PR-A or PR-B that subsequently converge to produce tissue- and cell-specific responses.[Citation42] In the ‘classical’ genomic mode of action binding of a ligand within the LBD, conformational changes are initiated, chaperone proteins dissociate and the PR translocates to the nucleus. Within the nucleus, ligand-bound PRs interact with the transcriptional machinery and bind as homo- or heterodimers to specific cis-acting PR response elements (PRE), typically located in the promoter regions of target genes. Robust, specific modulation of gene transcription, however, requires recruitment of additional co-regulatory proteins to a transcription complex that includes the DNA-bound receptor. The different co-regulatory factors are generally considered to act to enhance transcription (coactivators) or to decrease the level of transcriptional activation (corepressors).[Citation26] Over 300 co-regulators are reported to interact with PR, and it is the tissue-specific expression of the factors that orchestrates the impact of progesterone on expression of different sets of genes within target tissues.[Citation43]
It is now well established that binding of agonists or antagonists to distinct amino acid residues within the LBD of PR alters the conformation of the receptor protein resulting in recruitment of differing type(s) of co-regulatory proteins into the transcription complex. For example, following binding of agonists, coactivators capable of modifying core histone protein side chains via acetylation or methylation are recruited and the resultant change in histone proteins facilitates access of the transcription machinery. PR coactivators include members of the steroid receptor coactivator (SRC) family (SRC-1–3) and receptor-interacting protein 140.[Citation44] The importance of the SRC PR interactions has been elucidated by studies in KO mice demonstrating that steroid receptor coactivator-1 (SRC-1) is the primary coactivator of PR in the uterus but SRC-3 is important in the mammary gland.[Citation45] Lately, a new class of PR modulators the Kruppel-like factors (KLFs) [Citation46] have been described and the absence of some KLFs in distinct pathologies (e.g. KLF9 and KLF11 in endometriosis and leiomyoma) suggests roles for multiple KLFs in maintaining homeostasis in female reproductive tissues.
On the other hand, PR can also interact with corepressors and this generally occurs in the presence of ligands like mifepristone that act as antagonists. Crystal structures have shown binding of the nuclear receptor corepressor (NCoR), and the silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) to both PR-A and PR-B in the presence of asoprisnil, a synthetic PR receptor modulator that is a mixed agonist/antagonist and downregulates expression of PR in endometrium.[Citation47,Citation48] In the human endometrium, the expression of both PR isoforms and their corepressors (NCoR and SMRT) has been observed [Citation28,Citation49] and is modulated over the course of the menstrual cycle in a compartment-specific manner [Citation49] demonstrating the potential for stage-dependent gene repression.
Finally, additional cell-specific impacts of ligand-activated PR may be determined by the co-recruitment of coactivators and a number of additional transcription factors also expressed in endometrium some of which appear to play a key role in decidualization of stromal cells in preparation for establishment of pregnancy. Examples include members of the forkhead-box O (FOXO) protein family (FOXO1, FOXO3a), signal transducer, and activator of transcription (STAT5) and CCAAT enhancer-binding protein (C/EBPβ).[Citation50]
Ligand-bound nuclear PR receptors can also be transcriptionally active at endogenous promoters lacking a canonical PRE. Transcription of these genes seems to be facilitated through nuclear protein–protein interactions with other DNA-binding transcription factors such as NFκB,[Citation51] SP1, and AP-1.[Citation52] Levels of gene transcription can also be modulated by post-translational modification of PRs primarily through N-terminal phosphorylation, acetylation, SUMOylation, and ubiquitination.[Citation53,Citation54] These modifications alter PRs trafficking, transcriptional activity, and target-gene selectivity.[Citation55] Studies in cancer cells have shown that modifications involve phosphorylation through mitogenic protein kinases, CDK2, CK2, or MAPK of the receptor.[Citation56] PR has been shown to trigger Src-dependent phosphorylation signaling cascades like proliferative Ras/Raf/MEKK/MAK kinase pathway.[Citation7] The relevance for uterine tissue dysfunction remains poorly understood.
In addition to its direct effect on transcription, progesterone has been identified to influence the activity of many other signaling pathways by non-genomic (extranuclear) mechanisms in the cytoplasm.[Citation57] These rapid non-genomic effects triggered by progesterone binding to membrane-bound receptors exhibit an onset within seconds to minutes (see ) and are insensitive to transcription or translation inhibitors. A detailed description of non-genomic progesterone effects in different target tissues and potential membrane receptors involved has been reviewed by Gellersen et al.[Citation42]
Table 1. Non-genomic signaling pathways reported to be triggered by progesterone.
