1. Expert opinion
Acute myeloid leukemia (AML) is an aggressive cancer affecting mostly adult and elderly patients. The common clinical practice remains unchanged since decades, consisting of sequential courses of standard chemotherapy. As a result of this non-specific treatment, resistance and relapse frequently occur after treatment. Transplantation of donor-derived healthy stem cells represents another major line of intervention, aimed to replenish the bone marrow (BM) with normal hematopoietic cells. However, recent preclinical findings have evidenced a severe vascular pathology occurring in the BM of AML xenografts, causing vascular leakiness and mobilization of healthy stem cells to the periphery, chasing them away from their protective niche environment and therefore exposing them to drug toxicity. This vascular dysfunction could represent a severe obstacle to the success of the stem cell transplantation approach, limiting the out-competition effect of both patient residual and donor-derived stem cell on the leukemic clones (graft versus leukemia). Thus, finding a strategy to keep healthy stem cells in the BM could significantly improve treatment outcome. Hence, recent literature addressing the molecular mechanisms of stem cell anchorage to the niche highlighted nitric oxide (NO) as a potential valuable target. The choice of the best therapeutic strategy would require further characterization of the mechanisms regulating NO production in the BM microenvironment. Yet, the most challenging step would be to move these studies from the bench to the bedside for a clinical assessment of their efficacy in combination with standard chemotherapy.
2. Editorial
Adult hematopoietic stem cells (HSCs) mainly reside in the BM, interacting with stromal cells to sustain lifelong mature blood production. Emerging data depict a complex array of regulatory cells with predominant roles for mesenchymal stem cells, their osteoblastic cell derivatives, and endothelial cells in forming both perivascular and endosteal BM niches, which maintain HSCs. In leukemia, the HSC niche has recently emerged as an oncogenic unit and thus has provided potential new lead for target intervention. Leukemic cells can indeed turn BM niches into ‘leukemic niches,’ which support them and impair the maintenance of normal HSC. Recent evidences have highlighted the dramatic impact of AML on the BM stem cell niche. In preclinical setting, Passaro et al. have shown that AML causes several vascular abnormalities. Vascular wall barriers responsible for oxygen, nutrients, and drug delivery appear severely damaged, with increased vascular permeability and hypoxia, altered perfusion, and release of normal HSCs to periphery. At molecular level, vascular niche signature is altered, resulting in increased endothelial nitric oxide synthase (NOS3) activation and NO release in the BM [Citation1].
NO is a master regulator of multiple physiologic and pathologic functions. One of its major contribution to the maintenance of the systemic homeostasis is linked to the vasculature. Produced and released by endothelial cells, among the other function, it is responsible for the vascular tone regulation through smooth muscle relaxation [Citation2], vascular permeability [Citation3], and angiogenesis [Citation4]. Moreover, in solid tumors, NO production by cancer cells and associated endothelium participates in angiogenesis and hyper-permeability [Citation5], a pathologic phenotype of tumor vessels [Citation6]. Increased vessel number and abnormalities have also been reported in AML patient-derived biopsies in several studies, raising the interest for anti-angiogenic therapies also in hematologic malignancies [Citation7]. However, diagnostic techniques to detect vascular permeability in patients at large scale are still under development.
A less explored field points to NO as a potential regulator of the microvascular environment in terms of stem cell regulation [Citation8]. NO is a direct mediator of leukocytes mobilization [Citation9]. Recent studies reported a role for cell autonomous NO production within the HSC compartment in regulating stem cell motility and niche adhesion [10]. NO being a gaseous metabolite, it rapidly diffuses outside of the producing cell and enters the surrounding environment. Thus, it is worth speculating that a regulated NO production in the stem cell niche is also required to maintain the balance between anchorage and release. The BM niche is responsible for stem cell adhesion/anchorage and reduction of motility [Citation11]. The microvasculature, in particular, has classically been associated with the control of HSC traffic [Citation12]. Far from being a passive gateway, endothelial cells surrounding specialized vessels in the BM regulate HSC anchorage by producing a complex milieu of adhesion molecules, cytokines, growth factors, and extracellular matrix [Citation13,Citation14].
Inhibition of NO production in preclinical models of AML significantly improved treatment response by restoring normal vascular niche, improving drug response and at the same time preserving residual BM HSCs in the niche [Citation1]. In this scenario, inhibition of NO in clinical setting could be employed as a novel strategy in combination with leukemia treatment to improve patient survival ().
