Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer-related deaths in advanced countries and is on the rise in developing countries. It is a devastating disease with a median five-year survival rate of only 8% [Citation1]. The fundamental reason for such a poor survival rate is that so far no clinically effective targeted therapeutics have been developed for this disease [Citation2]. This situation calls for the need to focus on discovering novel signaling molecules that could lead to PDAC, and also to define the mechanistic role of these molecules in causing cancer progression, metastasis, and therapeutic resistance. The signaling molecules as well as their molecular mechanisms that contribute to the transformation of normal pancreatic cells to pancreatic adenocarcinoma cells have been widely studied [Citation3]. Approximately more than 95% of human pancreatic cancers carry oncogenic RAS mutations, specifically in K-RAS at codon 12 [Citation4]. This has been shown to erratically activate Pak1. P21 activated kinase 1 (Pak1)—a serine/threonine kinase that orchestrates cytoskeletal remodeling and cell motility—has been shown to function as downstream nodule for various oncogenic signaling pathways that promote cell proliferation, regulate apoptosis, and accelerate mitotic abnormalities, resulting in tumor formation and invasiveness. Recent reports showed that Pak1 per se is overexpressed and plays a crucial role in transformation in a wide variety of cancers and also causes drug resistance [Citation5,Citation6]. Despite the remarkable increase in information about the biology of human Pak1 in various malignancies since its discovery, the contribution and direct role of Pak1 signaling to the etiology of pancreatic cancer remains elusive, and this represents one of the core areas to focus on.
As a consequence of this, Pak1 expression pattern in human pancreatic cancer tissues was evaluated and it was found that Pak1 levels are significantly high in PDAC tissue [Citation4,Citation7]. Overexpression and downregulated Pak1 cell line model systems revealed explicit changes in transforming properties, and Pak1 diminished cells failed to form tumors in athymic mice [Citation7]. Quantitative PCR array-based approach identified fibronectin (FN), a component of the extracellular matrix, as a transcriptional target of Pak1 signaling. Further, in vitro studies revealed that Pak1 per se nuclear Pak1 contributes to invasive EMT phenotype via modulating NF-κB-p65-FN nexus in pancreatic cancer cells [Citation7]. This is one of the first molecular studies to examine the role of Pak1 pathway in the involvement of pancreatic cancer.
Tumor cell–stroma interactions are being increasingly recognized as important determinants of pancreatic tumor cell fate. It is well established that pancreatic cancer cells recruit pancreatic stellate cells (PSCs) via fibrogenic and mitogenic factors, which promote PSC activation, fibrosis, and ECM remodeling capability [Citation8]. As Pak1 modulates EMT markers like FN, E-cadherin, and vimentin, it is speculated that Pak1 might also play a role in activating PSCs, thereby causing desmoplasia, which will eventually lead to chemoresistance (). On the other hand, Pak1 being an established survival signaling molecule may also protect pancreatic cancer cells from adverse effects of chemotherapy via multiple signaling crosstalks. Therefore, combinatorial therapeutic strategies capable of managing these detrimental effects of Pak1 need to be tailored to effectively handle pancreatic cancer [Citation9]. Also, it was reported that targeting RAC-Pak1 signaling in RAS-driven cancers using Pak1 deletion or Pak1 inhibitor could efficiently regress tumor growth in vivo [Citation10]. As the earlier pharmacologic inhibitors of Pak1 are not very specific, new Pak1 selective inhibitors such as FRAX-597, G-5555, Novartis compound 3 etc. are been synthesized and are under evaluation for clinical efficacy [Citation11–Citation13]. In addition, as suggested by Baker et al. [Citation14], it would be worthwhile to identify a reliable Pak1 substrate-specific biomarker for Pak1 inhibitor antitumor activity. This will aid in high-throughput screening of inhibitors specific and selective to Pak1 on a reliable model system. We hope that all these strategies to handle Pak1 in tumor cells will eventually pave the way to PAKup pancreatic cancer to a certain extent.
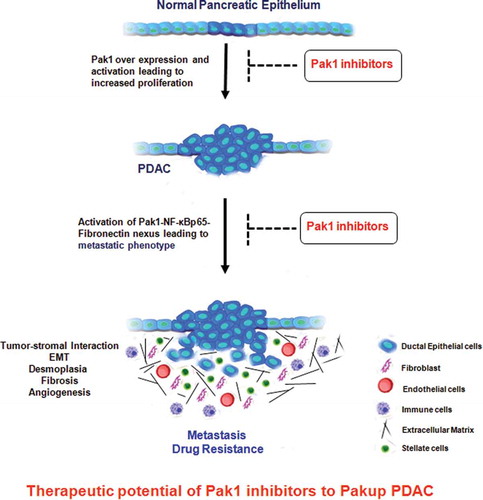
Figure 1. Pak1 over expression promotes proliferation, metastasis and drug resistance in pancreatic cancer through Pak1-NF-κBp65-Fibronectin pathway. Activation of this nexus would enhance tumorigenesis process by activating EMT, fibronectin production, fibrosis, desmoplastic reaction, increasing vascular formation and recruiting immune cells for tumor growth. Blocking of Pak1 activation and downstream nexus pathways with Pak1 inhibitors might play a therapeutic role for the treatment of pancreatic cancer.