Finally, the distinction between the rapid non-genomic kinase activation and genomic actions has become less certain. For example, results using breast cancer cells have demonstrated activated kinases can be recruited together with the phosphorylated nuclear PR into an integrated PRE-containing promotor.[Citation66] According to this model, rapid signaling may be a concurrent pathway integrated into the activation of the transcriptional machinery by nuclear PR,[Citation67] but further studies are required to validate this in nonmalignant cells.
3. Selective progesterone receptor modulators
3.1. Modulation of PR activity by selective progesterone receptor modulators
Selective progesterone receptor modulators (SPRMs) represent a new class of synthetic steroids, which can exert agonist, antagonist, or mixed effects on various progesterone target tissues in vivo upon PR binding [Citation68] (see ). They have many potential clinical applications in female reproduction and gynecological therapies like uterine fibroids but also in the treatment of some tumors.[Citation57]
Figure 2. Structure of common SPRMs.
Chemical structures of selective progesterone receptor modulators (SPRMs) in current clinical use or which have been in clinical development.
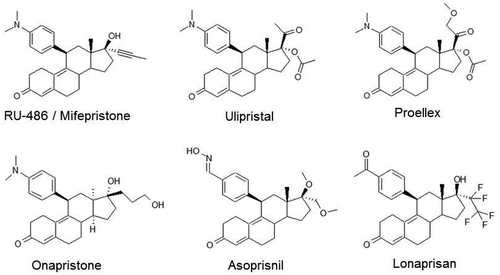
X-ray crystal structures of the PR bound to SPRM ligands revealed that the mode of binding differs between different molecules and depends on the agonistic or antagonistic nature of the interaction.[Citation69] The resulting effect on target genes appears to depend both on the cell type and availability of different co-regulators [Citation70] also confirmed by evaluation of protein–protein interactions with PR and SPRMs.[Citation71]
The first and one of the most widely used SPRMs with mixed agonistic/antagonistic properties and tissue-specific effects is mifepristone (RU486). Wardell et al. described the involvement of the amino-terminal domain of the PR and the phosphorylation of a serine for the partial agonist features of mifepristone.[Citation72]
With an in vitro chromatin transcription system that recapitulates PR-mediated transcription in vivo, Liu et al. [Citation44] have determined the molecular basis by which mifepristone regulates transcription in a cell-type-specific manner. Specifically, agonist-bound PR interacts only with coactivators such as SRC-1, whereas mifepristone-bound PR binds to both the coactivator SRC-1 and the corepressor silencing mediator for retinoid and thyroid hormone receptor (SMRT) so that the precise impact in different cell types may be influenced by the relative availability/abundance of these factors.
Afhuppe and colleagues [Citation73] compared different SPRMs and their ability of interaction with PR and co-regulators. Mifepristone, onapristone, and lonaprisan (ZK230211) differ in their induced interactions of PR with the NCoR. PR modulators with marked PR agonistic activity demonstrate induced interactions with the LX-H10 peptide (contains the LxxLL motif of coactivators) similarly to the ones observed with R5020 (promegestrone), whereas SPRMs with antagonistic behavior like lonaprisan do not show any recruitment of the LX-H10 peptide.[Citation74] In contrast to mifepristone, asoprisnil mediates the recruitment of coactivators to the PR in vitro. However, none of these compounds has a progesterone-like ability to oppose estrogen in the rat endometrium,[Citation47] again demonstrating the high degree of complexity of the system as a whole.[Citation70] There have been several recent reviews that provide informative summaries of the effects of SPRM administration.[Citation75–Citation77]
3.2. Impact of SPRMs upon leiomyoma growth and endometrial morphology
The mechanisms of fibroid growth reduction have been addressed in several in vitro studies but a clearer picture may be expected in the near future when fibroid biopsies from the different clinical studies using SPRMs are fully analyzed. So far, there is strong evidence that SPRMs induce apoptosis through activation of the tumor necrosis-related apoptosis-inducing ligand (TRAIL) pathways.[Citation78] Fibroids treated with ulipristal acetate (UPA) revealed upregulation of caspase 3 and downregulation of BCL2.[Citation79] Furthermore, ample evidence exists that strong expression of extracellular matrix in fibroids is reduced by SPRMs due to suppression of collagen synthesis (type I and III) and modulation of extracellular matrix enzymes like MMPs and TIMPs.[Citation80]
An interesting aspect in the pathomechanism of fibroid growth has recently been addressed by Bulun et al..[Citation81] They describe a paracrine pathway that may mediate progesterone-derived growth of leiomyoma tissue. Treatment of mature myometrial cells with estrogen and progesterone resulted in secretion of wingless type (WNT) ligands, translocation of β-catenin in neighboring leiomyoma stem-progenitor cells, and activation of gene expression critical for fibroid growth and proliferation. The importance of WNT/β-catenin signaling in formation of leiomyoma-like tumors and fibrogenesis has been shown in mice that express a constitutively active form of β-catenin in mesenchymal cells of the uterus.[Citation82]
In this context, it is noteworthy to mention that the mediator complex subunit 12 (MED12) gene, which has been previously demonstrated to regulate β-catenin/WNT signaling, has mutations in exon 2 in nearly 70% of uterine leiomyomas.[Citation83] The role(s) for such signaling pathways in the endometrium of women with fibroids has to date not been determined.