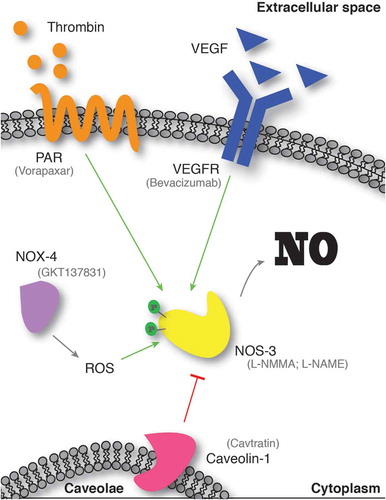
NO inhibitors have been developed for several applications, and many of them are under characterization in clinical trials [Citation15]. However, the specificity of these compounds represents an issue, with most of them selectively acting on NOS2 and only few specific for NOS3. The development of a specific peptide able to mimic the endogenous inhibiting function of caveolin-1 has been a valuable tool for investigating the mechanisms involved in NOS3 regulation and NO production, as well as a valuable inhibitor in preclinical studies [Citation16].
The efficacy of this peptide in inhibiting NO production suggests that a more functional approach would be to identify and target upstream regulators of NO production in endothelial cells. The choice of the best therapeutic target turns out to be very difficult, as the upstream regulation of NO production in endothelial cells is broad and complex (). Potential therapeutic targets can be found among pro-angiogenic factors produced by AML. The Vascular Endothelial Growth Factor/ Vascular Endothelial Growth Factor Receptor (VEGF/VEFGR) signaling pathway is the master regulator of vascular formation, endothelial cell proliferation and migration, as well as vascular permeability [Citation5], and it accounts for several Food and Drug Administration (FDA)-approved inhibitors [Citation17]. Moreover, inhibition of VEGF, being also a growth factor for AML cells, might have a dual therapeutic effect on both the leukemic compartment (direct) and the vascular function (indirect). However, targeting VEGF has been widely tested in clinical trials to inhibit AML-derived angiogenesis and it has only produced modest results [Citation18]. Similar to VEGF, other angiogenic cytokines have deceived the initial enthusiasm when brought to clinics [Citation19,Citation20]. Nevertheless, more selective approaches could be designed, targeting specific branches of these pathways. For instance, VEGFR2 (NRP1) is likely to be a more suitable target, as it specifically orchestrates the endothelial junction formation and maintenance and it can selectively regulate permeability during vasculature development [Citation21,Citation22]. From a different angle, hypoxia-activated NAPDH oxidases (e.g. NOX4) also represent an intriguing pathway to inactivate for inhibiting NO production, since they have been implicated in endothelial response to hypoxia and activation of NOS3 [Citation23]. Moreover, several inhibitors have been produced, one of them in clinical development [Citation24], thus representing a promising concrete strategy. Finally, another possibility to explore is targeting the Thrombin/PAR pathway, known regulators of NOS3 in endothelial cells via phosphorylation [Citation25], and recently identified upstream NO production also in HSCs [10]. Despite that several PAR inhibitors have been developed [Citation26], the effect of this signaling on stem cell mobilization has only been described in the HSC compartment, thus more data are required to support its effectiveness as a niche-based therapeutic intervention.
In conclusion, the implication of NO in vascular permeability and HSC mobilization during leukemia lead the way to promising paths toward clinical applications, with the possibility to enhance the efficacy of current treatment strategies and improve patient survival.
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Passaro D, Di Tullio A, Abarrategi A, et al. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell. 2017 Sep 11;32(3):324–341 e6. PubMed PMID: 28870739; PubMed Central PMCID: PMCPMC5598545.
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11–17;327(6122):524–526.. PubMed PMID: 3495737.
- Hatakeyama T, Pappas PJ, Hobson RW 2nd, et al. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol. 2006 Jul 1;574(Pt 1):275–281. PubMed PMID: 16675496; PubMed Central PMCID: PMCPMC1817804.
- Cooke JP. NO and angiogenesis. Atheroscler Suppl. 2003 Dec;4(4):53–60. PubMed PMID: 14664903.
- Fukumura D, Gohongi T, Kadambi A, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001 Feb 27 98(5):2604–2609. PubMed PMID: 11226286; PubMed Central PMCID: PMCPMC30185
- McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002 Sep 15;62(18):5381–5385. PubMed PMID: 12235011.