Expert opinion
Ample amount of PubMed data available on Pak1 point out to the fact that Pak1 is one of the extensively studied/investigated molecule in the field of oncology. Pak1—a serine threonine kinase—is amplified or overexpressed in several tumors. Pak1’s ability to modulate cytoskeletal remodeling and cell survival functions have triggered considerable interest among cancer researchers—to an extent that strategies targeting Pak1 may lead to the emergence of target specific drugs.
PDAC remains one of the most lethal cancers among all solid tumors. Recently, several reports [Citation4,Citation9,Citation11,Citation15] unraveled the role of Pak1 in the activation of pancreatic stellate cells (PSCs), its involvement in transforming normal pancreatic cells to PDAC and its ability to modulate drug resistance in pancreatic cancer. All these findings suggest that Pak1-specific inhibitors will prove to be a better adjuvant with the existing chemotherapy modality for PDAC. In addition, the reported difference in the expression levels of Pak1 between adjacent normal and human pancreatic tumor tissues could be exploited to develop drugs that selectively inhibit cancer cells.
The identification of unique Pak1 substrates as well as novel Pak1-interacting proteins combined with the availability of the crystal structure of Pak1 provides an ideal atmosphere to develop novel small molecules/inhibitors or peptides to interfere with Pak1 activity. However, till date, no specific Pak1-directed therapeutic drug has advanced to proof-of-concept studies in human trails, although several major pharmaceutical companies are currently attempting to produce such drugs that inhibit Pak1 kinase activity. One possible impeding factor which is difficult to achieve is the selectivity within the PAK family, because of sequence homology among the family members. Although some Pak1 selective inhibitors like IPA-3 [Citation9,Citation16–Citation19], FRAX-597 [Citation11,Citation20], G-5555 [Citation12], and Novartis compound 3 [Citation13] have been disclosed, the successful in vivo dose optimization for target specificity and stability is yet to be tested on larger group of animals.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30.
- Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6:321–337.
- Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806.
- Yeo D, He H, Baldwin GS, et al. The role of p21-activated kinases in pancreatic cancer. Pancreas. 2015;44:363–369.
- Radu M, Semenova G, Kosoff R, et al. PAK signalling during the development and progression of cancer. Nat Rev Cancer. 2014;14:13–25.
- Eswaran J, Li DQ, Shah A, et al. Molecular pathways: targeting p21-activated kinase 1 signaling in cancer–opportunities, challenges, and limitations. Clin Cancer Res. 2012;18:3743–3749.
- Jagadeeshan S, Krishnamoorthy YR, Singhal M, et al. Transcriptional regulation of fibronectin by p21-activated kinase-1 modulates pancreatic tumorigenesis. Oncogene. 2015;34:455–464.
- Haqq J, Howells LM, Garcea G, et al. Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies. Eur J Cancer. 2014;50:2570–2582.
- Jagadeeshan S, Subramanian A, Tentu S, et al. P21-activated kinase 1 (Pak1) signaling influences therapeutic outcome in pancreatic cancer. Ann Oncol. 2016;27:1546–1556.
- Chow HY, Jubb AM, Koch JN, et al. p21-activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012;72(22):5966–5975.
- Yeo D, He H, Patel O, et al. FRAX597, a PAK1 inhibitor, synergistically reduces pancreatic cancer growth when combined with gemcitabine. BMC Cancer. 2016;16:24.
- Ndubaku CO, Crawford JJ, Drobnick J, et al. Design of Selective PAK1 Inhibitor G-5555: improving properties by employing an unorthodox low-pK a polar moiety. ACS Med Chem Lett. 2015;6:1241–1246.
- Karpov AS, Amiri P, Bellamacina C, et al. Optimization of a dibenzodiazepine hit to a potent and selective allosteric PAK1 Inhibitor. ACS Med Chem Lett. 2015;6:776–781.
- Baker NM, Yee Chow H, Chernoff J, et al. Molecular pathways: targeting RAC-p21-activated serine-threonine kinase signaling in RAS-driven cancers. Clin Cancer Res. 2014;20:4740–4746. .
- Yeo D, He H, Phillips P, et al. Targeting PAK1 in pancreatic stellate cells increases pancreatic cancer survival. Pancreatology. 2016;16:S26–S27.
- Deacon SW, Beeser A, Fukui JA, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331.
- Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther. 2009;8:2559–2565.
- Wong LL, Lam IP, Wong TY, et al. IPA-3 inhibits the growth of liver cancer cells by suppressing PAK1 and NF-κB activation. PLoS One. 2013;8:e68843.
- Al-Azayzih A, Missaoui WN, Cummings BS, et al. Liposome-mediated delivery of the p21 activated kinase-1 (PAK-1) inhibitor IPA-3 limits prostate tumor growth in vivo. Nanomedicine. 2016;12:1231–1239.
- Licciulli S, Maksimoska J, Zhou C, et al. FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas. J Biol Chem. 2013;288:29105–29114.