The administration of all family members of the SPRM class of compound has been found to date to be accompanied with morphological changes within the endometrium described as PR modulator-associated endometrial changes (PAEC).[Citation84] These histological changes are recognized as a distinct histological entity and should not be confused with endometrial hyperplasia. SPRMs have been shown to induce a specific endometrial antiproliferative effect and the endometrial glandular epithelium shows reduced mitotic activity compared to the proliferative phase. Furthermore, evidence is accumulating that PAEC rapidly regress on cessation of treatment, although the rate of regression can be variable.[Citation85] Whilst PAEC are now well described and appear reversible, the mechanisms by which these develop are poorly understood.
UPA is a SPRM licensed in Europe for preoperative treatment of moderate-to-severe symptoms of uterine fibroids in adult women of reproductive age and also for intermittent treatment of moderate-to-severe symptoms of uterine fibroids in adult women of reproductive age.[Citation86] In common with other SPRMs, UPA significantly reduces menstrual bleeding and fibroid volume.[Citation85,Citation87]
The mechanisms responsible for these effects remain poorly understood. In keeping with other SPRMs, despite progesterone antagonism and maintenance of circulating estradiol levels, hyperplasia does not occur with any increased frequency although extensive cystic glandular dilatation is seen.[Citation88,Citation89] Although in vitro work describes antiproliferative and proapoptotic effects on leiomyoma cells,[Citation90] there are only limited data on the effects of SPRMs upon human endometrium.
Studies in nonhuman primates (macaques) have shown a suppressive effect specifically upon the endometrium with a reduction in proliferation markers and upregulation of the AR consistent with the ‘class effect’ of other SPRMs.[Citation91] In a long-term oral toxicity study with UPA,[Citation92] findings in the endometrium were similar to SPRM-associated endometrial changes described in SPRM-treated women. No adverse effects were observed that would raise concerns about potential premalignancy. Detailed human in vivo data still remain limited to small studies. Apoptosis indices are increased in the endometrium, but knowledge of impact upon proliferation and sex steroid receptor expression to date are again limited.
Thus far, there have been no reports of cytological atypia accompanying SPRM administration in the presence of a normal endometrial biopsy prior to drug therapy with SPRMs. Administration of low-dose mifepristone (RU486; 2–5 mg) for 120 days was observed to reduce endometrial proliferation markers.[Citation93] Administration of another SPRM with mixed agonist–antagonist activity, asoprisnil, had no reports of endometrial hyperplasia with administration limited to daily administration for 12 weeks. Oral daily doses of both 10 or 25-mg asoprisnil had no impact on markers of endometrial proliferation.
The discovery of the ‘endometrial antiproliferative effect’ of SPRMs was an important milestone in their development. This effect was initially observed in rabbit and primate endometrium. The finding of endometrial atrophy induced by the SPRM class of compound was not anticipated. The compounds were reported not to bind the estrogen receptor due to PR antagonist activity and thus the endometrium would have been expected to exhibit unopposed estrogenic effects and yet functional antiestrogenic effects were reported. The believed unique endometrial effects of SPRMs are specific to menstruating primates such as Old World monkeys and humans. Cynomolgus and rhesus macaques are good models, as their endometrium is similar to the human with respect to hormonal regulation and morphological changes during the menstrual cycle. Studies in nonhuman primates have been reported to show that SPRM administration in both spayed and intact macaques induced endometrial atrophy with stromal compaction and inhibited mitotic activity. These effects were observed following administration of mifepristone (RU486), ZK 230 211, and ZK 137 316.[Citation94] Studies with ZK 137 316 in the rhesus monkey also showed a dose-dependent degradation of the spiral arteries in the basal layer of the endometrium. It is notable that these profound morphological changes were observed in the presence of follicular phase estrogen levels. This functional antiestrogenic effect appears to be limited to the endometrium. The oviduct and vagina are reported to be unaffected, thereby providing evidence for an ‘endometrial antiproliferative effect’ and provides support that SPRMs may target the endometrium directly and this effect may possibly be via the endometrial vasculature. Dosage of SPRM administration may be important as some effects may be dose-dependent.[Citation94,Citation95]