- Cogle CR, Bosse RC, Brewer T, et al. Acute myeloid leukemia in the vascular niche. Cancer Lett. 2016 Oct 1;380(2):552–560. PubMed PMID: 25963886.
- Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003 Nov;9(11):1370–1376. PubMed PMID: 14556003.
- Chigaev A, Smagley Y, Sklar LA. Nitric oxide/cGMP pathway signaling actively down-regulates alpha4beta1-integrin affinity: an unexpected mechanism for inducing cell de-adhesion. BMC Immunol. 2011 May 17;12:28.
- Foster K, Lassailly F, Anjos-Afonso F, et al. Different motile behaviors of human hematopoietic stem versus progenitor cells at the osteoblastic niche. Stem Cell Reports. 2015 Nov 10;5(5):690–701. PubMed PMID: 26455414.
- Rafii S, Mohle R, Shapiro F, et al. Regulation of hematopoiesis by microvascular endothelium. Leukemia & Lymphoma. 1997 Nov;27(5–6):375–386. PubMed PMID: 9477120.
- Itkin T, Gur-Cohen S, Ja S, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016 Apr 21;532(7599):323–328. PubMed PMID: 27074509.
- Singh P, Hoggatt J, Kamocka MM, et al. Neuropeptide Y regulates a vascular gateway for hematopoietic stem and progenitor cells. J Clin Invest. 2017 Nov 13;127:4527–4540. PubMed PMID: 29130940.
- Vitecek J, Lojek A, Valacchi G, et al. Arginine-based inhibitors of nitric oxide synthase: therapeutic potential and challenges. Mediators Inflamm. 2012;2012:318087. PubMed PMID: 22988346; PubMed Central PMCID: PMCPMC3441039.
- Gratton JP, Lin MI, Yu J, et al. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003 Jul;4(1):31–39. PubMed PMID: 12892711.
- Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med. 2012 Oct 1;2(10). PubMed PMID: 23028128; PubMed Central PMCID: PMCPMC3475399. Doi: 10.1101/cshperspect.a006577.
- Gordon EM, Figueroa DM, Barochia AV, et al. High-density Lipoproteins and Apolipoprotein A-I: potential new players in the prevention and treatment of lung disease. Front Pharmacol. 2016;7:519. PubMed PMID: 28111549; PubMed Central PMCID: PMCPMC5216034.
- Ribatti D, Vacca A, Rusnati M, et al. The discovery of basic fibroblast growth factor/fibroblast growth factor-2 and its role in haematological malignancies. Cytokine Growth Factor Rev. 2007 Jun-Aug;18(3–4):327–334. PubMed PMID: 17537668.
- Aref S, Mabed M, Sakrana S, et al. Soluble hepatocyte growth factor (sHGF) and vascular endothelial growth factor (sVEGF) in adult acute myeloid leukemia: relationship to disease characteristics. Hematology. 2002 Oct;7(5):273–279. PubMed PMID: 12850814.
- Fantin A, Lampropoulou A, Senatore V, et al. VEGF165-induced vascular permeability requires NRP1 for ABL-mediated SRC family kinase activation. J Exp Med. 2017 Apr 3;214(4):1049–1064. PubMed PMID: 28289053; PubMed Central PMCID: PMCPMC5379968.
- Erskine L, Francois U, Denti L, et al. VEGF-A and neuropilin 1 (NRP1) shape axon projections in the developing CNS via dual roles in neurons and blood vessels. Development. 2017 Jul 1;144(13):2504–2516. PubMed PMID: 28676569; PubMed Central PMCID: PMCPMC5536872.
- Craige SM, Chen K, Pei Y, et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011 Aug 9;124(6):731–740. PubMed PMID: 21788590; PubMed Central PMCID: PMCPMC3589548.
- Altenhofer S, Radermacher KA, Kleikers PW, et al. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal. 2015 Aug 10;23(5):406–427. PubMed PMID: 24383718; PubMed Central PMCID: PMCPMC4543484.
- Tillery LC, Epperson TA, Eguchi S, et al. Featured Article: differential regulation of endothelial nitric oxide synthase phosphorylation by protease-activated receptors in adult human endothelial cells. Exp Biol Med (Maywood). 2016 Mar;241(6):569–580. PubMed PMID: 26729042; PubMed Central PMCID: PMCPMC4950326.
- Flaumenhaft R, De Ceunynck K. Targeting PAR1: now what? Trends Pharmacol Sci. 2017 Aug;38(8):701–716.. PubMed PMID: 28558960; PubMed Central PMCID: PMCPMC5580498.