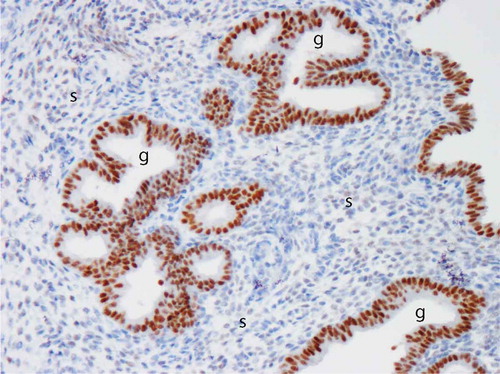
The SPRM, asoprisnil, suppresses endometrial bleeding and administration has a striking histological effect on the endometrial spiral arteries which develop an unusual appearance as prominent aggregations due to abnormally thick muscular walls.[Citation96] When global endometrial gene expression in asoprisnil-treated versus control women was performed, a most interesting and statistically significant reduction of inflammatory genes has been reported.[Citation48] The IL-15 pathway, known to play a key role in uterine NK cell development and function, was identified at the center of a pathway analysis and suppression of IL-15 by asoprisnil was also observed on mRNA level. Furthermore, immunostaining for the uterine NK cell marker CD56 revealed an impressive reduction in the asoprisnil-treated endometrium.[Citation48] In the normal cycling endometrium, IL-15 levels are progesterone-responsive. In the study of asoprisnil-treated endometrium, there is a downregulation of stromal PR expression, upregulation of glandular PR expression (see ), and a marked reduction in number of uterine NK cells. These observations with administration of a SPRM have provided support for a role for the IL-15 pathway in the complex interplay between endometrial stromal cells, uterine NK cells, and spiral arteries and an effect on both physiological and HMB.[Citation48,Citation97,Citation98]
Figure 3. Image of progesterone receptor (PR) immuno-reactivity in human endometrium after administration of a selective PR modulator (SPRM). Note intense positive (brown) immunostaining in the glandular epithelium (g) and virtual absence of immuno-reactivity in the stroma (s). Image kindly provided by Professor Alistair Williams, University of Edinburgh. Full color available online.
4. Clinical potential for SPRMs in management of benign gynecological disorders
Benign gynecological complaints such as HMB, fibroids, pelvic pain, and endometriosis have a very significant impact on quality of life and represent a large health-care burden. Each year in the United Kingdom, one million women seek help for HMB [Citation99] and endometriosis has a prevalence of 2–10% of women of reproductive age.[Citation100] There are a multitude of etiologies that can cause HMB.[Citation101] Many cases have uterine fibroids that disrupt everyday life and fibroids remain the leading indication for hysterectomy.[Citation102,Citation103] While there may be relief from HMB during pregnancy and lactation, and an end to the problem at menopause, women affected will tend to suffer the adverse impacts of HMB over what should be the prime years of their lives. This can accumulate to a lifetime loss of 5–7 years of ‘healthy’ life.
Along with the direct impact on the woman and her family, there are significant costs both to the economy and the health service. In the USA, lost work-hour costs are estimated between $1.55 and 17.2 billion annually and direct costs of $4.1–9.4 billion.[Citation104]
Current medical therapies often either fail to fully resolve symptoms or are associated with unacceptable side effects. As a result, many women opt for a definitive solution consequently hysterectomy remains the solution with the highest long-term patient satisfaction and cost-effectiveness.[Citation99] Many women however wish to avoid surgery and to retain fertility. This is pertinent given that nearly half of all UK-born babies are to women aged 30 or over.[Citation105] Furthermore, those from low-income backgrounds are less likely to receive a surgical treatment [Citation106] and as such may be further penalized by ineffective medical treatment. Finally, the recent RCOG HMB audit reported that at 1-year post-referral only 35% of all women (including those given surgery) were ‘satisfied’ (or better) at the prospect of current menstrual symptoms continuing, as currently experienced, for the next 5 years.[Citation106] There is thus a very substantial unmet need for long-term medical therapies that are effective, affordable, and without unwanted side effects.
The evidence of the impact of SPRMs on endometrial cell proliferation (reviewed above) and data from nonhuman primate studies have identified these compounds as offering significant potential for longer term medical therapy of HMB. Specifically, two members of this class, mifepristone and asoprisnil, have been shown to significantly reduce menstrual blood loss in association with fibroids but do not increase proliferation.[Citation107,Citation108] The impact of SPRM administration on menstrual bleeding in women without fibroids is not known.
The medical management of endometriosis is currently largely dependent upon administration of progestogens and estrogen deficiency has to date restricted long-term use of GnRH analogs. With each of these commonly used therapies, management is frequently limited by accompanying side effects and symptom control may be suboptimal. Studies with SPRM administration undertaken in women with endometriosis indicate potential clinical utility for symptom relief.[Citation109,Citation110] For example, mifepristone administration (50 mg for 6 months) in patients with endometriosis has been reported to have a significant effect on symptoms and extent of disease.[Citation110] In a randomized, placebo-controlled study, asoprisnil (5, 10, or 25 mg) was administered for 12 weeks to women with a laparoscopic diagnosis of endometriosis who complained of moderate or severe pain. A significant reduction in non-menstrual pelvic pain and dysmenorrhea compared to placebo was reported [Citation111] that would be consistent with reports of a tissue-specific suppression of endometrial prostaglandin production.
5. Conclusion
Studies on HMB are hampered by the lack of an appropriate in vivo model for fibroid-associated endometrial bleeding limiting our understanding of the interplay between fibroids and endometrium.
At present, the biggest challenge facing researchers who are keen to develop regimes using SPRMs as long-term treatments for women with debilitating benign gynecological conditions is a paucity of data related to the mechanisms underpinning the development of PAEC.
6. Expert opinion
Progesterone acting via its cognate receptors (PR-A, PR-B) plays a central role in regulation of uterine function making PR an attractive therapeutic target.
PRs classical genomic mode of action has been studied in detail and the impact of ligand binding, conformational change, and coactivator and -repressor recruitment on cell and tissue-specific patterns of gene expression is now better understood.
The regulation/impact of non-genomic progesterone pathways has recently been described as via the trigger of SRC-dependent phosphorylation signaling like proliferative RAS/RAF/MEKK/MAPK kinase pathway. The level of interaction and integration of these extranuclear signaling cascades with classical genomic signaling remains a topic for further studies.
A number of SPRMs have been developed which have a range of agonistic and antagonistic profiles when compared with progesterone. Mechanistic studies have identified distinct patterns of co-regulator recruitment that may in part explain their unique impact on cell function. Here, more molecular details will emerge in the future as the number of molecular studies on human tissues will increase as more SPRMs enter the market and it would be interesting to follow whether the systematic analysis of expression profiles of different fibroid and endometrial cellular components holds potential to generate a gene expression fingerprint which would be suitable to allow differentiation of SPRMs on their mechanistic action toward the different uterine cellular compartments.
Clinical studies indicate SPRMs hold promise for treatment of fibroids and associated HMB. Extensive in vitro studies on primary cells and small-scale investigations of biopsies from clinical studies highlighted inhibition of cell proliferation and an increase of the expression of proapoptotic markers and pathways and suppression of extracellular matrix synthesis as the molecular mechanism behind reduction of fibroid volume.
A link to progesterone action for fibroid growth has been offered by identification of distinct leiomyoma stem-progenitor cell populations processing paracrine signals from adjacent myometrium induced by progesterone. One of the key components mediating progesterone action in fibroid cells is the WNT/β-catenin pathway. Dissecting the paracrine mechanism involved with leiomyoma growth may also shed further light into the molecular action of SPRMs and may also lead to new treatments beyond SPRMs.
New fundamental insights into the etiology of fibroids will arise from the latest development in research regarding the MED12 mutation which is a driver mutation for fibroids with very high prevalence rate. Very recently, MED12-mutant mice have been generated, which will offer an opportunity to dissect downstream signaling of Med12 and investigate effects on tumor growth but also on AUB from adjacent endometrial tissue. Whether potential factors might be identified causative for induction of HMB will be an exciting area to follow.
In contrast to fibroid shrinkage, the cellular mechanisms by which SPRMs control endometrial bleeding are still poorly understood. Reduction in the numbers of uterine NK cells and their complex interaction with the spiral arteries and endometrial stroma cells maybe one possible explanation for SPRMs’ effect on bleeding suppression.
The administration of the SPRM class of compound is accompanied with morphological changes within the endometrium described as PAEC. These histological changes are recognized as a distinct histological entity and should not be confused with endometrial hyperplasia. However, whilst PAEC are well described and appear reversible, a greater understanding of molecular and cellular mechanisms underpinning these histological features is required.
Article highlights
Abnormal progesterone responses are implicated in a wide spectrum of benign human reproductive disorders, including abnormal uterine bleeding (AUB; including heavy menstrual bleeding [HMB]), fibroids (leiomyomas) and endometriosis.
Progesterone acting via its cognate receptors (PR-A, PR-B) plays a central role in regulation of uterine function making PR an attractive therapeutic target.
Differential recruitment of PR and associated transcriptional co-regulators to gene promoters are critical to tissue selective impacts of progesterone, for example, whereas it inhibits growth of the endometrium it stimulates growth of fibroids (leiomyomas).
Selective progesterone receptor modulators (SPRMs) represent a new class of synthetic ligands, which can exert agonist, antagonist or mixed effects on various progesterone target tissues.
Administration of all family members of the SPRM class of compound have been found to date to be accompanied with morphological changes within the endometrium described as progesterone receptor modulator associated endometrial changes (PAEC). These histological changes are recognized as a distinct histological entity.
Whilst PAEC are now well described and appear reversible, the mechanisms by which these develop are poorly understood.
This box summarizes key points contained in the article.
Declaration of interests
A Wagenfeld is an employee of Bayer Pharma Ag. H O. D. Critchley has acted as a consultant for Bayer Pharma Ag, AbbVie Incorporated, Preglem SA, Gedeon Ritcher, and Vifor Pharma Ag; and received research support from Bayer Pharma Ag, Medical Research Council UK and the National Institute for Health Research. P Saunders has received research support from Bayer Pharma AG, and Medical Research Council UK. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
We are most grateful to Mrs Sheila Milne for her help with manuscript preparation and Mr Ronnie Grant for graphical assistance. We thank Professor Alistair Williams, University of Edinburgh, for provision of the histological image in .
References
- Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826.
- Salazar EL, Calzada L. The role of progesterone in endometrial estradiol- and progesterone-receptor synthesis in women with menstrual disorders and habitual abortion. Gynecol Endocrinol. 2007;23:222–225.
- Maybin JA, Critchley HO. Progesterone: a pivotal hormone at menstruation. Ann NY Acad Sci. 2011;1221:88–97.
- Bulun SE. Uterine fibroids. N Engl J Med. 2013;369:1344–1355.
- Li X, O’Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278:39261–39264.
- Patel B, Elguero S, Thakore S, et al. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21:155–173.
- Mani SK, Oyola MG. Progesterone signaling mechanisms in brain and behavior. Front Endocrinol (Lausanne). 2012;3:7.
- Obr AE, Edwards DP. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol. 2012;357:4–17.
- Maybin JA, Critchley HO. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update. 2015;21:748–761.
- Brosens JJ, de Souza NM, Barker FG. Uterine junctional zone: function and disease. Lancet. 1995;346:558–560.
- Fusi L, Cloke B, Brosens JJ. The uterine junctional zone. Best Pract Res Clin Obstet Gynaecol. 2006;20:479–491.
- Mote PA, Balleine RL, McGowan EM, et al. Heterogeneity of progesterone receptors A and B expression in human endometrial glands and stroma. Hum Reprod. 2000;15(Suppl 3):48–56.
- Critchley HO, Saunders PT. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci. 2009;16:191–199.
- Mesiano S. Myometrial progesterone responsiveness. Semin Reprod Med. 2007;25:5–13.
- Lessey BA, Killam AP, Metzger DA, et al. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334–340.
- Ingamells S, Campbell IG, Anthony FW, et al. Endometrial progesterone receptor expression during the human menstrual cycle. J Reprod Fertil. 1996;106:33–38.
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453.
- Das SK. Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction. 2009;137:889–899.
- Goddard LM, Murphy TJ, Org T, et al. Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell. 2014;156:549–562.
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857.
- Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839.
- Burris TP, Solt LA, Wang Y, et al. Nuclear receptors and their selective pharmacologic modulators. Pharmacol Rev. 2013;65:710–778.
- Pieber D, Allport VC, Bennett PR. Progesterone receptor isoform A inhibits isoform B-mediated transactivation in human amnion. Eur J Pharmacol. 2001;427:7–11.
- Ellmann S, Sticht H, Thiel F, et al. Estrogen and progesterone receptors: from molecular structures to clinical targets. Cell Mol Life Sci. 2009;66:2405–2426.
- Hagan CR, Knutson TP, Lange CA. A common docking domain in progesterone receptor-B links DUSP6 and CK2 signaling to proliferative transcriptional programs in breast cancer cells. Nucleic Acids Res. 2013;41:8926–8942.
- O’Malley BW. Coregulators: from whence came these “master genes”. Mol Endocrinol. 2007;21:1009–1013.
- Mangal RK, Wiehle RD, Poindexter AN 3rd, et al. Differential expression of uterine progesterone receptor forms A and B during the menstrual cycle. J Steroid Biochem Mol Biol. 1997;63:195–202.
- Wang H, Critchley HO, Kelly RW, et al. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod. 1998;4:407–412.
- Kawaguchi K, Fujii S, Konishi I, et al. Immunohistochemical analysis of oestrogen receptors, progesterone receptors and Ki-67 in leiomyoma and myometrium during the menstrual cycle and pregnancy. Virchows Arch A Pathol Anat Histopathol. 1991;419:309–315.
- Viville B, Charnock-Jones DS, Sharkey AM, et al. Distribution of the A and B forms of the progesterone receptor messenger ribonucleic acid and protein in uterine leiomyomata and adjacent myometrium. Hum Reprod. 1997;12:815–822.
- Fujimoto J, Hirose R, Ichigo S, et al. Expression of progesterone receptor form A and B mRNAs in uterine leiomyoma. Tumour Biol. 1998;19:126–131.
- Bombail V, MacPherson S, Critchley HO, et al. Estrogen receptor related beta is expressed in human endometrium throughout the normal menstrual cycle. Hum Reprod. 2008;23:2782–2790.
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, et al. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754.
- Conneely OM, Mulac-Jericevic B, Lydon JP, et al. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103.
- Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–778.
- Conneely OM, Mulac-Jericevic B, DeMayo F, et al. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355.
- Samalecos A, Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology. 2008;149:5872–5887.
- Thomas P, Zhu Y, Pace M. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: a review with some new findings. Steroids. 2002;67:511–517.
- Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312.
- Hanna R, Pang Y, Thomas P, et al. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J Endocrinol. 2006;190:247–260.
- Krietsch T, Fernandes MS, Kero J, et al. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRalpha, beta, and gamma) localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol. 2006;20:3146–3164.
- Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15:119–138.
- Scarpin KM, Graham JD, Mote PA, et al. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal. 2009;7:e009.
- Liu Z, Auboeuf D, Wong J, et al. Coactivator/corepressor ratios modulate PR-mediated transcription by the selective receptor modulator RU486. Proc Natl Acad Sci U S A. 2002;99:7940–7944.
- Han SJ, DeMayo FJ, Xu J, et al. Steroid receptor coactivator (SRC)-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol Endocrinol. 2006;20:45–55.
- Simmen RC, Heard ME, Simmen AM, et al. The Kruppel-like factors in female reproductive system pathologies. J Mol Endocrinol. 2015;54:R89–R101.
- Madauss KP, Grygielko ET, Deng SJ, et al. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol. 2007;21:1066–1081.
- Wilkens J, Male V, Ghazal P, et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J Immunol. 2013;191:2226–2235.
- Gregory CW, Wilson EM, Apparao KB, et al. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab. 2002;87:2960–2966.
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372.
- Kalkhoven E, Wissink S, van der Saag PT, et al. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271:6217–6224.
- Shatnawi A, Tran T, Ratnam M. R5020 and RU486 act as progesterone receptor agonists to enhance Sp1/Sp4-dependent gene transcription by an indirect mechanism. Mol Endocrinol. 2007;21:635–650.
- Hagan CR, Lange CA. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med. 2014;12:32.
- Faus H, Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006;60:520–528.
- Abdel-Hafiz HA, Horwitz KB. Post-translational modifications of the progesterone receptors. J Steroid Biochem Mol Biol. 2014;140:80–89.
- Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–1037.
- Chabbert-Buffet N, Meduri G, Bouchard P, et al. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005;11:293–307.
- Boonyaratanakornkit V, McGowan E, Sherman L, et al. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21:359–375.
- Cai W, Zhu Y, Furuya K, et al. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. 2008;55:127–138.
- Ballare C, Vallejo G, Vicent GP, et al. Progesterone signaling in breast and endometrium. J Steroid Biochem Mol Biol. 2006;102:2–10.
- Vares G, Sai S, Wang B, et al. Progesterone generates cancer stem cells through membrane progesterone receptor-triggered signaling in basal-like human mammary cells. Cancer Lett. 2015;362:167–173.
- Lu J, Reese J, Zhou Y, et al. Progesterone-induced activation of membrane-bound progesterone receptors in murine macrophage cells. J Endocrinol. 2015;224:183–194.
- Balasubramanian B, Portillo W, Reyna A, et al. Nonclassical mechanisms of progesterone action in the brain: I. Protein kinase C activation in the hypothalamus of female rats. Endocrinology. 2008;149:5509–5517.
- Balasubramanian B, Portillo W, Reyna A, et al. Nonclassical mechanisms of progesterone action in the brain: II. Role of calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinology. 2008;149:5518–5526.
- Bashour NM, Wray S. Progesterone directly and rapidly inhibits GnRH neuronal activity via progesterone receptor membrane component 1. Endocrinology. 2012;153:4457–4469.
- Vicent GP, Ballare C, Nacht AS, et al. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell. 2006;24:367–381.
- Faivre E, Skildum A, Pierson-Mullany L, et al. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426.
- Madauss KP, Stewart EL, Williams SP. The evolution of progesterone receptor ligands. Med Res Rev. 2007;27:374–400.
- Lusher SJ, Raaijmakers HC, Vu-Pham D, et al. Structural basis for agonism and antagonism for a set of chemically related progesterone receptor modulators. J Biol Chem. 2011;286:35079–35086.
- Chabbert-Buffet N, Pintiaux A, Bouchard P. The immninent dawn of SPRMs in obstetrics and gynecology. Mol Cell Endocrinol. 2012;358:232–243.
- Berrodin TJ, Jelinsky SA, Graciani N, et al. Novel progesterone receptor modulators with gene selective and context-dependent partial agonism. Biochem Pharmacol. 2009;77:204–215.
- Wardell SE, Narayanan R, Weigel NL, et al. Partial agonist activity of the progesterone receptor antagonist RU486 mediated by an amino-terminal domain coactivator and phosphorylation of serine400. Mol Endocrinol. 2010;24:335–345.
- Afhuppe W, Sommer A, Muller J, et al. Global gene expression profiling of progesterone receptor modulators in T47D cells provides a new classification system. J Steroid Biochem Mol Biol. 2009;113:105–115.
- Giangrande PH, Kimbrel EA, Edwards DP, et al. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–3115.
- Im A, Appleman LJ. Mifepristone: pharmacology and clinical impact in reproductive medicine, endocrinology and oncology. Expert Opin Pharmacother. 2010;11:481–488.
- Hoellen F, Griesinger G, Bohlmann MK. Therapeutic drugs in the treatment of symptomatic uterine fibroids. Expert Opin Pharmacother. 2013;14:2079–2085.
- Benagiano G, Bastianelli C, Farris M, et al. Selective progesterone receptor modulators: an update. Expert Opin Pharmacother. 2014;15:1403–1415.
- Yoshida S, Ohara N, Xu Q, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28:260–273.
- Horak P, Mara M, Dundr P, et al. Effect of a selective progesterone receptor modulator on induction of apoptosis in uterine fibroids in vivo. Int J Endocrinol. 2012;2012:436174.
- Xu Q, Ohara N, Liu J, et al. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Mol Hum Reprod. 2008;14:181–191.
- Bulun SE, Moravek MB, Yin P, et al. Uterine leiomyoma stem cells: linking progesterone to growth. Semin Reprod Med. 2015;33:357–365.
- Tanwar PS, Lee HJ, Zhang L, et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81:545–552.
- Mäkinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334:252–255.
- Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21:591–598.
- Donnez J, Vazquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101:1565–1573.e18.
- Esmya EMC 5 mg Tablets (ulipristal acetate). [cited 2016 Feb 2]. Available from: http://www.medicines.org.uk/emc/medicine/26068
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409–420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421–432.
- Williams AR, Bergeron C, Barlow DH, et al. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol. 2012;31:556–569.
- Attardi BJ, Burgenson J, Hild SA, et al. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol. 2004;88:277–288.
- Brenner RM, Slayden OD, Nath A, et al. Intrauterine administration of CDB-2914 (Ulipristal) suppresses the endometrium of rhesus macaques. Contraception. 2010;81:336–342.
- Pohl O, Williams AR, Bergeron C, et al. A 39-week oral toxicity study of ulipristal acetate in cynomolgus monkeys. Regul Toxicol Pharmacol. 2013;66:6–12.
- Baird DT, Brown A, Critchley HO, et al. Effect of long-term treatment with low-dose mifepristone on the endometrium. Hum Reprod. 2003;18:61–68.
- Slayden OD, Brenner RM. Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol. 2004;67:393–409.
- Brenner RM, Slayden OD. Progesterone receptor antagonists and the endometrial antiproliferative effect. Semin Reprod Med. 2005;23:74–81.
- Williams AR, Critchley HO, Osei J, et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod. 2007;22:1696–1704.
- Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–930.
- Wilkens J, Williams AR, Chwalisz K, et al. Effect of asoprisnil on uterine proliferation markers and endometrial expression of the tumour suppressor gene, PTEN. Hum Reprod. 2009;24:1036–1044.
- NICE. Clinical Guideline 44; Heavy menstrual bleeding 2007; [cited 2016 Feb 2]. Available from: http://www.nice.org.uk/nicemedia/pdf/CG44FullGuideline.pdf
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258.
- Munro MG, Critchley HO, Broder MS, et al. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3–13.
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298.
- Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit. 2008;14:CR24–CR31.
- Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211.e1–211.e9.
- ONS. Who is having babies? 2009; [cited 2016 Feb 2]. Available from: http://webarchive.nationalarchives.gov.uk/20140721132900/http://www.statistics.gov.uk/pdfdir/births1209.pdf
- RCOG. National heavy menstrual bleeding audit final report. London: RCOG; 2014.
- Engman M, Granberg S, Williams AR, et al. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod. 2009;24:1870–1879.
- Wilkens J, Chwalisz K, Han C, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab. 2008;93:4664–4671.
- Chwalisz K, Garg R, Brenner RM, et al. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373–388.
- Kettel LM, Murphy AA, Morales AJ, et al. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertil Steril. 1996;65:23–28.
- Chwalisz K, Perez MC, Demanno D, et al. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438.
